Abstract
Background
Studies have demonstrated that blood pressure (BP) control can be improved when clinical pharmacists assist with patient management. The purpose of this study was to evaluate the effectiveness of a physician/pharmacist intervention to improve BP control.
Methods
This was a prospective, cluster-randomized controlled clinical trial with clinics randomized to control (n=3) or intervention (n=3) groups. The study enrolled 402 patients with uncontrolled hypertension (mean age 58.3 years). Clinical pharmacists made drug-therapy recommendations to physicians based on national guidelines. Research nurses performed BP measurements and 24-hour BP monitoring.
Results
Guideline adherence scores increased from 49.4 ± 19.3 at baseline to 53.4 ± 18.1 at 6 months (9% increase) in the control group and from 40.4 ± 22.6 to 62.8 ± 13.5 (57% increase) in the intervention group (p=0.089 adjusted comparison between groups). Mean BP decreased 6.8/4.5 and 20.7/9.7 mm Hg in the control and intervention groups, respectively, (p<0.05 for between-group systolic BP (SBP) comparison). The adjusted difference in SBP was −12.0 (95% CI: −24.0, 0.0) mm Hg, while the difference in diastolic BP (DBP) was −1.8 (CI: −11.9, 8.3). The 24-hour BP levels showed similar effect sizes. BP was controlled in 29.9% of patients in the control group and 63.9% in the intervention group (adjusted odds ratio 3.2; CI: 2.0, 5.1; p<0.001).
Conclusions
A physician/pharmacist collaborative intervention achieved significantly better mean BP and overall BP control rates when compared to a control group. Additional research should be conducted to evaluate efficient strategies to implement team-based chronic disease management.
Keywords: hypertension management, clinical trial, pharmacist management, blood pressure control, guideline adherence
Introduction
Studies suggest that medications are frequently not intensified when BP remains uncontrolled, termed clinical inertia.1–5 One strategy to improve BP control is team-based care involving clinical pharmacists.6–17 However, many of these studies were small single site studies, did not control for important patient, physician or clinic variables and/or did not use an unbiased BP measurement.
The purpose of this study was to evaluate if a physician\pharmacist collaborative model in community-based medical offices could improve BP control. We hypothesized that this model would achieve better guideline adherence, lower mean BP and higher rates of BP control when compared to a control group.18, 19
Methods
This was a prospective, cluster-randomized controlled trial in six community-based family medicine residency programs in Davenport, Des Moines (2 offices), Mason City, Sioux City and Waterloo, Iowa, randomized to either control (n=3) or intervention (n=3) group using a table of random numbers. Preliminary data were collected at baseline including clinic characteristics and an audit of medical records for the most recent BP from a random sample of patients with hypertension. Educational lectures, published national guidelines,19 and clinical trial evidence20 were provided to physicians in all six clinics before the study.
The study was approved by the University of Iowa Institutional Review Board (IRB) and the local IRBs for the six clinics. The recruitment process was identical in control and intervention offices. A research nurse employed by each office reviewed lists and clinic schedules for patients with diagnostic codes for hypertension and approached patients to participate. The research nurse either telephoned patients who met the study criteria or approached them during a regular clinic visit.
Patients in the active study groups provided written informed consent. Males or females over 21 years of age with a diagnosis of essential hypertension taking 0–3 antihypertensives were eligible if they did not have diabetes and their SBP was between 140–179 mm Hg or DBP 90–109 mm Hg. Patients with diabetes with a SBP between 130–179 mm Hg SBP or DBP 80–109 mm Hg were eligible. Exclusion criteria included: BP medication or dose change within four weeks of the baseline visit, BP values ≥ 180/110 mm Hg, evidence of hypertensive urgency or emergency, myocardial infarction or stroke (6 months prior to screening), New York Heart Association Class III or IV heart failure, unstable angina, serious renal or hepatic disease, pregnancy, poor prognosis (life expectancy less than 3 years), dementia or cognitive impairment.
We also performed a medical record audit of hypertensive patients from intervention clinics who met the same inclusion criteria but who did not receive the intervention, termed the passive observation group. The goal of evaluating this group was to determine if BP improved throughout the entire practice.
The primary aim was to evaluate if guideline adherence improved more in the intervention group than the control group, using a tool validated for this study.21–23 Once the patient completed the trial, the research nurses performed a structured medical record abstraction process for all patients that included all clinic progress notes, laboratory values, medications and records from hospitalizations or emergency department visits. For patients in the passive observation group, an index date was selected and data were abstracted for six months before and six months after the index date. An investigator visited each clinic and evaluated a sample of the case abstracts against the medical record to ensure that data were being completely abstracted by each research nurse. Guideline adherence was determined by the percent of 22 eligible criteria met by each patient using a computerized algorithm developed by the investigators.21–23
The research nurses were trained to measure BP using standardized guidelines and certified to properly measure BP at baseline and then once yearly.24, 25 BP was measured three times with an automated Omron HEM 907-XL device. The second and third values were averaged and used as the study BP. At the end of the baseline visit, the research nurse placed a 24-hour monitor set to measure BP every 20 minutes during the day and every 30 minutes during sleep (SpaceLabs 90217-A, SpaceLabs Medical, Redmond, Washington).26 These baseline 24-hour results were not made available to the physician or clinical pharmacist until the patient completed the trial. Finally, patients in both groups were given written information on hypertension from NHLBI.
The following data were collected at the baseline visit: patient age, height, weight, gender, race, educational degree, insurance status, household income, marital status, smoking status, alcohol intake and history of co-existing conditions. Race and ethnicity was self-declared by the patient. The nurse personally administered a validated self-reported questionnaire of medication adherence27, 28 and a questionnaire developed for another study on symptoms that might indicate adverse reactions (potential range from 0–188).8, 29
Patients returned at 3 and 6 months for repeat BP measurements. At the 6-month visit, the nurses performed all of the same procedures as performed at the baseline visit including adverse reaction and medication adherence surveys. Patients received $100 if they completed both 24-hour BP measurements to reimburse them for the inconvenience of wearing the monitors. Patients were telephoned prior to clinic visits to encourage adherence with study visits.
The intervention was modeled after our other studies.6, 8 Intervention physicians and pharmacists underwent teambuilding exercises conducted by two investigators using previous described strategies.8, 30 All six offices employed clinical pharmacists who had been at the office for at least 8 years. The clinical pharmacists had all received a doctor of pharmacy (PharmD) degree and completed a clinical pharmacy residency in primary care. In 5/6 sites the clinical pharmacists were funded 50% by the medical office (to provide family medicine physician resident education and patient care) and 50% from the College of Pharmacy (for pharmacy student teaching). In one control site, the clinical pharmacist was funded entirely by the medical office’s health system. The majority of the pharmacists’ time was spent on pharmacy student, medical resident and staff physician education on drug therapy with a minority of their time devoted to direct patient management prior to the study. All of the pharmacists were well versed in hypertension treatment. However, two initial 90-minute training sessions were provided to the intervention pharmacists by one investigator to ensure that a consistent intervention was provided.
All study visits with intervention pharmacists occurred in the medical office and pharmacists were encouraged to assess medications and BP at baseline, one month plus over the telephone at 3 months and more frequently if necessary. The pharmacists made recommendations consistent with national guidelines.18, 19 BP control was defined as an office BP <130/80 mm Hg for patients with diabetes or chronic kidney disease and <140/90 mm Hg for all other patients.18 Physicians and pharmacists in the intervention offices decided how to best implement the intervention and they were not required to perform the suggested intervention visits for this pragmatic trial. The pharmacists almost always provided face-to-face recommendations to the patient’s physician. The pharmacist provided physician education, if necessary, and all therapy changes were approved by the physician.
Clinical pharmacists in control sites abstained from providing care for study patients but continued to answer general treatment questions from physicians. Patients in the control group also received BP measurements at baseline, 3 and 6 months. The primary care physician determined when office visits for routine care or BP should occur.
Data analysis
Data were entered into case report forms by the research nurses. Individual data elements were double-entered into an Access® database and analyzed by a separate data management team chaired by the biostatistician (JDD).
Power calculations were performed based on a two-sample t-test to compare SBP, assuming effect sizes of 6–10 mm Hg SBP with a standard deviation of 16–19 mm Hg.9, 14, 31, 32 With a sample size of 200 patients per group (400 total), an α = 0.05 two-sided test would have 88–100% power. Because this approach ignores random effects due to physician and clinic, this may overstate the power.
Descriptive statistics (means, standard deviations) were performed on baseline data and comparisons between groups were made using Student’s t-test and Fisher’s Exact test. Preliminary analysis revealed that the response variables were correlated within-subject, but there was no significant clustering within physicians, and very little clustering within clinic, similar to our previous findings in another study.8 However, we included clinic as a random effect in our analyses of response variables, consistent with the study design. For continuous responses (BP), likelihood-based mixed models with random patient and clinic effects were fit in SAS Proc Mixed to incorporate all available data from baseline through 6 months in an intention-to-treat analysis. For BP control, a Generalized Estimating Equation (GEE) model using the binomial distribution and the logit link was fit in SAS Proc Genmod, accommodating the correlations within clinics. For both models (mixed and GEE), contrasts were estimated in order to test for the treatment effect adjusted for the following baseline values: BP, age, gender, race, education, insurance status, household income, marital status, smoking status, alcohol intake, body mass index, number of co-existing conditions, number of antihypertensive medications and medication adherence. Medication adherence was determined using the instrument validated by Morisky.27, 28 Poor medication adherence was defined as answering yes to 3 or more of the 5 questions.
Results
The general operations of all six sites were similar and office served as the model office for a distinct family medicine residency program. All six programs met the institutional requirements of the Accreditation Committee for Graduate Medical Education (ACGME) and the program requirements for family practice set out by the ACGME and its Residency Review Committee (RRC). All faculty physicians were Board Certified in Family Practice. The faculty physicians spent an 3.4 (both mean and mode, range 2–5) half-days per week seeing patients in their clinic. The scheduled time in the clinic for first-, second- and third-year family medicine physician residents was: 1–2 half-days, 2–4 half-days and 3–5 half-days per week, respectively. The remainder of the family medicine residents’ time was spent on clinical rotations in the affiliated hospital. Providers in each office admitted patients to a distinct community hospital that operated and funded a significant portion of each residency program. Table 1 outlines the demographics and staffing at the participating clinics. Prior to the study, there was a wide range for BP control rates (28.6%–70.0%) that was higher in the control sites (55.7%) than intervention sites (41.9%). These values compared with BP control rates of 28–48% in managed care plans at the same time period.33
Table 1.
Clinic Characteristics at Baseline Prior to the Study
| Office A | Office B | Office C | Office D | Office E | Office F | |
|---|---|---|---|---|---|---|
| Randomized | Control | Control | Control | Intervention | Intervention | intervention |
| Outpatient Visits per year | 27,000 | 20,700 | 18,389 | 38,343 | 29,236 | 13,612 |
| Faculty physicians | 5 | 6 | 5 | 8 | 6 | 4 |
| Resident physicians | 17 | 30 | 17 | 17 | 24 | 18 |
| Clinical pharmacists | 1 | 1 | 1 | 1 | 1 | 1 |
| Pharmacy residents | 1 | 0 | 0 | 1 | 0 | 0.1* |
| Chart audit BP control (n) | 70% (50) | 50% (50) | 46.9% (49) | 28.6% (49) | 49% (49) | 48% (50) |
Patient, pharmacist and physician data were collected for the year 2000 and BP data in 2001 as preliminary data for the study grant application.
- one resident per year spent 5 weeks in this office.
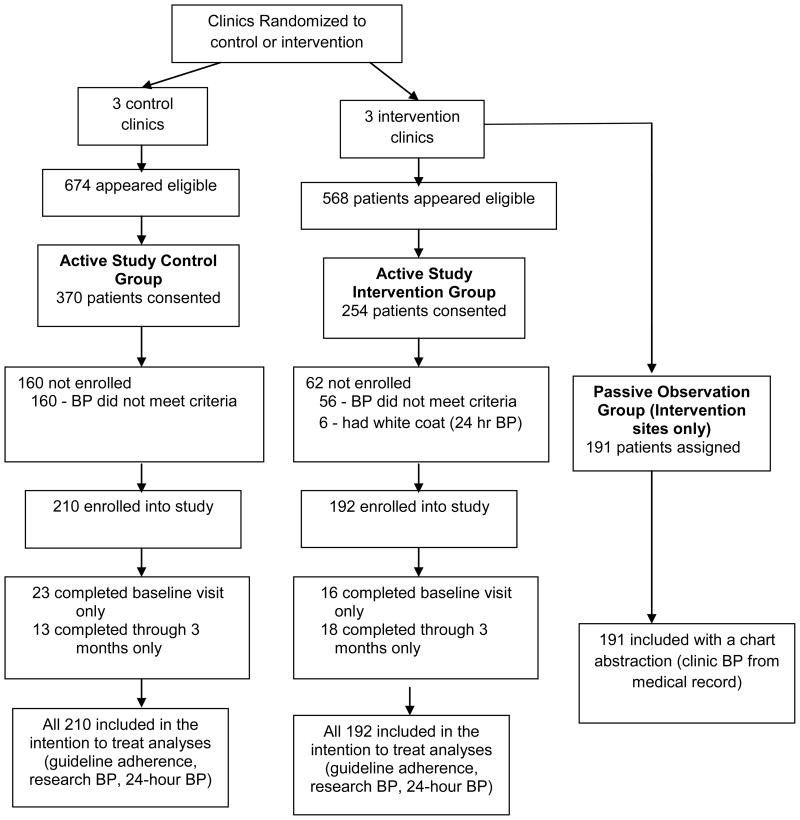
Patient recruitment began in January 2004 and the last patient completed the trial in March 2008 (Table 2). In the control group, 173/210 (82.4%) patients completed the 3-month visit and 174/210 (82.9%) completed the 6-month visit while 173/192 (90.1%) patients in the intervention group completed the 3-month visit and 158/192 (82.3%) the 6-month visit (Figure 1). Pharmacists made 1.6 ± 1.4 additional visits/contacts per patient including 83.9% of the specified 1-month telephone call and these contacts were controlled for in the analyses. When adjusted for the intervention effect, the within-clinic interclass correlation coefficient (ICC) at 6 months was 1.7% for SBP, 11.3% for DBP and 10.4% for adherence scores.
Table 2.
Patient Demographics at Baseline
| Control (n=210) Number (%) or Mean (± SD) | Intervention (n=192) Number (%) or Mean (± SD) | P-value for difference | |
|---|---|---|---|
| Gender: Female | 117 (55.7) | 120 (62.5) | 0.187 |
| Male | 93 (44.3) | 72 (37.5) | |
| Race: Caucasian | 163 (77.6) | 165 (85.9) | 0.0394 |
| Hispanic | 0 | 8 (4.2) | |
| African-American | 41 (19.5) | 13 (6.8) | |
| American Indian | 2 (1.0) | 1 (0.5) | |
| >1 race or other | 4 (1.9) | 5 (2.6) | |
| Age (years) | 59.2 (±13.8) | 57.3 (±14.3) | 0.193 |
| Married | 91 (43.3) | 130 (67.7) | <0.001 |
| Education beyond high school | 76 (36.2) | 65 (33.9) | 0.753 |
| Household income < $25,000 | 109 (51.9) | 41 (21.4) | <0.001 |
| Insurance status | <0.001 | ||
| Individual or group plan | 68 (32.4) | 108 (56.3) | |
| Medicare/Medicaid | 85 (40.5) | 71 (37.0) | |
| Self-pay or other | 57 (27.1) | 13 (6.8) | |
| BMI (kg/m2) | 34.2 (±8.7) | 32.1 (±6.8) | 0.010 |
| Smoker (within last 15 years) | 86 (41.0) | 65 (33.9) | 0.150 |
| More than 2 alcoholic drinks/wk | 8 (3.8) | 5 (2.6) | 0.575 |
| Family History of premature CV event | 50 (23.8) | 37 (19.8) | 0.278 |
| Diabetes Mellitus | 80 (38.1) | 38 (19.8) | <0.001 |
| Hx Stroke or TIA | 16 (7.6) | 12 (6.3) | 0.696 |
| Hx Myocardial infarction | 13 (6.2) | 0 (0.0) | 0.002 |
| Coronary artery bypass grafting | 5 (2.4) | 3 (1.6) | 0.726 |
| Heart failure | 4 (1.9) | 1 (0.5) | 0.374 |
| Angina | 12 (5.7) | 1 (0.5) | 0.003 |
| Peripheral arterial disease | 4 (1.9) | 4 (2.1) | 1.000 |
| Chronic kidney disease | 16 (7.6) | 11 (5.7) | 0.551 |
| Left-ventricular hypertrophy | 3 (1.4) | 3 (1.6) | 1.000 |
| At least one co-existing condition | 200 (95.2) | 173 (90.1) | 0.051 |
| Number of co-existing conditions | 3.6 (± 2.2) | 2.8 (±1.8) | <0.001 |
| At least one antihypertensive | 192 (91.4) | 127 (66.2) | <0.001 |
| Number of antihypertensive medications | 1.9 (±1.0) | 1.3 (±1.2) | <0.001 |
| Low self-reported medication adherence (score ≥3) | 19 (9.1) | 17 (8.9) | 1.000 |
Figure 1.
Flow of patients through the study protocol
Patients in the control group were significantly less likely to be married (p<0.001), more likely to have had household income below $25,000 (p<0.001), self-pay for their care (p<0.001), diabetes mellitus (p<0.001), a history of MI (p=0.002) or angina (p=0.003), more co-existing conditions (p<0.001), and more antihypertensive medications (p<0.001) at baseline (Table 2). These variables were covariates in the analyses.
Primary Outcomes
Mean guideline adherence scores improved modestly (9%) in the control group from 49.4 ± 19.3 at baseline to 53.4 ± 18.1 at 6 months (Table 3). Mean guideline adherence scores improved 57% from 40.4 ± 22.6 to 62.8 ± 13.5 in the intervention group (between group comparisons: p=0.043 for unadjusted, p=0.089 for adjusted). After adjustment for the covariates, the mean difference between groups for the improvement in the guideline adherence score was 9.6 (95% CI: −2.3, 21.4) (Table 4).
Table 3.
Clinic BP, 24-hour blood pressure, BP control, and Adherence Scores
| Variable | Baseline | 3 Month | 6 Month |
|---|---|---|---|
| Control (n=210) | |||
| Systolic BP | 150.6 ±14.1 † | 146.1 ±19.6 | 143.8 ±20.5 † |
| Diastolic BP | 83.6 ±12.3 | 81.5 ±14.0 | 79.1 ±14.3 |
| BP control (%)* | 0 | 25.4% | 29.9% § |
| 24 hr average systolic BP | 137.9 ±15.8 | -- | 131.5 ±17.7 |
| 24 hr average diastolic BP | 77.2 ±10.7 | -- | 73.7 ±10.7 |
| Total guideline adherence score (% criteria met) | 49.4 ±19.3 | -- | 53.4 ±18.1# |
| Intervention (n=192) | |||
| Systolic BP | 153.6 ±12.8 † | 134.8 ±14.6 | 132.9 ±15.5 † |
| Diastolic BP | 87.4 ±11.9 | 79.9 ±11.3 | 77.7 ±11.2 |
| BP control (%)* | 0 | 49.7% | 63.9% § |
| 24 hr average systolic BP | 136.2 ±14.6 | -- | 121.1 ±13.7 |
| 24 hr average diastolic BP | 78.5 ±11.7 | -- | 70.2 ±8.7 |
| Total guideline adherence score (% criteria met) | 40.4 ±22.6 | -- | 62.8 ±13.5# |
- BP control defined as <130/80 mm Hg for diabetics and <140/90 for non-diabetic patients. p-values are based on between-group differences adjusted for baseline values for age, gender, race, education, insurance status, household income, marital status, smoking status, alcohol intake, body mass index, the number of co-existing conditions, number of antihypertensive medications, and medication adherence:
- p<0.050;
- p<0.010;
- p<0.001,
- p=0.043 (crude) and p=0.089 (adjusted).
Table 4.
Unadjusted and adjusted effects of intervention vs. control at 6 months.
| Outcome | Crude Effect (CI) | Adjusted Effect (CI) |
|---|---|---|
| SBP | −11.9 (−21.6, −2.2) | −12.0 (−24.0, 0.0) |
| DBP | −3.6 (−10.7, 3.4) | −1.8 (−11.9, 8.3) |
| BP Control | 4.2 (2.6, 6.7) | 3.2 (2.0, 5.1) |
| 24-hr Average SBP | −8.0 (−17.8, 1.9) | −10.3 (−23.7, 3.1) |
| 24-hr Average DBP | −3.9 (−9.7, 1.8) | −3.1 (−9.0, 2.8) |
| Total adherence score | 11.1 (0.4, 21.7) | 9.6 (−2.3, 21.5) |
Effects are reported as differences (95% CI’s) for BP levels and guideline adherence scores and as Odds Ratios (95% CI’s) for BP control in the intervention group compared to the control group. Covariates for adjustment included the baseline values of response, age, gender, race, education, insurance status, household income, marital status, smoking status, alcohol intake, body mass index, the number of co-existing conditions, number of antihypertensive medications, and medication adherence.
BP was controlled in significantly more patients in the intervention group (63.9%) than the control group (29.9%) (p<0.001) with an odds ratio of 3.2 (95% CI: 2.0, 5.1) after adjustment for covariates. BP was controlled in 32.4% of non-diabetics in the control group and 68.8% in the intervention group (adjusted odds ratio of 3.9; CI: 3.1, 5.0; p<0.001). BP was controlled in 26.1% of patients with diabetes in the control group and 45.5% in the intervention group (adjusted odds ratio of 4.7; CI: 1.7, 13.1; p=0.003).
BP was reduced 6.8/4.5 mm Hg in the control group and 20.7/9.7 mm Hg in the intervention group (between-group, p<0.05 for SBP). The mean difference (control group minus the intervention group) in 6-month SBP was −12.0 (95% CI: −24.0, 0.0) mm Hg after adjustment (Table 4), while the adjusted mean difference in DBP was −1.8 (CI: −11.9, 8.3) mm Hg. The mean difference in 24-hour BP was −10.3 (CI: −23.7, 3.1) mm Hg for SBP and −3.1 (CI: −9.0, 2.8) mm Hg for DBP.
We performed a sensitivity analysis to determine the robustness of our findings in the presence of informative dropout. First, we repeated our analysis under a scenario that all 70 subjects who dropped out had uncontrolled BP at the end of the study and found that BP control rates in the intervention and control groups would be 52.6% and 24.8%, respectively, (adjusted OR 3.2; CI: 2.0, 5.2, p<0.001). More pessimistically, we considered the scenario where all 34 dropouts in the intervention group had uncontrolled BP and all 36 dropouts in the control group had controlled BP. In this situation, the respective BP control rates would be 52.6% and 41.9%, (adjusted OR 3.0 CI: 2.0, 4.5; p<0.001).
Secondary Outcomes
We identified 197 patients for the passive observation group from the three intervention clinics. We did not compare this group statistically to the active intervention groups because the outcome measures were different (research nurse measured BP in study patients versus chart-recorded BP in the passive observation group). The mean SBP at baseline in the passive observation group (149.2 ± 15.8 mm Hg) was lower than the active control group (150.6 ± 14.1 mm Hg) and the active intervention group (153.6 ± 12.7 mm Hg). By six months, the mean SBP in the passive observation group from intervention clinics was 139.3 ± 16.9 mm Hg compared to 143.8 ± 20.5 mm Hg in the control and 132.9 ± 15.4 mm Hg in the intervention group. BP control rates at 6 months were: control group 29.9%, passive observation group 39.1% and the active intervention group 63.9% which supports the initial hypothesis that BP control within intervention sites could be improved more broadly in patients who did not receive the intervention.
The intervention pharmacists made 771 recommendations, of which 742 (96%) were accepted by physicians. The mean increase in the number of antihypertensives from baseline was higher in the intervention group than in the control group (1.1 vs. 0.3; p<0.001). There were 1,140 documented antihypertensive changes in the 402 subjects, of which 562 (49%) were new medications, 333 (29%) were increases in dosage, 195 (17%) were decreases in dosage, and 49 (4%) were cessation of current medications. The mean of overall changes was higher in the active intervention group (3.6 vs. 2.2 changes per subject; P=0.001), as was the number of new medications (1.9 vs. 1.0; P<0.001), and, possibly, the number of discontinued medication (0.6 vs. 0.3; P=0.053). The number of dosage changes did not vary significantly between groups. The mean number of antihypertensives was not different in the intervention (2.4 ± 1.1) and the control groups (2.2 ± 1.1) at the end of the study (p=0.219).
The percentage of patients with poor self-reported medication adherence declined from 18.7 ± 22.0% to 14.7 ± 20.9 in the control group and from 17.3 ± 27.5 to 14.6 ± 25.4% in the intervention group (p=0.602 and p=0.979, respectively).
Symptom scores were higher at baseline in the control group (42.1 ± 24.2) compared to the intervention group (28.0 ± 23.0) (p<0.001). In spite of the increase in medications in both groups, symptom scores declined at 6 months to 39.2 ± 24.2 in the control group (p=0.073 vs. baseline) and 16.6 ± 12.5 in the intervention (p<0.001 vs. baseline or between groups at 6 months).
Discussion
This team-based approach to the management of BP was highly effective.17, 32 Studies involving pharmacists found control rates of 45–70% and a difference of approximately 8–14 mm Hg in systolic BP.16, 34 The present pragmatic study achieved BP control (64%) and SBP (12 mm Hg) at the higher end of this range, but lower BP control than our previous efficacy study (89%).8 However, that study used a 9-month intervention with more required visits with the pharmacist than the present study. The 6-month BP control in that study was 73% compared to 64% in the present trial. These differences could be due to incomplete implementation of the intervention, a less potent intervention, differences in the patient populations or other factors. Nonetheless, the intervention was effective and was consistent with the chronic care model in which the physician utilizes team-based care.35–39 As with our previous study, there were more medication additions in the intervention group (1.1 medications) than in the control group (0.3 additions) which is one likely reason for better BP control in the intervention group since medication adherence did not differ.
The changes in 24-hour BP values showed reductions similar to those of the clinic BP values. However, the large difference between groups was not statistically significant due to the large number of patients who refused the repeat 24-hour monitoring in this pragmatic trial. Therefore, the power was low for the 24-hour results.
There was greater improvement in the guideline adherence score in the intervention (57%) group than the control group (9%) which was significant in unadjusted analyses, but not quite significant for adjusted analyses. This may be due to the notable within-clinic clustering of adherence scores, which would tend to compromise power in our cluster-randomized design and may have been compounded by adjusting for so many baseline covariates. Both groups still had room for improvement, with over one third of the eligible criteria not met in the intervention group and nearly half of the eligible criteria not met in the control group at the end of the study.
It is important to note that the patients in the control group did not receive usual care. Instead, they were informed of their BP, the goal BP they needed to achieve and they were given written information on managing BP. In addition, all physicians received educational sessions on strategies to improve BP control. These approaches, along with increased surveillance by the research nurses, achieved BP control in 29.9% of patients with previously uncontrolled BP in the control group.
This study had several strengths including the use of standardized clinic BP measurements,24 intention-to-treat analyses and control of numerous baseline covariates. In addition, this was only the second study of team-based care to use 24-hour BP monitoring.8 This study randomized by clinic which avoided contamination that might occur with randomization by patient or physician and had one of the largest sample sizes of team-based care for BP.
There were several limitations of the study including a small number of randomized clinics which may have compromised power for adherence scores and BP levels. As an additional concern, there were more non-Caucasians, more with lower income, higher BMI and more coexisting conditions including diabetes in the control group, which could make it more difficult to achieve improvements in BP or led to lower medication adherence. However, we adjusted for all these variables in our analyses. BP was controlled in 46% of patients with diabetes in the intervention group and 26% in the control group suggesting that at least the imbalance of diabetics between groups probably did not influence the findings. This study can only be generalized to community-based family medicine offices. BP control rates might be different in other practice settings or when patients are not required to attend specific research visits. Also, this study had a higher dropout rate than our previous efficacy study. Even so, the BP reductions were still significantly greater in the intervention group and our sensitivity analysis suggests the dropouts would not have changed the conclusions. Finally, since the study only enrolled patients with diagnosed hypertension, the intervention strategy and results cannot be generalized to patients who are unaware of their hypertension or those yet to receive a diagnosis of hypertension.
Conclusion
This study used an intervention involving physician\pharmacist collaboration and found a significant improvement in BP control compared to the control group. This study suggests that clinics or health systems with clinical pharmacists should consider reallocation of duties to provide more direct patient management to significantly improve BP control. Future studies of this model should include more clinics with greater geographic, ethnic and socioeconomic diversity since these populations are likely to respond differently to the intervention.
Acknowledgments
The authors acknowledge the assistance and support from physicians, pharmacists, nurses and staff from the following family medicine residency programs: Broadlawns Family Health Center (Des Moines), East Des Moines Family Care Center, Mercy Family Practice Center (Mason City), Northeast Iowa Family Practice Center (Waterloo), Quad Cities Genesis Family Medicine Center (Davenport), Siouxland Family Practice Center (Sioux City). The authors also acknowledge the assistance of the Data Safety and Monitoring Board (DSMB) including, Drs. Henry R. Black, MD (chair), Clinical Professor, New York University, School of Medicine, George L. Bakris, MD, Professor of Medicine, University of Chicago Pritzker School of Medicine, Kathryn Chaloner, PhD, Professor and Head, Department of Biostatistics, College of Public Health, University of Iowa, and Daniel W. Jones, MD, Chancellor, University of Mississippi. Members of the DSMB were provided with honoraria from the NHLBI grant budget for their time to conduct meetings twice a year.
Funding for this project was supported by the National Heart, Lung, and Blood Institute, RO1 HL070740. Drs. Carter, Doucette and Chrischilles are supported by the Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics Cooperative Agreement #5U18HSO16094. Dr. Carter and Ms. Franciscus are also supported by the Center for Research in Implementation in Innovative Strategies in Practice (CRIISP), Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (HFP 04-149).The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00201019
References
- 1.Milchak JL, Carter BL, James PA, Ardery G. Measuring adherence to practice guidelines for the management of hypertension: an evaluation of the literature. Hypertension. 2004;44:602–608. doi: 10.1161/01.HYP.0000144100.29945.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter BL, Malone DC, Ellis SL, Dombrowski RC. Antihypertensive Drug Utilization in Hypertensive Veterans With Complex Medication Profiles. J Clin Hypertens. 2000;2:172–180. [PubMed] [Google Scholar]
- 3.Hill MN, Levine DM, Whelton PK. Awareness, use, and impact of the 1984 Joint National Committee consensus report on high blood pressure. American Journal of Public Health. 1988;78:1190–1194. doi: 10.2105/ajph.78.9.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naiman DJ, Barker LR. Adherence to the JNC VI guidelines for the treatment of hypertension in the resident clinic. Maryland Medical Journal. 1999;48:307–311. [PubMed] [Google Scholar]
- 5.Nelson CR, Knapp DA. Trends in antihypertensive drug therapy of ambulatory patients by US office-based physicians. Hypertension. 2000;36:600–603. doi: 10.1161/01.hyp.36.4.600. [DOI] [PubMed] [Google Scholar]
- 6.Carter BL, Barnette DJ, Chrischilles E, Mazzotti GJ, Asali ZJ. Evaluation of hypertensive patients after care provided by community pharmacists in a rural setting. Pharmacotherapy. 1997;17:1274–1285. [PubMed] [Google Scholar]
- 7.Borenstein JE, Graber G, Saltiel E, et al. Physician-pharmacist comanagement of hypertension: a randomized, comparative trial. Pharmacotherapy. 2003;23:209–216. doi: 10.1592/phco.23.2.209.32096. [DOI] [PubMed] [Google Scholar]
- 8.Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens. 2008;10:260–271. doi: 10.1111/j.1751-7176.2008.07434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson SR, Slaughter R, Halapy H. Pharmacists’ ability to influence outcomes of hypertension therapy. Pharmacotherapy. 1997;17:140–147. [PubMed] [Google Scholar]
- 10.Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. 2006;296:2563–2571. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]
- 11.McKenney JM, Slining JM, Henderson HR, Devins D, Barr M. The effect of clinical pharmacy services on patients with essential hypertension. Circulation. 1973;48:1104–1111. doi: 10.1161/01.cir.48.5.1104. [DOI] [PubMed] [Google Scholar]
- 12.Monson R, Bond CA, Schuna A. Role of the clinical pharmacist in improving drug therapy. Clinical pharmacists in outpatient therapy. Arch Intern Med. 1981;141:1441–1444. [PubMed] [Google Scholar]
- 13.Solomon DK, Portner TS, Bass GE, et al. Clinical and economic outcomes in the hypertension and COPD arms of a multicenter outcomes study. Journal of the American Pharmaceutical Association. 1998;38:574–585. doi: 10.1016/s1086-5802(16)30371-0. [DOI] [PubMed] [Google Scholar]
- 14.Vivian EM. Improving blood pressure control in a pharmacist-managed hypertension clinic. Pharmacotherapy. 2002;22:1533–1540. doi: 10.1592/phco.22.17.1533.34127. [DOI] [PubMed] [Google Scholar]
- 15.Zillich AJ, Sutherland JM, Kumbera PA, Carter BL. Hypertension outcomes through blood pressure monitoring and evaluation by pharmacists (HOME study) J Gen Intern Med. 2005;20:1091–1096. doi: 10.1111/j.1525-1497.2005.0226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter BL, Rogers M, Daly J, Zheng S, James PA. Quality improvement strategies for hypertension: The potency of team-based care interventions. Arch Intern Med. 2009 doi: 10.1001/archinternmed.2009.316. (in press October 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646–657. doi: 10.1097/01.mlr.0000220260.30768.32. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 20.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 21.Milchak JL, Carter BL, Ardery G, Dawson JD, Harmston M, Franciscus CL. Physician adherence to blood pressure guidelines and its effect on seniors. Pharmacotherapy. 2008;28:843–851. doi: 10.1592/phco.28.7.843. [DOI] [PubMed] [Google Scholar]
- 22.Ardery G, Carter BL, Milchak JL, et al. Explicit and implicit evaluation of physician adherence to hypertension guidelines. J Clin Hypertens. 2007;9:113–119. doi: 10.1111/j.1524-6175.2007.06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milchak JL, Carter BL, Ardery G, et al. Development of explicit criteria to measure adherence to hypertension guidelines. J Hum Hypertens. 2006;20:426–433. doi: 10.1038/sj.jhh.1002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 25.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien E, Coats A, Owens P, et al. Use and interpretation of ambulatory blood pressure monitoring: recommendations of the British hypertension society. BMJ. 2000;320:1128–1134. doi: 10.1136/bmj.320.7242.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Morisky DE, Levine DM, Green LW, Shapiro S, Russell RP, Smith CR. Five-year blood pressure control and mortality following health education for hypertensive patients. Am J Public Health. 1983;73:153–162. doi: 10.2105/ajph.73.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber CA, Leloux MR, Carter BL, Farris KB, Xu Y. Reduction in adverse symptoms as blood pressure becomes controlled. Pharmacotherapy. 2008;28:1104–1114. doi: 10.1592/phco.28.9.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farris KB, Cote I, Feeny D, et al. Enhancing primary care for complex patients. Demonstration project using multidisciplinary teams. Can Fam Physician. 2004;50:998–1003. [PMC free article] [PubMed] [Google Scholar]
- 31.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) SHEP Cooperative Research Group. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 32.Carter BL, Zillich AJ, Elliott WJ. How pharmacists can assist physicians with controlling blood pressure. J Clin Hypertens. 2003;5:31–37. doi: 10.1111/j.1524-6175.2003.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The State of Managed Care Quality. Health Employer Data and Information Set. National Committee for Quality Assurance; 2000. [Accessed May 17, 2001. ]. http://www.ncqa.org/communication. [Google Scholar]
- 34.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 35.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20:64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 36.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74:511–544. [PubMed] [Google Scholar]
- 37.Wagner EH, Glasgow RE, Davis C, et al. Quality improvement in chronic illness care: a collaborative approach. Jt Comm J Qual Improv. 2001;27:63–80. doi: 10.1016/s1070-3241(01)27007-2. [DOI] [PubMed] [Google Scholar]
- 38.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 39.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]