Abstract
Background
Risk models to predict prostate cancer on biopsy, whether they include only prostate-specific antigen (PSA) or other markers, are intended for use in all men of screening age. Yet the association between PSA and cancer likely depends on a man’s recent screening history.
Methods
To examine the effect of prior screening on prostate cancer risk prediction using a previously reported four-kallikrein panel: total, free, and intact PSA, and kallikrein-related peptidase 2 (hK2). The study cohort comprised 1241 men in Gothenburg, Sweden, undergoing biopsy for elevated PSA during their second or later visit for the European Randomized study of Screening for Prostate Cancer. We calculated the predictive accuracy of a four-kallikrein panel.
Results
Total PSA was not predictive of prostate cancer. A previously published four-kallikrein model increased predictive accuracy compared to total PSA and age alone (area-under-the-curve [AUC] 0.66 vs. 0.51; p<0.001), but was poorly calibrated and missed many cancers. A model developed with recently screened men gave important improvements in discrimination (AUC 0.67 vs. 0.56; p<0.001). Use of this model would reduce the number of biopsies by 413 per 1000 men with elevated PSA, miss 60 of 216 low-grade (Gleason ≤6) cancers, but miss only 1 of 43 high-grade cancers.
Conclusions
Prior participation in PSA-screening dramatically changes the performance of statistical models predicting biopsy outcome. A four-kallikrein panel can predict prostate cancer in men with a recent screening history, providing independent replication that multiple kallikrein-forms contribute important diagnostic value in men with elevated PSA.
Keywords: prostate cancer, screening, prostate specific antigen, kallikreins, molecular markers
Introduction
Death from cancer almost invariably results from disseminated disease; successful resection of a tumor detected before it becomes disseminated should therefore prevent cancer-specific mortality. Hence, early detection of cancer remains the one of the most promising strategies for cancer control. For prostate cancer, a widespread early detection strategy involves analysis of blood markers, with approximately 50% of American men at risk undergoing regular testing for prostate specific antigen (PSA) 1. Indeed, interim reports from two large randomized studies of PSA-based screening for prostate cancer were recently published2, 3. One showed a modest 20% reduction in cancer mortality for previously unscreened men participating in the European Randomized Screening Study of Prostate Cancer (ESRPC)2. The current lack of mortality reduction in the PLCO study could - at least in part - be attributed to wide use of PSA-screening among the men both randomized to screening or selected as controls in the US3. For many years, PSA was largely considered a binary test. Patients above a threshold level of PSA, such as 4 ng/ml, are considered positive, and recommended for biopsy; patients with PSA below the threshold are told that they are at low risk and not in need of further work-up 4.
Recent data have demonstrated some of the shortcomings of PSA as a basis for biopsy decisions. The positive predictive value of an elevated PSA in the 20–30% range 4, implying that a large number of men receive unnecessary biopsy. In addition, PSA is insufficiently sensitive, such that men with low PSA may nonetheless harbor cancer 5. These findings have prompted the search for new markers of prostate cancer. Using a data set from the Gothenburg section of ERSPC, we recently reported that a panel of four kallikrein markers — total PSA, free PSA, intact PSA and human kallikrein-related peptidase 2 (hK2) — was strongly predictive of biopsy outcome in men with elevated PSA 6. We estimated that using the model to determine referral to biopsy would reduce biopsy rates by 573 per 1000 men with elevated PSA and would miss only a small number of cancers (42 per 1000 men with elevated PSA). Moreover, most of the cancers missed were the low grade cancers most likely to constitute overdiagnosis.
Our initial results were based on the first round of the ERPSC, wherein a very small proportion had been previously screened (~3%). It is therefore unclear how well our results would apply to men undergoing regular screening. For example, an initial PSA test would identify both recently developing cancers and those that were established for many years; a repeat test would find only recent cancers as the long-established cancers had been removed from the risk set. Accordingly, we hypothesize that PSA will have higher predictive value for initial than repeat testing. Yet risk models to predict prostate cancer on biopsy, whether they include only PSA or other markers, appear to be intended for use in all men of screening age, irrespective of screening history. For example, the most well known model, the PCPT risk calculator 7, does not include PSA screening history as an input.
In this study, we evaluate the predictive accuracy of PSA and of our published four-kallikrein panel, developed on patients biopsied during their first visit of the ERSPC, when applied to men biopsied during their second and subsequent visits. We then compare our results to a model developed using the second and subsequent visit data set. The difference in results between these two models will address the question of whether recent screening affects the properties of statistical models for the prediction of prostate cancer.
Methods
Study Cohort
The design of the Swedish arm of the ERSPC in Gothenburg, Sweden has been previously reported 6, 8. In brief, the study cohort consisted of a random sample of 19,911 men; ~62% of all males living in Gothenburg, Sweden, who were born between 01/01/1930 and 12/31/1944. Approximately one-half were randomly selected to be invited to screening (n=9,957) and the participation rate was 75% (n=7,510) in the screening arm. Following an initial PSA test, men not diagnosed with cancer and aged <70 were invited for up to six additional biennial screens. The current cohort includes screens conducted through 12/31/06. The rate at which participants in the screening arm continued to undergo subsequent screening was very high, with 90% of men participating in at least 3 screening rounds, until a cancer diagnosis, or age 70. Data were obtained under a waiver approved by the medical ethics committee at the University of Gothenburg. Men with an elevated PSA level in serum (defined as either ≥ 2.54 during 2005–2006; 2.89 during 1999–2004; or 3.39 ng/ml during 1995–1998) were invited to undergo subsequent clinical examination by an experienced urologist and were highly compliant (≈89%). This examination consisted of transrectal ultrasound guided laterally directed sextant biopsy and digital rectal examination (DRE). Biopsy specimens were evaluated by a single pathologist, with tumors classified according to the 1997 International Union Against Cancer staging system 9 and graded according to the Gleason grading system 10.
All biomarker measurements used for statistical analysis are in accordance with WHO calibration standards. Laboratory methods were as for prior publication 6, with the exception being that the measurement assays were changed in 2004 to reflect WHO PSA calibration standards. A correction factor provided by the assay manufacturer was applied to measurements taken in earlier years. Accordingly, the threshold for clinical examination varied slightly between 2.54 and 3.39 ng/ml.
For the statistical analysis, the first screening round (‘round 1’) was defined as the first time that the man participated in the study irrespective of calendar year. The second screening round was defined as that occurring approximately two years after the first screening round; screening rounds 3–6 were similarly defined.
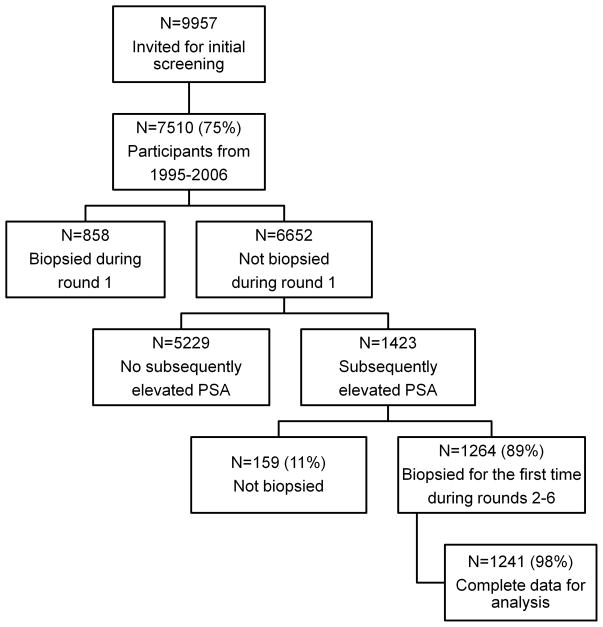
The current analysis includes 1241 men biopsied during screening rounds 2–6 and who were not biopsied in any previous screening round. Our main analysis includes only the first biopsy for each participant. The findings are therefore applicable to recently screened men with an elevated PSA and with no prior biopsy. High-grade cancer was defined as biopsy Gleason score 7 or higher. Figure 1 shows the flow of participants through the study. The PSA threshold that prompted biopsy was 3.39 ng/ml in 6% (n=75), 2.89 ng/ml in 73% (n=902), and 2.54 ng/ml in 21% (n=264).
Figure 1.
Flow of participants
The principal aim was to determine whether the additional kallikrein markers (free PSA, intact PSA, and hK2) could provide value additional to PSA when deciding whether a biopsy would be advisable. We considered two settings: a laboratory sending blood results to a doctor and a clinical consultation between a patient and a doctor following news of an elevated PSA. Accordingly, the base model for the laboratory setting included age and total PSA; for the clinical setting, the base model also included the DRE result (note, however, that only men with elevated PSA underwent DRE). In all cases, the levels of markers used were those taken closest to but before the time of the biopsy.
For the current analysis, we took two statistical approaches. First, we evaluated the performance of the models previously developed for never screened men 6 when applied to men with a recent screening history. To do this, we calculated the predicted probability of a positive biopsy for each recently screened man from the previously developed models. We then computed the AUC of each model for the recently screened men. Second, we hypothesized that recently screened men differ from never screened men such that they require a different statistical model. We therefore refit a multivariable model using the current sample as the data set 6. In this analysis, all AUCs were calculated by repeated 10-fold cross validation. The models previously developed for never screened men consisted of the same exact predictor variables as the models currently developed for recently screened men; the only difference between the models was the estimated coefficients for each predictor, which results from fitting the models in separate cohorts.
For all analyses, the point estimates of the AUCs were obtained by taking the bootstrap mean, and confidence intervals for all AUCs were obtained from bootstrap percentile intervals, with 2000 bootstrap replications. To test for a difference between the AUCs, we calculated the difference in the AUCs of the full and base models within each bootstrap replication. The null hypothesis is that the difference in the AUCs equals zero; accordingly, a two-sided p-value to test the null hypothesis is defined as the proportion of replications where the differences was less than or equal to zero, multiplied by two. To examine the association between PSA and risk of prostate cancer between men biopsied during round 1 (data from previous paper) and those biopsied during screening rounds 2–6 (data from current paper), we fitted separate logistic regression models by group, using only total PSA as the predictor variable. To account for any non-linear relationships between biomarker levels and risk, the biomarkers were entered into all models using restricted cubic splines with knots at the tertiles.
The clinical effects of the models were characterized by decision curve analysis 11. This method estimates a “net benefit” for prediction models by summing the benefits (true positives) and subtracting the harms (false positives), where the latter is weighted by a factor related to the relative harm of a missed cancer vs. an unnecessary biopsy. The weighting is derived from the threshold probability of prostate cancer at which a patient would choose to be biopsied. As this threshold probability can vary from patient to patient, net benefit is calculated across a range of probabilities; we chose 10–40% as a reasonable range. The interpretation of a decision curve is straightforward: the model with the highest net benefit at a particular threshold probability should be chosen. Correction for overfit was by repeated 10-fold cross-validation. All statistical analyses were conducted using Stata 10.0 (StataCorp LP, College Station, TX).
Results
In total, 1241 participants received a first biopsy during screening rounds 2–6, of which 322 (26%) were diagnosed with prostate cancer (Table 1). The 1241 biopsied participants include 120 (10%) who, in an earlier screening round, had had an elevated PSA (≥3 ng/ml) but declined biopsy; their initial elevated PSA levels ranged from 3 to 9 ng/ml, with 63% (n=75) being between 3 and 4 ng/ml. As is typical among recently screened men, the overwhelming majority of cancers diagnosed were Gleason 6 or less (n=269 or 84%), while 45 (14%) were Gleason 7 and only 8 (2%) were Gleason 8 or higher.
Table 1.
Characteristics of 1241 participants who underwent biopsy in rounds 2–6 of the ERSPC Gothenburg
| Cancer detected on biopsy |
||
|---|---|---|
| No | Yes | |
| N=919 | N=322 | |
| Age at biopsy (years) | 63 (60, 66) | 64 (61, 67) |
| Round of biopsy | ||
| 2 | 296 (32%) | 72 (22%) |
| 3 | 268 (29%) | 88 (27%) |
| 4 | 168 (18%) | 75 (23%) |
| 5 | 85 (9%) | 47 (15%) |
| 6 | 102 (11%) | 40 (12%) |
| Previous elevated PSA and refused biopsy | 89 (10%) | 31 (10%) |
| Total PSA (ng/ml) | 3.51 (3.11, 4.15) | 3.51 (3.11, 4.29) |
| Free PSA (ng/ml) | 0.74 (0.59, 1.00) | 0.69 (0.51, 0.90) |
| Intact PSA (ng/ml) | 0.39 (0.28, 0.52) | 0.39 (0.25, 0.52) |
| Human kallikrein 2 (ng/ml) | 0.046 (0.029, 0.066) | 0.055 (0.038, 0.081) |
| Abnormal DRE | 55 (6%) | 69 (21%) |
| Biopsy Gleason grade | ||
| ≤6 | n/a | 269 (84%) |
| 7 | n/a | 45 (14%) |
| ≥8 | n/a | 8 (2%) |
| Clinical stage | ||
| T1C | n/a | 252 (78%) |
| T2A | n/a | 48 (15%) |
| T2B | n/a | 9 (3%) |
| T2C | n/a | 4 (1%) |
| T3 | n/a | 8 (2%) |
| Unknown | n/a | 1 (0.3%) |
DRE, digital rectal examination
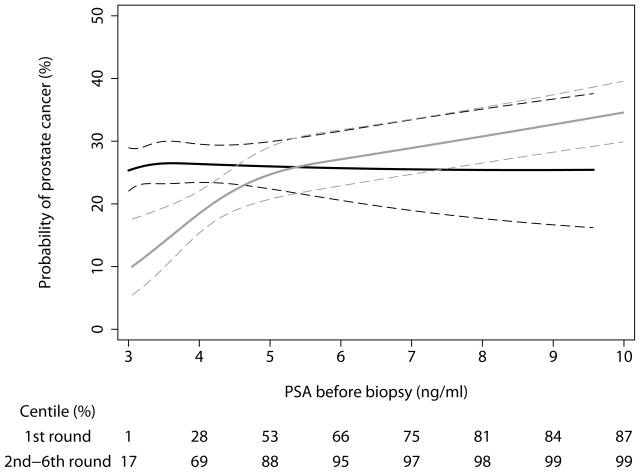
The predicted probability of a positive biopsy based on total PSA is shown in Figure 2, separately for men biopsied during round 1 (data from previous paper) and those biopsied for the first time during subsequent screening rounds (data from current paper). In unscreened men with PSA ≥3 ng/ml, risk of cancer rises in parallel with increases in PSA above 3 ng/ml; for men with prior PSA screens, the relationship between a PSA elevation beyond 3 ng/ml and cancer risk is essentially flat. In other words, there was no significant relationship between PSA and cancer risk in men with recent PSA screening and with a subsequently elevated PSA (p=0.9). As might be expected, the distribution of total PSA differed markedly between the two groups: 88% of previously screened men, but only 53% of those without prior screening, had a total PSA in the range of 3–5 ng/ml (i.e. raised only slightly above the biopsy threshold); conversely, 13% of unscreened men had high PSA (>10 ng/ml) compared to only 1% of those previously screened.
Figure 2.
Predicted probability of a positive biopsy by total PSA, separately for men biopsied during the first round (never screened: grey lines) and for those biopsied for the first time during subsequent screening rounds (recently screened: black lines). Dashed lines are 95% confidence intervals.
We first evaluated the performance of the models previously developed for men without prior screening, when they were applied to the recently screened men (Table 2). The base laboratory model, which included age and total PSA, had little if any discrimination for diagnosis of prostate cancer in recently screened men (AUC 0.51; 95% confidence interval [95%CI] 0.49, 0.55). A statistically significant enhancement was observed when all new markers were included in a multivariable model (AUC 0.66; 95% CI 0.62, 0.69). Similar enhancements were observed for the clinical model, where DRE result was also included in the base model.
Table 2.
Predictive accuracy (area-under-the-curve) of the kallikrein models in recently screened men, with 95% C.I. The base model for the laboratory model includes age and total PSA, and for the clinical model includes age, total PSA, and DRE result. The full model includes the base model plus free PSA, intact PSA, and hK2.
| Model | Laboratory Model | Clinical Model | ||
|---|---|---|---|---|
| Any cancer | High-grade cancer | Any cancer | High-grade cancer | |
| Models developed with never screened men and applied to recently screened men | ||||
| Base | 0.514 (0.487, 0.546) | 0.581 (0.493, 0.671) | 0.569 (0.530, 0.608) | 0.675 (0.585, 0.762) |
| Full | 0.656 (0.621, 0.693) | 0.781 (0.714, 0.844) | 0.678 (0.643, 0.714) | 0.800 (0.732, 0.859) |
| P value versus base | <0.001 | <0.001 | <0.001 | 0.004 |
| Models developed with recently screened men and applied to recently screened men | ||||
| Base model | 0.564 (0.521, 0.603) | 0.658 (0.557, 0.747) | 0.622 (0.580, 0.661) | 0.717 (0.625, 0.800) |
| Full model | 0.674 (0.636, 0.711) | 0.819 (0.751, 0.879) | 0.697 (0.661, 0.734) | 0.828 (0.769, 0.878) |
| P value versus base | <0.001 | <0.001 | 0.005 | 0.009 |
Using the full models developed with never screened men, we determined the number of biopsies conducted and cancers identified per 1000 men with elevated PSA if these models were used to make decisions as to which men with a recent screening history should be subject to biopsy (Table 3). We used a threshold probability of 20%. That is, we assume that a participant would agree to have a biopsy if his predicted probability was 20% or higher. This value was chosen as it is slightly lower than the positive predictive value of a PSA ≥3 ng/ml in this cohort (26%). Using the full models developed with never screened men, the number of biopsies performed would be greatly reduced (by 730–850 per 1000 men with elevated PSA); however, in doing so we would miss an important number of cancers (as many as 196, three-quarters of the total), including many high-grade tumors (23, half of the total).
Table 3.
Biopsies conducted and cancers detected per 1000 men with elevated PSA and with recent screening, using as a threshold for biopsy a 20% or higher probability of cancer. Cancers with biopsy Gleason score 7 or higher were considered high grade.
| No. biopsies: | No. cancers: | No. high-grade cancers: | ||||
|---|---|---|---|---|---|---|
| performed | reduced (%) | caught | missed (%) | caught | missed (%) | |
| Biopsy all | 1000 | n/a | 259 | n/a | 43 | n/a |
| Models developed with never screened men and applied to recently screened men | ||||||
| Laboratory model | ||||||
| Risk ≥ 20%: total PSA, age | 245 | 755 (76%) | 72 | 187 (72%) | 20 | 23 (53%) |
| Risk ≥ 20%: kallikrein panel, age | 268 | 732 (73%) | 106 | 153 (59%) | 31 | 12 (28%) |
| Clinical model | ||||||
| Risk ≥ 20%: total PSA, age, DRE | 146 | 854 (85%) | 63 | 196 (76%) | 20 | 23 (53%) |
| Risk ≥ 20%: kallikrein panel, age, DRE | 258 | 742 (74%) | 113 | 146 (56%) | 32 | 11 (26%) |
| Models developed with recently screened men and applied to recently screened men | ||||||
| Laboratory model | ||||||
| Risk ≥ 20%: total PSA, age | 857 | 143 (14%) | 234 | 25 (10%) | 42 | 1 (2%) |
| Risk ≥ 20%: total PSA, free PSA, age | 724 | 276 (28%) | 211 | 48 (19%) | 41 | 2 (5%) |
| Risk ≥ 20%: kallikrein panel, age | 643 | 357 (36%) | 212 | 47 (18%) | 41 | 2 (5%) |
| Clinical model | ||||||
| Risk ≥ 20%: total PSA, age, DRE | 728 | 272 (27%) | 207 | 52 (20%) | 39 | 4 (9%) |
| Risk ≥ 20%: total PSA, free PSA, age, DRE | 631 | 369 (37%) | 194 | 65 (25%) | 38 | 5 (12%) |
| Risk ≥ 20%: kallikrein panel, age, DRE | 587 | 413 (41%) | 199 | 60 (23%) | 42 | 1 (2%) |
| Alternative clinical strategies applied to recently screened men | ||||||
| Total PSA ≥ 4 ng/ml | 305 | 695 (70%) | 84 | 175 (68%) | 22 | 21 (49%) |
| Total PSA ≥ 4 ng/ml or positive DRE | 367 | 633 (63%) | 116 | 143 (55%) | 29 | 14 (33%) |
To overcome the poor decision analytic properties for the full models developed on recently screened men when applied to recently screened men, we then developed new models for prostate cancer diagnosis using the data from our current cohort of recently screened men. The AUC of the laboratory base model (PSA and age) was 0.56 (upper limit of 95% CI 0.60) and the clinical base model (also including DRE) was 0.62 (upper limit of 95% CI 0.66). These AUCs were higher than those obtained when the models were developed with never screened men, suggesting that the operating characteristics of total PSA differs between never screened and recently screened men. The predictive accuracy of the full laboratory model was 0.67 with lower limit of 95% CI 0.64 (0.11 higher than base model; p<0.001), and the full clinical model was 0.70 with lower limit of 95% CI 0.66 (0.08 higher than base model; p<0.005), which were similar to the increments obtained with the models developed with never screened men. Table 3 shows that the models developed with recently screened men have good decision analytic characteristics: biopsying men according to the clinical full model would reduce the number of biopsies by 41%, while missing 60 cancers out of 259 per 1000 (23%) and only 1 biopsy Gleason 3+4 cancer of 43 high-grade cancers (biopsy Gleason ≥7). All of the 60 cancers missed were clinical stage T1C.
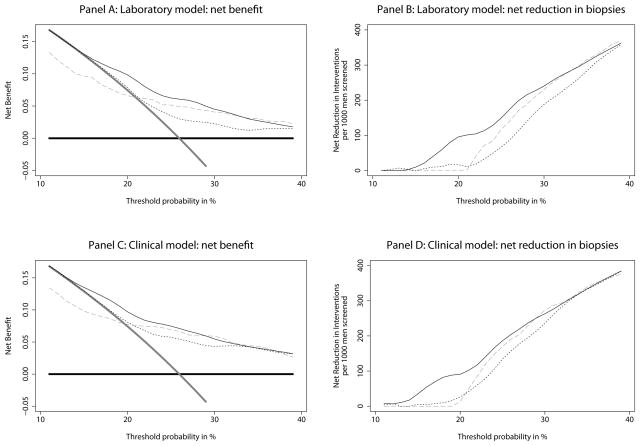
Figure 3 gives decision curves for our models. Although results are close for very risk-averse men — those who would opt for biopsy for risks of cancer below 15% — the net benefit of the four-kallikrein panel model for previously screened men is superior to the alternative strategies of either biopsying all men, no men, or biopsying on the basis of total and free PSA, across the full range of threshold probabilities for both clinical and laboratory models. The models developed with never screened men and applied to recently screened men had net benefits that were generally inferior to those of the new models and were particularly poor where threshold probabilities were low.
Figure 3.
Decision curves, showing the estimated clinical benefit of various biopsy strategies. Panels A and B: Laboratory models. Thick grey line: biopsy all men. Thick black line: biopsy no man. Thin black line: biopsy on the basis of the laboratory kallikrein model (age, total PSA, free PSA, intact PSA, and hK2). Dotted line: biopsy on the basis of free PSA, total PSA and age. Grey dashed line: biopsy using the laboratory kallikrein model developed for unscreened men. Panels C and D: Clinical models. Thick grey line: biopsy all men. Thick black line: biopsy no man. Thin black line: biopsy on the basis of the clinical kallikrein model (age, DRE, total PSA, free PSA, intact PSA, and hK2). Dotted line: biopsy on the basis of free PSA, total PSA, age and DRE. Grey dashed line: biopsy using the clinical kallikrein model developed for unscreened men.
We performed a sensitivity analysis to investigate whether our results depended on the biopsy scheme used for the ERSPC. Participants underwent a transrectal ultrasound guided laterally directed sextant biopsy, which may have missed cancers that could have been detected with an extended biopsy scheme. To examine the effect of biopsy scheme, we considered the results of repeat biopsies among those with an initial negative biopsy: we assumed that cancers detected initially with an extended biopsy would have been detected with repeat sextant biopsies within 4 years of the initial biopsy. The sensitivity analyses included 802 participants with an initial biopsy before 12/31/2002; these participants were eligible for a subsequent biopsy in the 4-year period ending 12/31/2006 (the last screen date in the data set). In this subgroup, 609 (76%) had an initial negative biopsy. Approximately 40% (248/609) had a subsequent biopsy within 4 years, which was positive in 91 men (37%). This increase in positive biopsy rates is broadly comparable to what has been reported for direct comparisons between sextant and more extended biopsy 12–14. The analysis was repeated considering those 91 participants as positive at their initial biopsy. This resulted in a predictive accuracy that was slightly lower, but importantly the enhanced predicted accuracy contributed by use of the full kallikrein model was unchanged (laboratory model: base 0.52 vs full 0.66, increment=0.14; clinical model: base 0.55 vs full 0.67, increment=0.12; both p<0.01). These results clearly indicate that the models are likely to be robust, independent of the biopsy scheme.
Discussion
We have found that a panel of four kallikrein forms can help predict the result of prostate biopsy in men with an elevated PSA who were subject to recent screening. The increment in predictive accuracy compared to a base model of total PSA and age was similar between our original publication (increase in AUC of 0.152 6) and this data set (increase of 0.110). As no data from the original publication were included in this data set, we take our results as providing an independent replication.
Nonetheless, our findings support our hypothesis that the predictive accuracy of total PSA changes dramatically for men who have undergone recent screening. The predictive accuracy of age plus total PSA was 0.68 when applied to men who had never been screened 6, compared to only 0.56 — little better than a coin flip — in our current, equally highly representative screening study cohort. This finding is similar to that of Eggener et al, who report an AUC of 0.58 for a model including age and PSA in men undergoing regular screening 15. This AUC is slightly higher than our finding, largely because Eggener et al’s cohort included a wider range of ages. The AUC of PSA alone in our cohort was 0.51, compared to 0.52 for Eggener et al. (data from authors).
The dramatically altered relationship between PSA and prostate cancer risk in recently screened men who had elevated PSA during subsequent screening rounds has important consequences for statistical modeling. In men who have been recently screened, a PSA ≥3 ng/ml likely increases risk of cancer 4, 7; however, rises above 3 ng/ml do not appear to increase risk further (Figure 2). A statistical model developed on unscreened men has reasonable discrimination when applied to those recently screened; in other words, those with higher risks from the model are indeed more likely to harbor cancer than those with lower risks. However, the model is poorly calibrated, in other words, the absolute level of risk is inaccurate. As a result, only about 1 in 3 men have a predicted risk from the model in excess of 20%, such that use of this threshold as a criterion for biopsy would miss approximately 75% of cancers.
Accordingly, we recommend that different statistical models be used to predict the risk of prostate cancer, depending on whether a man has been subject to recent screening. PSA would be strongly weighted in a model applied to an unscreened man, but not in the model to be applied to a man with recent PSA tests. This view has not been incorporated in published statistical models for prostate cancer. Indeed, of 36 prostate cancer prediction models described in a recent review16, none appears to take PSA screening history into account. The best known and most widely used prediction tool, the “PCPT risk calculator”, was developed using data from the Prostate Cancer Prevention Trial 7. Although the inputs for the model include race, family history, DRE, age and PSA, it is the PSA level and DRE that drive risk. Our data, as well as those of Eggener et al., suggest that PSA level does not discriminate recently screened men with cancer from those without cancer at biopsy.
The results of the ERPSC suggest that although PSA screening can reduce prostate cancer mortality, this comes at a high cost in terms of the number of men needing to be screened, biopsied and treated to prevent one death2. Our data on the kallikrein panel suggests an approach for shifting the benefit to harm ratio of PSA screening. Not only would use of the panel reduce the number of biopsies by 30 – 40%, but the vast majority of the small number of cancers that would be missed would be the low stage, low grade, cancers typically thought to constitute overdiagnosis.
Although our study independently replicates the higher predictive accuracy of the kallikrein panel compared to PSA alone, our new statistical models for previously screened men have not been independently validated. We therefore plan to evaluate our models in other ERSPC cohorts.
Conclusions
We have found that adding information on kallikreins other than PSA can help predict the result of biopsy in men with elevated PSA who have a recent screening history. Our models can therefore be used to determine which men should be advised to have biopsy and which might be advised to continue screening, but defer biopsy until there was stronger evidence of malignancy. The predictive accuracy of PSA alone is dramatically affected by screening history, and we therefore recommend that different predictive models be used for men with and without recent PSA tests. This recommendation has considerable implications for current predictive models, none of which appear to incorporate information on recent screening history.
Acknowledgments
We thank Ms. Gun-Britt Eriksson and Kerstin Håkansson for expert assistance with immunoassays.
Funding: National Cancer Institute [P50-CA92629 SPORE]; Swedish Cancer Society [project no. 3455], Swedish Research Council (Medicine) [project no. 20095]; the European Union 6th Framework contract [LSHC-CT-2004-503011 (P-Mark)]; the Academy of Finland [Project 206690]; and the Sidney Kimmel Center for Prostate and Urologic Cancers.
Footnotes
Conflict of Interest Statement: Dr. Hans Lilja holds patents for free PSA and hK2 assays, and is a co-inventor on a patent for intact/nicked PSA-assay.
References
- 1.Thompson IM, Ankerst DP, Chi C, Lucia MS, Goodman PJ, Crowley JJ, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. Jama. 2005;294(1):66–70. doi: 10.1001/jama.294.1.66. [DOI] [PubMed] [Google Scholar]
- 2.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 3.Andriole GL, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8(4):268–78. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 5.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239–46. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 6.Vickers AJ, Cronin MS, GA A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Gothenburg, Sweden. BMC Medicine. 2008;6:19. doi: 10.1186/1741-7015-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98(8):529–34. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 8.Hugosson J, Aus G, Lilja H, Lodding P, Pihl CG. Results of a randomized, population-based study of biennial screening using serum prostate-specific antigen measurement to detect prostate carcinoma. Cancer. 2004;100(7):1397–405. doi: 10.1002/cncr.20126. [DOI] [PubMed] [Google Scholar]
- 9.Sobin LH, Wittekind C. UICC TNM classification of malignant tumors. New York: John Wiley & Sons; 1997. [Google Scholar]
- 10.Gleason DF. Veterans Administration Cooperative Urological Research Group. Histologic grading and staging of prostatic carcinoma. In: Tannenbaum M, editor. Urologic pathology: the prostate. Philadelphia: Lea & Febiger; 1977. pp. 171–98. [Google Scholar]
- 11.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–74. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durkan GC, Sheikh N, Johnson P, Hildreth AJ, Greene DR. Improving prostate cancer detection with an extended-core transrectal ultrasonography-guided prostate biopsy protocol. BJU Int. 2002;89:33–9. doi: 10.1046/j.1464-4096.2001.01488.x. [DOI] [PubMed] [Google Scholar]
- 13.Eskicorapci SY, Baydar DE, Akbal C, Sofikerim M, Gunay M, Ekici S, et al. An extended 10-core transrectal ultrasonography guided prostate biopsy protocol improves the detection of prostate cancer. Eur Urol. 2004;45:444–9. doi: 10.1016/j.eururo.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Guichard G, Larre S, Gallina A, Lazar A, Faucon H, Chemama S, et al. Extended 21-sample needle biopsy protocol for diagnosis of prostate cancer in 1000 consecutive patients. Eur Urol. 2007;52:430–5. doi: 10.1016/j.eururo.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 15.Eggener SE, Yossepowitch O, Roehl KA, Loeb S, Yu X, Catalona WJ. Relationship of prostate-specific antigen velocity to histologic findings in a prostate cancer screening program. Urology. 2008;71(6):1016–9. doi: 10.1016/j.urology.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Schroder F, Kattan MW. The comparability of models for predicting the risk of a positive prostate biopsy with prostate-specific antigen alone: a systematic review. Eur Urol. 2008;54(2):274–90. doi: 10.1016/j.eururo.2008.05.022. [DOI] [PubMed] [Google Scholar]