Abstract
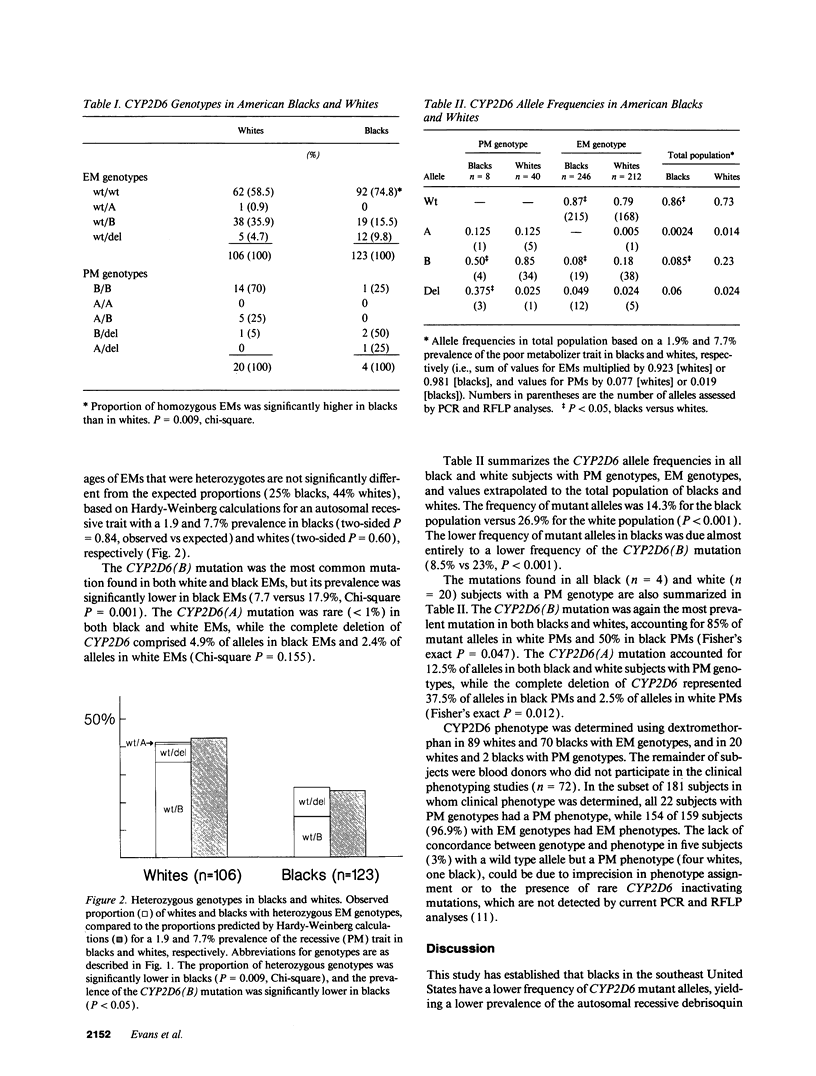
Debrisoquin hydroxylase (CYP2D6) is a cytochrome P450 enzyme that catalyzes the metabolism of > 30 commonly prescribed medications. Deficiency in CYP2D6 activity, inherited as an autosomal recessive trait, was found to be significantly less common in American blacks (1.9%) than whites (7.7%). To determine the genetic basis for this difference, inactivating CYP2D6 mutations were assessed by allele-specific PCR amplification and RFLP analyses of genomic DNA from 126 unrelated whites and 127 unrelated blacks. Blacks had a twofold lower frequency (8.5 versus 23%, P = 0.001) of the CYP2D6(B) mutation (point mutation at intron 3/exon 4 splice site), while complete deletion of the CYP2D6 gene (5.5% blacks, 2.4% whites), and the CYP2D6(A) mutation (single nucleotide deletion in exon 5; 0.24% blacks, 1.4% whites) were not different between the two groups. The prevalence of heterozygous genotypes was significantly lower in blacks (25 versus 42% of extensive metabolizers, P = 0.009), consistent with the observed prevalence of the deficient trait in blacks and whites. We conclude that the same CYP2D6 mutations lead to a loss of functional expression in blacks and whites, but American blacks have a lower prevalence of the deficient trait due to a lower frequency of the CYP2D6(B) mutation. This could explain racial differences in drug effects and disease risk.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong M., Daly A. K., Cholerton S., Bateman D. N., Idle J. R. Mutant debrisoquine hydroxylation genes in Parkinson's disease. Lancet. 1992 Apr 25;339(8800):1017–1018. doi: 10.1016/0140-6736(92)90537-d. [DOI] [PubMed] [Google Scholar]
- Ayesh R., Idle J. R., Ritchie J. C., Crothers M. J., Hetzel M. R. Metabolic oxidation phenotypes as markers for susceptibility to lung cancer. Nature. 1984 Nov 8;312(5990):169–170. doi: 10.1038/312169a0. [DOI] [PubMed] [Google Scholar]
- Broly F., Gaedigk A., Heim M., Eichelbaum M., Morike K., Meyer U. A. Debrisoquine/sparteine hydroxylation genotype and phenotype: analysis of common mutations and alleles of CYP2D6 in a European population. DNA Cell Biol. 1991 Oct;10(8):545–558. doi: 10.1089/dna.1991.10.545. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Gram L. F. Clinical significance of the sparteine/debrisoquine oxidation polymorphism. Eur J Clin Pharmacol. 1989;36(6):537–547. doi: 10.1007/BF00637732. [DOI] [PubMed] [Google Scholar]
- Caporaso N. E., Tucker M. A., Hoover R. N., Hayes R. B., Pickle L. W., Issaq H. J., Muschik G. M., Green-Gallo L., Buivys D., Aisner S. Lung cancer and the debrisoquine metabolic phenotype. J Natl Cancer Inst. 1990 Aug 1;82(15):1264–1272. doi: 10.1093/jnci/82.15.1264. [DOI] [PubMed] [Google Scholar]
- Dahl M. L., Johansson I., Palmertz M. P., Ingelman-Sundberg M., Sjöqvist F. Analysis of the CYP2D6 gene in relation to debrisoquin and desipramine hydroxylation in a Swedish population. Clin Pharmacol Ther. 1992 Jan;51(1):12–17. doi: 10.1038/clpt.1992.2. [DOI] [PubMed] [Google Scholar]
- Devesa S. S., Blot W. J., Fraumeni J. F., Jr Declining lung cancer rates among young men and women in the United States: a cohort analysis. J Natl Cancer Inst. 1989 Oct 18;81(20):1568–1571. doi: 10.1093/jnci/81.20.1568. [DOI] [PubMed] [Google Scholar]
- DiLella A. G., Woo S. L. Cloning large segments of genomic DNA using cosmid vectors. Methods Enzymol. 1987;152:199–212. doi: 10.1016/0076-6879(87)52021-3. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Spannbrucker N., Steincke B., Dengler H. J. Defective N-oxidation of sparteine in man: a new pharmacogenetic defect. Eur J Clin Pharmacol. 1979 Sep;16(3):183–187. doi: 10.1007/BF00562059. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Woolhouse N. M. Inter-ethnic difference in sparteine oxidation among Ghanaians and Germans. Eur J Clin Pharmacol. 1985;28(1):79–83. doi: 10.1007/BF00635712. [DOI] [PubMed] [Google Scholar]
- Evans W. E., Relling M. V. Concordance of P450 2D6 (debrisoquine hydroxylase) phenotype and genotype: inability of dextromethorphan metabolic ratio to discriminate reliably heterozygous and homozygous extensive metabolizers. Pharmacogenetics. 1991 Dec;1(3):143–148. [PubMed] [Google Scholar]
- Evans W. E., Relling M. V., Petros W. P., Meyer W. H., Mirro J., Jr, Crom W. R. Dextromethorphan and caffeine as probes for simultaneous determination of debrisoquin-oxidation and N-acetylation phenotypes in children. Clin Pharmacol Ther. 1989 May;45(5):568–573. doi: 10.1038/clpt.1989.74. [DOI] [PubMed] [Google Scholar]
- Gaedigk A., Blum M., Gaedigk R., Eichelbaum M., Meyer U. A. Deletion of the entire cytochrome P450 CYP2D6 gene as a cause of impaired drug metabolism in poor metabolizers of the debrisoquine/sparteine polymorphism. Am J Hum Genet. 1991 May;48(5):943–950. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J., Skoda R. C., Kimura S., Umeno M., Zanger U. M., Nebert D. W., Gelboin H. V., Hardwick J. P., Meyer U. A. Characterization of the common genetic defect in humans deficient in debrisoquine metabolism. Nature. 1988 Feb 4;331(6155):442–446. doi: 10.1038/331442a0. [DOI] [PubMed] [Google Scholar]
- Heim M., Meyer U. A. Genotyping of poor metabolisers of debrisoquine by allele-specific PCR amplification. Lancet. 1990 Sep 1;336(8714):529–532. doi: 10.1016/0140-6736(90)92086-w. [DOI] [PubMed] [Google Scholar]
- Iyun A. O., Lennard M. S., Tucker G. T., Woods H. F. Metoprolol and debrisoquin metabolism in Nigerians: lack of evidence for polymorphic oxidation. Clin Pharmacol Ther. 1986 Oct;40(4):387–394. doi: 10.1038/clpt.1986.195. [DOI] [PubMed] [Google Scholar]
- Kagimoto M., Heim M., Kagimoto K., Zeugin T., Meyer U. A. Multiple mutations of the human cytochrome P450IID6 gene (CYP2D6) in poor metabolizers of debrisoquine. Study of the functional significance of individual mutations by expression of chimeric genes. J Biol Chem. 1990 Oct 5;265(28):17209–17214. [PubMed] [Google Scholar]
- Kimura S., Umeno M., Skoda R. C., Meyer U. A., Gonzalez F. J. The human debrisoquine 4-hydroxylase (CYP2D) locus: sequence and identification of the polymorphic CYP2D6 gene, a related gene, and a pseudogene. Am J Hum Genet. 1989 Dec;45(6):889–904. [PMC free article] [PubMed] [Google Scholar]
- Lee J. T., Kroemer H. K., Silberstein D. J., Funck-Brentano C., Lineberry M. D., Wood A. J., Roden D. M., Woosley R. L. The role of genetically determined polymorphic drug metabolism in the beta-blockade produced by propafenone. N Engl J Med. 1990 Jun 21;322(25):1764–1768. doi: 10.1056/NEJM199006213222502. [DOI] [PubMed] [Google Scholar]
- Lennard M. S., Silas J. H., Freestone S., Ramsay L. E., Tucker G. T., Woods H. F. Oxidation phenotype--a major determinant of metoprolol metabolism and response. N Engl J Med. 1982 Dec 16;307(25):1558–1560. doi: 10.1056/NEJM198212163072505. [DOI] [PubMed] [Google Scholar]
- Mahgoub A., Idle J. R., Dring L. G., Lancaster R., Smith R. L. Polymorphic hydroxylation of Debrisoquine in man. Lancet. 1977 Sep 17;2(8038):584–586. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Goto F., Ray W. A., McAllister C. B., Jacqz E., Wilkinson G. R., Branch R. A. Interethnic differences in genetic polymorphism of debrisoquin and mephenytoin hydroxylation between Japanese and Caucasian populations. Clin Pharmacol Ther. 1985 Oct;38(4):402–408. doi: 10.1038/clpt.1985.194. [DOI] [PubMed] [Google Scholar]
- Park Y. H., Kullberg M. P., Hinsvark O. N. Quantitative determination of dextromethorphan and three metabolites in urine by reverse-phase high-performance liquid chromatography. J Pharm Sci. 1984 Jan;73(1):24–29. doi: 10.1002/jps.2600730107. [DOI] [PubMed] [Google Scholar]
- Reed T. E. Caucasian genes in American Negroes. Science. 1969 Aug 22;165(3895):762–768. doi: 10.1126/science.165.3895.762. [DOI] [PubMed] [Google Scholar]
- Relling M. V., Cherrie J., Schell M. J., Petros W. P., Meyer W. H., Evans W. E. Lower prevalence of the debrisoquin oxidative poor metabolizer phenotype in American black versus white subjects. Clin Pharmacol Ther. 1991 Sep;50(3):308–313. doi: 10.1038/clpt.1991.141. [DOI] [PubMed] [Google Scholar]
- SUNAHARA S., URANOM, OGAWAM Genetical and geographic studies on isoniazid inactivation. Science. 1961 Nov 10;134(3489):1530–1531. doi: 10.1126/science.134.3489.1530. [DOI] [PubMed] [Google Scholar]
- Schmid B., Bircher J., Preisig R., Küpfer A. Polymorphic dextromethorphan metabolism: co-segregation of oxidative O-demethylation with debrisoquin hydroxylation. Clin Pharmacol Ther. 1985 Dec;38(6):618–624. doi: 10.1038/clpt.1985.235. [DOI] [PubMed] [Google Scholar]
- Sindrup S. H., Brøsen K., Bjerring P., Arendt-Nielsen L., Larsen U., Angelo H. R., Gram L. F. Codeine increases pain thresholds to copper vapor laser stimuli in extensive but not poor metabolizers of sparteine. Clin Pharmacol Ther. 1990 Dec;48(6):686–693. doi: 10.1038/clpt.1990.212. [DOI] [PubMed] [Google Scholar]
- Skoda R. C., Gonzalez F. J., Demierre A., Meyer U. A. Two mutant alleles of the human cytochrome P-450db1 gene (P450C2D1) associated with genetically deficient metabolism of debrisoquine and other drugs. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5240–5243. doi: 10.1073/pnas.85.14.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger U. M., Vilbois F., Hardwick J. P., Meyer U. A. Absence of hepatic cytochrome P450bufI causes genetically deficient debrisoquine oxidation in man. Biochemistry. 1988 Jul 26;27(15):5447–5454. doi: 10.1021/bi00415a010. [DOI] [PubMed] [Google Scholar]





