Abstract
Study Objective
To evaluate blood pressure (BP) control following discontinuation of a physician\pharmacist collaborative intervention.
Design
BP was previously measured at baseline and at the end of a 9-month cluster-randomized intervention trial. This study abstracted medical record data for mean BP and BP control at 18 months (9 months after the discontinuation of the intervention) and at 27 months (18 months after discontinuation of the intervention).
Setting
Five primary care medical offices operated by a university health system.
Patients
Subjects with hypertension who were enrolled in a previous controlled trial and who consented to have data abstracted for an additional 18 months following the end of that study.
Intervention
A physician\pharmacist collaborative intervention to improve BP control was withdrawn after 9 months and BP and the change in BPs following withdrawal of the intervention were evaluated.
Measurements
A research nurse measured BP during the 9-month interventional study. BP values were then abstracted from the medical record for the 18 month period after the end of that study.
Main Results
104 patients had BP values at all 4 time periods. At baseline, systolic BP (SBP) was 152.5 ± 9.5 and 150.1 ± 9.6 mm Hg in the intervention and control groups, respectively (p=0.22). At 9 months, SBP decreased to 124.5 ± 10.7 and 132.0 ± 15.1 mm Hg (p=0.0038 between groups) and BP was controlled in 78.5% and 48.7% in the intervention and control groups, respectively (p=0.0017). By 18 months, SBP had deteriorated to 131.0 ± 12.2 and 143.3 ± 17.5 mm Hg (p<0.001) and BP control rates deteriorated to 53.9% and 30.8% in the intervention and control groups, respectively (p=0.02). By 27 months, SBP was 131.3 ± 13.0 and 141.2 ± 15.8 mm Hg (p=0.0008) and BP control was 55.3% and 35.9% in the intervention and control groups, respectively (p=0.05).
Conclusions
This study found a sustained effect on BP control up to 18 months following discontinuation of a pharmacist intervention. However, BP control deteriorated at a similar rate in both the intervention and control group but remained significantly higher in the intervention group. This study suggests that continued interventions by pharmacists may be necessary to maintain high rates of BP control, especially in those patients who lose BP control.
Keywords: hypertension management, clinical trial, pharmacist management, team-care, blood pressure control
Introduction
One of the most effective strategies to improve BP control involves team-based care that includes collaborative care or independent management by a pharmacist.1–10 We conducted a systematic review of the literature that included 37 controlled clinical studies of either nurse or pharmacist-assisted BP management.10 The reduction in SBP when a pharmacist was involved was 9.3 mm Hg and 4.8 mm Hg in studies involving nurses. The odds ratio (OR) and 95% confidence interval (CI) for the difference between the intervention and control group for controlled BP were: nurses OR=1.69 (CI = 1.48, 1.93), pharmacists within primary care clinics OR=2.17 (CI = 1.75, 2.68) and community pharmacists OR=2.89 (CI = 1.83, 4.55).10
We recently reported the results of a prospective, cluster-randomized efficacy trial in 179 patients with previously uncontrolled BP conducted in five clinics that was included in the systematic review.4 BP control was achieved in 89% of patients following a 9-month physician\pharmacist collaborative intervention compared to 53% in the control group as defined by the 7th Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC-7).11 After that study began, our group was funded by AHRQ to develop a Center for Education and Research on Therapeutics (CERT). As part of that award, patients from both control and intervention groups from our hypertension study were invited to sign additional consent to participate in an extended post-intervention follow-up period which is the basis for this paper. There are few studies that have evaluated the effect of discontinuing a pharmacist intervention for hypertension.6, 7 In a paper published in 1973, McKenney conducted a study within a community pharmacy and compared BP in a control group (n=25) to a study group (n=25) that received pharmacist interventions to improve BP control. This study evaluated BP before, during and after the pharmacist intervention and found that BP control deteriorated when the pharmacist management was discontinued.7 BP was controlled in 20% of patients in the control group after the intervention but only 14% after the intervention was discontinued. In the intervention group, BP control was 79% during the intervention but it deteriorated to 42% after the intervention was discontinued.
The purpose of the present study was to evaluate whether BP control deteriorated following the discontinuation of a physician\pharmacist collaborative intervention from a cluster randomized controlled clinical trial. We hypothesized that mean BP values and BP control as defined by JNC-7 would deteriorate but that there would be some residual benefit 18 months following the discontinuation of the intervention.
Methods
The design of the clinical study was previously published.4 Briefly, the prospective, cluster-randomized controlled efficacy trial involved five primary care clinics that were randomized to either a control (n=3) or intervention (n=2) group. One control and one intervention clinic were residency training sites but only patients cared for by faculty physicians were included in the study. Educational lectures were provided to physicians in all five clinics (control and intervention sites) by one pharmacist investigator (BLC) immediately before patients were enrolled. The majority of participating physicians attended these training sessions. Handouts, slides and the express version of the JNC-7 guidelines11 were supplied to all physicians including those who were unable to attend these sessions. A validated 26-item knowledge survey of the JNC-7 guidelines was administered at baseline prior to patient recruitment and at the end of the initial clinical trial to determine if physician learning occurred during the course of the study.12
Patients aged 21 to 85 years with uncontrolled hypertension and without diabetes were eligible if their clinic BP was between 145–179 mm Hg systolic BP or 95–109 mm Hg diastolic BP. Patients with diabetes or chronic kidney disease were eligible if they had a clinic BP between 135–179 mm Hg systolic BP or 85–109 mm Hg diastolic BP. Values 5 mm Hg above accepted goal levels were chosen to eliminate patients near their BP goal. Exclusion criteria included: BP medication or dose change within four weeks of the baseline visit, enrollment in the 24-hour BP monitoring consult service within the previous 6 months, severe hypertension (BPs ≥ 180/110 mm Hg), evidence of hypertensive urgency or emergency, myocardial infarction or stroke (6 months prior to screening), New York Heart Association Class III or IV heart failure, unstable angina, serious renal or hepatic disease, pregnancy, poor prognosis (life expectancy less than 3 years), dementia or cognitive impairment. The initial study was an intention-to-treat analysis and all enrolled patients were evaluated.4 The present study included patients from the parent study who consented to have additional data abstracted from their medical record after the 9-month interventional study and who had BP values at both 9 and 18 months after discontinuation of the intervention‥
The study was approved by the University of Iowa Institutional Review Board and all patients signed informed consent. Physicians also signed consent so that a knowledge survey could be administered. The knowledge instrument was previously validated.12 Two different research nurses, each dedicated to patients in either control sites or intervention sites, collected the following data at the baseline visit: patient age, gender, race, educational degree, insurance status, household income, marital status, smoking status, alcohol intake and history of co-existing conditions. They measured the patient’s height and weight, calculated a basal metabolic index (BMI), recorded all antihypertensive medications, doses and dates of last refills and performed a pill count of BP medications to calculate medication adherence. Patients in both groups were provided written information on hypertension from the National Heart Lung and Blood Institute. The research nurses encouraged all patients (control and intervention) to follow the lifestyle modifications (diet, exercise, stopping smoking) as described in these resources. Patients were given their BP value at each visit and were given their desired goal BP.
Research nurses were specially trained to measure BP using the American Heart Association guidelines and the process used in large clinical trials.13, 14 Specifically, the nurses measured the subjects’ BP three times at each data collection visit with a mercury sphygmomanometer using standardized techniques. The second and third values were averaged and used as the research BP. The nurses were certified quarterly in their ability to accurately position patients and measure BP to ensure consistent and valid readings. Patients returned at 2, 4, 6, 8 and 9 months for repeat research BP measurements. Patients were instructed to return all of their BP medications at every study visit. The research nurses performed pill counts at each visit so that medication adherence could be calculated.
Five clinical pharmacists provided the original clinical trial intervention collaboratively with physicians in their clinics. The clinical pharmacists followed the JNC-7 guidelines and provided strategies to improve BP control, methods to optimize therapy and strategies to improve medication adherence. The intervention protocol specified a patient interview at baseline by the clinical pharmacist and to assess the patient’s regimen, suggest a goal BP and provide recommendations to improve BP control to the physician. BP control was defined as an office BP <130/80 mm Hg for patients with diabetes or chronic kidney disease and <140/90 mm Hg for all other patients.11, 15 The clinical pharmacists were encouraged to see the patients at each scheduled research visit at baseline and 2, 4, 6 and 8 months and they provided an average of 1.1 additional visits, usually between the baseline and 2-month visit. Additional details about the intervention and the types of medication changes made for the larger study sample (n=179) during the intervention have previously been published.4, 16
After this study began, the investigators received funding from AHRQ for a Center for Education and Research on Therapeutics (CERT) and one of the projects in that grant was to evaluate the effect of discontinuation of the pharmacist intervention on BP. Patients from the clinical trial who agreed to participate signed a new consent form that allowed the investigators to collect data for an additional 18 months following the completion of the original clinical trial (i.e. 27 months after initiation of the intervention). One research nurse constructed case abstracts of relevant medical record data using techniques we have developed.17–19 She abstracted data for 18 months following the clinical trial including BP, laboratory values and all clinic visits. BP values in the post-clinical trial phase were obtained from the medical record. If more than one BP value was recorded in the medical record on a given clinic visit, the lowest value was used. In order to determine a patient’s BP at the 18 and 27 month time periods, the BP value recorded in the medical record that was closest to those index dates were used in the analyses.
Data management and statistical analysis
All patient data were entered into case report forms by the research nurses. Individual data elements were double-entered into an Access® database by a data management team that included data technicians, the data manager and the biostatistician (JDD).
Descriptive statistics (means, standard deviations, and percentages) of patient demographic and health-related variables were calculated at baseline for each group. Baseline comparisons between the groups were made using Student’s t-test and Fisher’s Exact test. Preliminary analysis revealed that the response variables were correlated within-subject, but no significant clustering due to clinics or physicians was observed.
Results
Patient recruitment for the previous study began in January 2004 and 179 patients (78 and 101 in the control and intervention groups respectively) were enrolled in the initial 9-month clinical trial. The present study began more than a year after the initiation of the initial clinical trial so many patients were unavailable. Twenty two patients were not available for the present study: 18 patients (8 intervention and 10 control) dropped in the first 9 months, 2 patients in the intervention group moved, 1 patient in the intervention group changed their source of medical care and one patient in the control group died. Another 28 patients refused consent for the additional chart audit following the 9-month clinical trial (9 intervention, 19 control). Of the 129 patients who signed consent, 104 patients had complete BP data at all 4 time intervals specified in this analysis (39 control and 65 intervention). Our protocol required evaluating only those who had BP data points that could be evaluated at baseline, 9-month (end of the pharmacist intervention), 18 months (9 months following discontinuation of the intervention) and at 27 months (18 months following discontinuation of the intervention). However, we evaluated BP for the 25 patients who signed consent but who had incomplete BP data to be sure that their results did not differ from those with complete data. Patient enrollment in both the parent study and the present report were uneven since the study randomized the intervention by clinic and not by patient to avoid contamination at the physician level. Baseline demographic data for both groups are shown in Table 1. All baseline demographics were no different between groups except BMI was significantly higher and medication adherence significantly lower in the intervention group than the control group.
Table 1.
Patient Demographics at Baseline
| Control (n=39) Number (%) or Mean (± SD) |
Intervention (n=65) Number (%) or Mean (± SD) |
p-value for difference |
|
|---|---|---|---|
| Gender: Female | 26 (66.7) | 37 (56.9) | 0.32 |
| Male | 13 (33.3) | 28 (43.1) | |
| Race: Caucasian | 38 (97.4) | 59 (90.8) | 0.19 |
| Non-Caucasian | 1 (2.6) | 6 (9.2) | |
| Age (years | 62.77 (±11.1) | 58.94 (±13.2) | 0.13 |
| Married | 22 (56.4) | 36 (55.4) | 0.92 |
| Education beyond high School |
25 (64.1) | 39 (60.0) | 0.68 |
| Household income < $25,000 | 8 (20.5) | 11 (16.9) | 0.65 |
| Insurance status | |||
| Individual or group plan | 34 (87.2) | 53 (81.5) | 0.29 |
| Medicare/Medicaid | 5 (12.8) | 8 (12.3) | |
| Self-pay or other | 0 (0.0) | 4 (6.2) | |
| BMI (kg/m2) | 29.15 (±6.0) | 31.99 (±7.2) | 0.04 |
| Smoker (within last 15 years) | 7 (18.0) | 11 (16.9) | 0.89 |
| More than 2 alcoholic drinks / Week |
9 (23.1) | 21 (32.3) | 0.31 |
| Family History of premature CV event |
10 (25.6) | 22 (33.9) | 0.38 |
| Diabetes Mellitus | 11 (28.2) | 17 (26.2) | 0.82 |
| Hx Stroke or TIA | 4 (10.3) | 4 (6.2) | 0.47 |
| Hx Myocardial infarction | 4 (10.3) | 6 (9.2) | 0.86 |
| Coronary artery bypass | 5 (12.8) | 3 (4.6) | 0.15 |
| Grafting | |||
| Heart failure | 1 (2.6) | 3 (4.6) | 1.0 |
| Angina | 1 (2.6) | 4 (6.2) | 0.65 |
| Peripheral arterial disease | 3 (7.7) | 5 (7.7) | 1.0 |
| Chronic kidney disease | 8 (20.5) | 15 (23.1) | 0.76 |
| Left-ventricular hypertrophy | 6 (15.4) | 5 (7.7) | 0.32 |
| At least one co-existing Condition* |
39 (100) | 61 (93.9) | 0.11 |
| Number of co-existing Conditions* |
3.31 (±1.7) | 2.74 (±2.1) | 0.16 |
| At least one antihypertensive | 30 (76.9) | 57 (87.7) | 0.15 |
| Number of antihypertensive | 1.33 (±1.0) | 1.63 (± 1.0) | 0.14 |
| Medications | |||
| Baseline medication Adherence (%) |
83.97 (±24.1) | 71.59 (±24.0) | 0.02 |
BMI = body mass index, CV = cardiovascular, Hx = history, TIA = transient ischemic attack
includes any of the listed co-existing conditions including diabetes mellitus.
The five clinics involved in this study and the pharmacist qualifications have previously been described and are not reported here.4 However, as previously reported, when adjusted for the intervention effect, the within-clinic interclass correlation coefficient (ICC) for SBP at 9 months was 0.0084 (within-clinic variance, 139.1; between-clinic variance, 1.2; clinic effect; p=0.416). When adjusted for all relevant baseline covariates, the ICC went from 0.0084 down to 0.0010. Similarly, the physician effects were very small, as the within-physician ICC was 0.0097, within-physician variance was 138.4, and between-physician variance was 1.4 (physician effect; p=0.418). When adjusted for baseline covariates, the between-physician ICC went from 0.0097 down to 0.0005. These results demonstrate that there was no clustering of effect by clinic or physician meaning that differences in clinic procedures or physician behavior/knowledge did not influence the results.
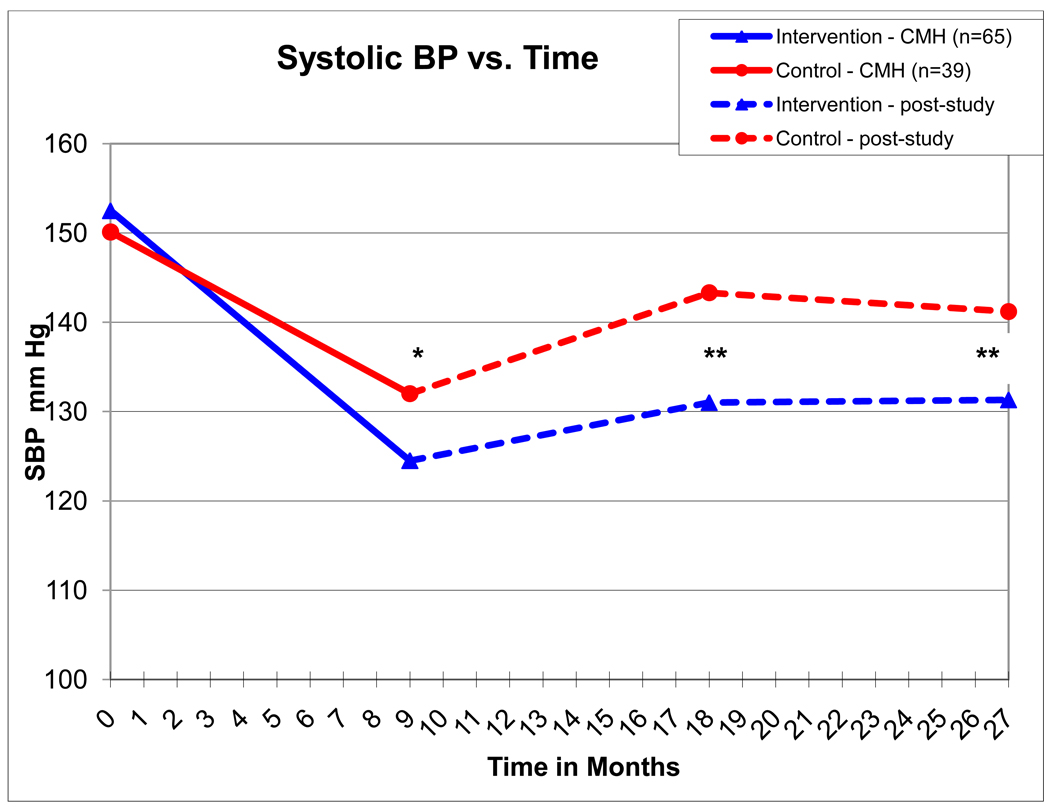
For the entire group of 129 patients who initially signed consent for the present study, the baseline mean systolic BP (SBP) was 152.7 ± 9.2 and 150.0 ± 9.7 mm Hg in the intervention and control groups, respectively (p=0.25) at baseline. These results were nearly identical for the 104 patients who had complete follow-up BP data at the four milestone dates where mean systolic BP (SBP) was 152.5 ± 9.5 and 150.1 ± 9.6 mm Hg in the intervention and control groups, respectively (p=0.22) at baseline (Table 2 and Figure 1).
Table 2.
Blood pressure values and blood pressure control at each time period
| Variable | Baseline | 9 month (clinical trial) |
18 month (9-month post- Clinical trial) |
27 month (18-month post clinical Trial) |
|---|---|---|---|---|
| Control (n=39) | ||||
| SBP | 150.1 ±9.6 | 132.0 ±15.1* | 143.3 ±17.5** | 141.2 ±15.8** |
| DBP | 85.4 ±10.7 | 79.1 ±11.7 | 75.7 ±9.5 | 77.1 ±11.3 |
| BP control (%)† | 0 | 48.7%# | 30.8%## | 35.9%§ |
| Intervention (n=65) | ||||
| SBP | 152.5 ±9.5 | 124.5 ±10.7* | 131.0 ±12.2** | 131.3 ±13.0** |
| DBP | 85.5 ±12.2 | 75.2 ±10.2 | 77.1 ±8.7 | 76.3 ±11.7 |
| BP control (%)* | 0 | 78.5%# | 53.9%## | 55.3%§ |
BP control defined as <130/80 mm Hg for patients with diabetes or chronic kidney disease and <140/90 for non-diabetic patients.
Between group differences:
p = 0.0038;
p < 0.001;
p=0.0017,
p=0.02,
p=0.05
Figure 1.
Solid lines depict systolic BP as measured by research nurses in the 9-month prospective study, hatched lines depict systolic BP as measured in the clinic and reported in the medical record.
* - p = 0.0038; ** - p < 0.001;
By the end of the 9-month intervention study, mean SBP decreased to 124.5 ± 10.7 in the intervention group and 132.0 ± 15.1 mm Hg in the control group (p=0.0038 between groups) when the 104 patients with complete data are evaluated (Table 2 and Figure 1). The results were nearly identical when evaluating all 129 patients who signed informed consent 124.2 ± 10.5 in the intervention group and 132.2 ± 13.7 mm Hg in the control group.
The remainder of this report will report the 104 patients with complete data at all time periods. Mean SBP deteriorated to 131.0 ± 12.2 and 143.3 ± 17.5 mm Hg (p<0.001) at 18 months (9 months following the completion of the 9-month clinical trial) in the intervention and control groups respectively. Mean SBP stabilized at 27 months (18 months following completion of the 9-month clinical trial) to 131.3 ± 13.0 and 141.2 ± 15.8 mm Hg (p<0.001) in the intervention and control groups, respectively. Diastolic BP values are displayed in Table 2. There were no significant differences between groups for DBP.
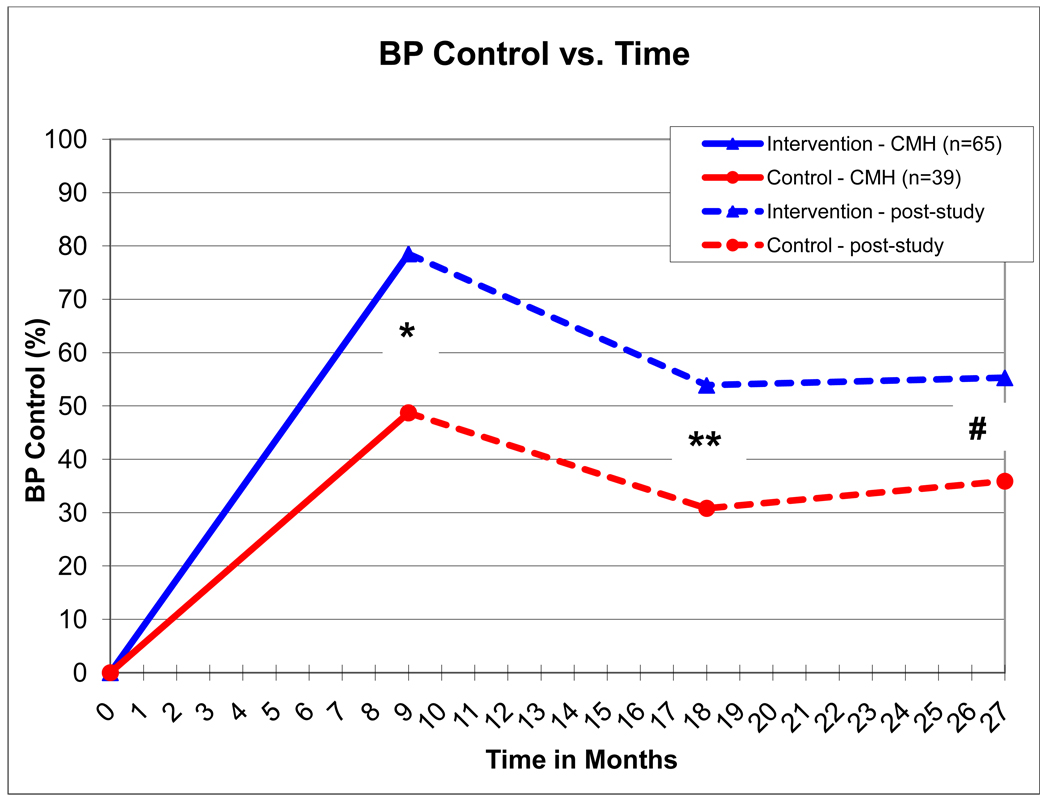
BP was controlled in 78.5% and 48.7% in the intervention and control groups, respectively (p=0.0017) at the end of the 9 month intervention study. BP control rates deteriorated to 53.9% and 30.8% in the intervention and control groups, respectively (p=0.02) at the 18-month time point. BP control was 55.3% and 35.9% in the intervention and control groups, respectively (p=0.05) at the 27-month time point.
Medication adherence, as determined by pill counts, was significantly better in the control group 84.0 ± 24.1% compared to 71.6 ± 24.0% in the intervention group at baseline (p=0.0248). Medication adherence improved in both groups by the 9-month study visit to 92.6 ± 11.8% in the control group and 98.9 ± 12.2% which were not significantly different between groups at the end of the intervention (p=0.1986).
There was no evidence that there was increased physician knowledge of the JNC-7 guidelines over time or as a function of the pharmacist intervention. At baseline, the percent of correct answers was 70.3% for physicians in the control group (n=14) compared to 63.5% in the intervention group (n=27, p=0.076). Several physicians failed to complete the knowledge survey at the end of the study. None-the-less, the results were very similar to baseline with 73.7% correct answers by control physicians (n=6) compared to 62.1% for physicians in the intervention group (n=21) (p=0.0055 between groups). Since the physicians in the intervention group had significantly lower correct answers compared to the control physicians, there is no evidence that improved physician knowledge was responsible for the better BP control in the intervention group.
Discussion
This study found sustained BP control for up to 18 months after a physician\pharmacist collaborative intervention was discontinued when compared to a control group. However, BP control deteriorated in both the control and intervention groups. The absolute difference between groups was 30% at the end of the 9-month intervention and remained 20% at the 18-month period following discontinuation of the intervention. These findings suggest a long-term, sustained effect of the pharmacist intervention. The fact that BP control was 49% in the control group and 79% in the intervention group at the end of the 9- month intervention period is impressive. These control rates are similar to the 54% and 89% control rates, respectively, in the entire 179 subjects in the original study.4 In addition, the demographic features in Table 1 were also very similar for the entire population from the original study (data not shown). Therefore, the sample that was available and agreed to participate in the present analysis was representative of the larger sample.
There was no change in physician knowledge scores from baseline to the end of the initial study and the scores in the intervention group were significantly lower than the control group. Therefore, changes in physician knowledge did not appear to influence the results. Both groups of physicians received didactic education and written copies of the JNC-7 guidelines. Our findings support other research that demonstrated that typical educational activities has limited effect on physician knowledge or BP control.20, 21 Medication adherence was significantly better in the control group at baseline but there was no difference between groups by the 9-month visit. This finding suggests differences in medication adherence probably had limited influence on the differences between groups. The likely reason for better BP control in the intervention group is likely due to the more intensive use of antihypertensives to overcome suboptimal therapy.4, 16
There are several reasons that the deterioration in BP control may have occurred. First, medication adherence could have slipped in both groups once they completed the initial clinical trial. However, the parallel deterioration in both intervention and control groups is highly suggestive of a similar mechanism operating in both groups. The initial 9-month intervention study had intensive observation and patient participation which may have caused a “Hawthorne effect” in which patients who know they are being studied behave differently and tend to have better results for outcomes such as BP. Removal of any Hawthorne effect at the end of the 9-month intervention study could have led to BP deterioration. A second potential reason for BP deterioration is the need for greater numbers of antihypertensive medications with long-term follow-up. Studies have demonstrated that the need for additional antihypertensive medication increases over time, perhaps due to changes in weight, diet or other factors as subjects age.22 Whatever the reason, these data suggest that additional “booster” interventions may be required in the 40% of subjects in the intervention group whose BP control is lost. It is not known how often the pharmacist should reengage in the intervention, nor when, but we are conducting two ongoing studies to evaluate such strategies. However, our data suggest that so long as BP remains uncontrolled, or at any point when BP becomes uncontrolled, the pharmacist should re-engage with frequent visits to adjust medications and increase doses.
BP deterioration has implications for the health system as well as patient risk. Health systems are attempting to achieve high control rates for chronic diseases in order to compete for pay for performance and/or with other health plans in the marketplace. It will be important for such health systems to determine how to most efficiently utilize health care teams to achieve optimal chronic disease care.
A potential limitation in the present study is that research BP measurements were made during the 9-month clinical trial by trained research nurses while the post-intervention BP measurements were performed by different office nurses (or physicians) using usual clinic procedures. Since clinic BP measurements are often performed inaccurately,23 the BP values and BP control rates at the 18 and 27 month periods may not be directly comparable to the baseline and 9-month values. Nonetheless, our analysis is a between group evaluation, not a comparison to baseline or 9-month, per se. The same procedures were used in both groups at the 18 and 27 month time points, so the differences between groups should still be valid. In addition, since many of the errors in BP measurement in the typical office tend to elevate BP (failure to achieve adequate rest, patient on examination table without feet on the floor or the back supported), the actual BP control rates for the 18 and 27-month periods could actually be slightly better than reported here. Because the initial 9-month study was an efficacy trial, it is likely there was some Hawthorne effect in both groups that were removed at the 18 and 27-month evaluations. But again, the main evaluation was between groups and they remained significantly different at each time period. We did not capture other medications or non-prescription medications in this evaluation which could have changed over time and contributed to some of the deterioration in BP in both groups. Finally, the study was relatively small with few patients from minority groups. The small sample resulted in some uneven characteristics at baseline. It should be noted that variables that could influence BP were worse in the intervention group (BMI was higher and medication adherence was lower in the intervention group) supporting the conclusion that the pharmacist intervention was the primary reason for the findings. Two ongoing clinical trials are being conducted to overcome these limitations. One study in a Veterans Affairs medical center is administering this pharmacist intervention to all patients for six months and then patients will be randomized to have the intervention discontinued or continued for 24 months. The other study in 27 primary care clinics around the U.S. has randomized clinics to a 9-month intervention, 24-month intervention or a control group and all patients will be followed for 24 months. This study will recruit large numbers of African Americans and Hispanics. These studies should help to determine they types of additional pharmacist intervention that are needed to achieve long-term BP control. Results from these studies are expected in 2013–2014.
Conclusion
This study found significantly better BP control following a physician\pharmacist collaborative model that was maintained for 18 months after the intervention was discontinued when compared to a control group. However, even though BP control rates remained 20% higher in the intervention group, BP deteriorated in both groups. Our findings suggest that the clinical pharmacists should become re-engaged with patients who lose BP control, but the optimal and most efficient approach needs to be studied in a prospective trial.
Figure 2.
Solid lines depict systolic BP as measured by research nurses in the 9-month prospective study, hatched lines depict systolic BP as measured in the clinic and reported in the medical record.
* - p = 0.0017; ** - p = 0.02; # - p=0.05
Acknowledgments
Support:
Funding for this project was supported by the National Heart, Lung, and Blood Institute, RO1 HL69801and the Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics Cooperative Agreement #5U18HSO16094. Dr. Carter and Ms. Franciscus is also supported by the Center for Research in Implementation in Innovative Strategies in Practice (CRIISP), Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (HFP 04-149). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Abbreviations
- BP
blood pressure
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
Footnotes
Prior presentation: Presented at the 2008 Annual meeting of the North American Primary Care Research Group meeting, November 15, 2008.
Trial Registration: clinicaltrials.gov Identifier: NCT00201045
Conflict of Interest Statement:
Barry L. Carter – no conflicts of interest
William R. Doucette – no conflicts of interest
Carrie L. Franciscus – no conflicts of interest
Gail Ardery – no conflicts of interest
Karen M. Kluesner – no conflicts of interest
Elizabeth A. Chrischilles – no conflicts of interest
References
- 1.Carter BL, Barnette DJ, Chrischilles E, Mazzotti GJ, Asali ZJ. Evaluation of hypertensive patients after care provided by community pharmacists in a rural setting. Pharmacotherapy. 1997;17:1274–1285. [PubMed] [Google Scholar]
- 2.Bond CA, Monson R. Sustained improvement in drug documentation, compliance, and disease control. A four-year analysis of an ambulatory care model. Arch Intern Med. 1984;144:1159–1162. [PubMed] [Google Scholar]
- 3.Borenstein JE, Graber G, Saltiel E, et al. Physician-pharmacist comanagement of hypertension: a randomized, comparative trial. Pharmacotherapy. 2003;23:209–216. doi: 10.1592/phco.23.2.209.32096. [DOI] [PubMed] [Google Scholar]
- 4.Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens. 2008;10:260–271. doi: 10.1111/j.1751-7176.2008.07434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson SR, Slaughter R, Halapy H. Pharmacists' ability to influence outcomes of hypertension therapy. Pharmacotherapy. 1997;17:140–147. [PubMed] [Google Scholar]
- 6.Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. 2006;296:2563–2571. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]
- 7.McKenney JM, Slining JM, Henderson HR, Devins D, Barr M. The effect of clinical pharmacy services on patients with essential hypertension. Circulation. 1973;48:1104–1111. doi: 10.1161/01.cir.48.5.1104. [DOI] [PubMed] [Google Scholar]
- 8.Vivian EM. Improving blood pressure control in a pharmacist-managed hypertension clinic. Pharmacotherapy. 2002;22:1533–1540. doi: 10.1592/phco.22.17.1533.34127. [DOI] [PubMed] [Google Scholar]
- 9.Zillich AJ, Sutherland JM, Kumbera PA, Carter BL. Hypertension outcomes through blood pressure monitoring and evaluation by pharmacists (HOME study) J Gen Intern Med. 2005;20:1091–1096. doi: 10.1111/j.1525-1497.2005.0226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter BL, Rogers M, Daly J, Zheng S, James PA. Quality improvement strategies for hypertension: The potency of team-based care interventions. Arch Intern Med. 2009 doi: 10.1001/archinternmed.2009.316. (in press, October 26, 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 12.Carter BL, Hartz A, Bergus G, et al. Relationship between physician knowledge of hypertension and blood pressure control. J Clin Hypertens (Greenwich) 2006;8:481–486. doi: 10.1111/j.1524-6175.2006.05601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 14.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.Von Muenster SJ, Carter BL, Weber CA, et al. Description of pharmacist interventions during physician-pharmacist co-management of hypertension. Pharm World Sci. 2008;30:128–135. doi: 10.1007/s11096-007-9155-6. [DOI] [PubMed] [Google Scholar]
- 17.Ardery G, Carter BL, Milchak JL, et al. Explicit and implicit evaluation of physician adherence to hypertension guidelines. J Clin Hypertens. 2007;9:113–119. doi: 10.1111/j.1524-6175.2007.06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milchak JL, Carter BL, Ardery G, et al. Development of explicit criteria to measure adherence to hypertension guidelines. J Hum Hypertens. 2006;20:426–433. doi: 10.1038/sj.jhh.1002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milchak JL, Carter BL, Ardery G, Dawson JD, Harmston M, Franciscus CL. Physician adherence to blood pressure guidelines and its effect on seniors. Pharmacotherapy. 2008;28:843–851. doi: 10.1592/phco.28.7.843. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein MK, Lavori P, Coleman R, Advani A, Hoffman BB. Improving adherence to guidelines for hypertension drug prescribing: cluster-randomized controlled trial of general versus patient-specific recommendations. Am J Manag Care. 2005;11:677–685. [PubMed] [Google Scholar]
- 21.Milchak JL, Carter BL, James PA, Ardery G. Measuring adherence to practice guidelines for the management of hypertension: an evaluation of the literature. Hypertension. 2004;44:602–608. doi: 10.1161/01.HYP.0000144100.29945.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 23.Villegas I, Arias IC, Botero A, Escobar A. Evaluation of the technique used by healthcare workers for taking blood pressure. Hypertension. 1995;26:1204–1206. doi: 10.1161/01.hyp.26.6.1204. [DOI] [PubMed] [Google Scholar]