Abstract
The discovery that the SRY gene induces male sex in humans and other mammals led to speculation about a possible equivalent for female sex. But females are proving to be more complicated. Several master genes appear to be autonomously involved, and female sex determination seems to remain relatively labile. Partial loss of function of the transcription factor FOXL2 leads to premature ovarian failure in women; and in animal models, Foxl2 is required for folliculogenesis as well as for maintenance, and possibly induction, of female sex determination. In the germ line, oocytes form apparently normally even in the absence of Foxl2, dependent on genes that include female-specific factors such as Fig-alpha, Nobox, etc. In the soma, ablation of Foxl2 or the independently expressed gene Wnt4 (likely downstream of Rspo1) can produce partial testis differentiation in XX mice, and the double knockout results in the formation of tubules and spermatogonia. This indicates that at least two autonomous ovarian pathways are required to antagonize testis differentiation in females, a finding that is being increasingly corroborated by studies in goats and non-mammalian vertebrates. In recent expression profiling of mouse ovaries that lack Foxl2 alone or in combination with Wnt4 or Kit/c-Kit, we found that following Foxl2 loss, early testis genes (including the downstream effector of Sry, Sox9) and several novel ovarian genes were consistently dysregulated during embryo-fetal development. The results support the proposal of dose-dependent Foxl2 function and anti-testis action. A partial working model for somatic development and sex determination is presented in which Sox9 is direct antagonist of Foxl2 in the supporting cell lineage.
Keywords: Sex determination, Ovary, Testis, Foxl2, Sex reversal, Gonadal development
I. Male/Female differences in “master gene” regulation of gonadal development
The need for a specific gene that activates male sex determination seems obvious in mammals. Males are heterogametic and the Y chromosome is dominant, thus a male signal (Sry) has to divert an otherwise “chromosomally” default female pathway (XX or X0) (Jost, 1972; McLaren, 1988; Goodfellow and Darling, 1988; Burgoyne, 1988). For the same reason, an equivalent signal for the female option of sex determination seems quite superfluous. Certainly, it seems reasonable that active male gene repression in the females may help to preserve femaleness against (mutational or otherwise accidental) sources of sexual cell fate instability. However, many chromosomally unlinked genes could well do the job. Current findings support both the unique master gene model for males and the polymorphous genetic model for females (Brennan J and Capel, 2004; Wilhelm and Koopman, 2006; Park SY and Jameson, 2005; Yao, 2005).
In males, the Y-linked male signal for testis determination, the “master gene” Sry or its downstream effector Sox9, are adequate to catalyze a powerful cascade leading from the bipotential gonad to the testis and spermatocytes (Koopman et al, 1991; Vidal VPI et al, 2001). In females (XX or XO), in the absence of the male signal, development of the bipotential gonad is rather directed toward the ovary. However, studies in a number of laboratories have increasingly shown that by contrast to the testis, where one gene can determine the sex of both germ cells and soma, in the ovary, there is no obvious single peremptory gene (represented by question marks in the schematic of Fig. 1 and see below). Instead, a cohort of master genes are responsible in parallel for specific lineages: the partially defined pathways lead, more or less independently, to oocytes or to various female somatic cell types (Kocer et al, 2009; Kim et al., 2006; Jeays-Ward et al, 2003; Parma et al, 2006; Tomizuka et al, 2008; Maatouk et al, 2008). We focus here on our work with Foxl2 (boxed in Fig. 1), a winged helix forkhead transcription factor that is not active in the testis but is specifically expressed in the granulosa cell lineage and is indispensible for ovary histogenesis (Ottolenghi et al, 2005; Loffler et al, 2003).
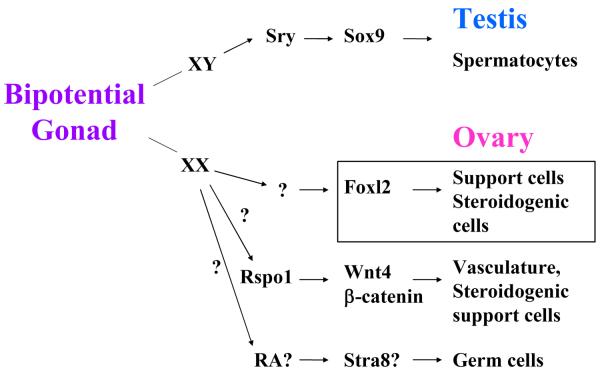
Figure 1.
Development of testis and ovary from the bipotential gonad. In the XY gonad a single master gene (Sry) drives a cascade of events, such as the activation of Sox9, that lead to male sex determination and testis development. In the XX gonad, several genes that are independent from one another, are responsible for female sex determination and the maturation of different cell lineages. Question marks represent either unknown factors or putative candidates for a particular lineage.
The Foxl2 gene was first identified as mutated in a syndrome involving risk of premature ovarian failure (as well as an eyelid malformation: BPES) (Crisponi et al., 2001). Women who have loss of function of one allele of the gene are prone to early menopause. The primary point where Foxl2 acts was clear in a mouse model in which the gene was completely ablated (Uda et al, 2004). In the knockout the fetal ovary is blocked in development. Follicle formation fails completely, and no steroid-forming cells or specialized vasculature form. This accounts for defective or deregulated growth of somatic cells and oocytes, respectively (Uda et al., 2004; Schmidt et al., 2004). The actions of Foxl2 are characteristically relatively independent of other pathways, as shown in Fig. 1. In particular, the expression of Foxl2 occurs whether or not oocytes are present (Fig. 2) – and conversely, oocytes seem to form and enter meiosis indifferent to the absence of Foxl2 (Ottolenghi et al, 2005), although gene expression profiling suggests that the timing of meiosis is somewhat delayed (Garcia et al, 2009 in press).
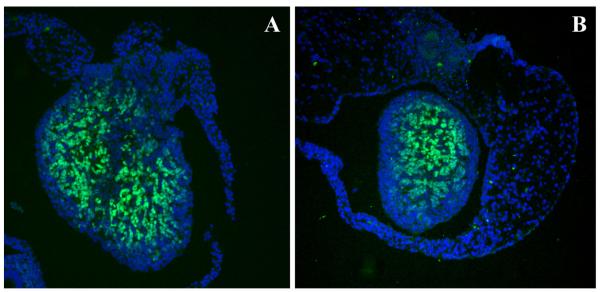
Figure 2.
Expression of Foxl2 in newborn ovaries, assessed by immunohistochemistry. The level of expression of Foxl2 (green) is similar in the wildtype ovary (A) and in the c-kit knockout ovary that is devoid of oocytes (B). DAPI stains all nuclei blue.
Comparable results for other “modular” genes involved in ovary formation have been reported, particularly for Rspo1 and for Wnt4 for somatic lineages and for Lhx8 for oocytes (Choi et al, 2008 and see below). In all of these cases, knockout models have been compared by our group and others to assess the requirement of each gene for the expression of others. The compilation of expression profiling data has facilitated the inference of a possible gene regulatory network, including the identification of additional candidate genes with putatively independent functions in development and sex determination (Garcia-Ortiz et al, 2009 in press).
II. Extent of irreversibility of sex determination
In contrast to testis, not only does the ovary have different master genes in different cell lineages, but ovarian somatic sex remains relatively labile, with the potential to switch to a testis state. Traditionally, the process of secondary partial sex reversal affecting the ovarian soma around birth or later in life has been regarded as distinct from primary sex reversal (Jost, 1972; Burgoyne, 1988). Indeed, several factors, such as loss of oocytes or excess anti-mullerian hormone, have been repeatedly implicated in late onset partial anomalies, but have never led to complete early sex reversal (Guicon and Magre, 2006; Lyet et al, 1995). By contrast, we have shown that Foxl2 mutations affect both processes in animal models, suggesting that the maintenance of female sex determination in ovarian somatic cells may rely on the continuous expression of critical gene(s) that include Foxl2.
When Foxl2 is ablated, interruption of ovary histogenesis is unequivocally accompanied by the initiation of partial sex reversal: Sox9 floods the ovary, and granulosa cells take on a Sertoli-like appearance, with the attendant synthesis of the entire array of testis-specific markers (Fig. 3). In the absence of Foxl2, the phenotypic effect occurs only after birth (Fig. 4), when follicles ordinarily form (Ottolenghi et al, 2005); but subtle changes in expression patterns in the gonad are seen as early as E13, when Foxl2 would ordinarily already be expressed (Garcia et al, 2009 in press).
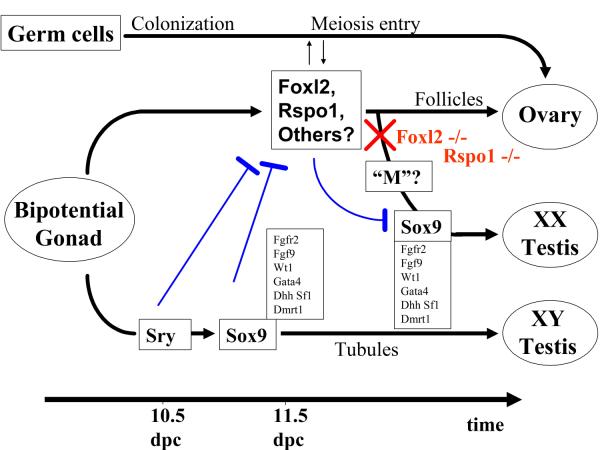
Figure 3.
Model of sex determination in mammals. Sry begins to be expressed in somatic cells around 10.5 dpc (days post coitum) and immediately induces the activation of Sox9, followed by the expression of a cascade of testis-specific markers that direct the development of the testis from the bipotential gonad in XY individuals. In the female, where Sry is not present, Foxl2, Rspo1 and other ovarian genes begin to be expressed around 11.5 dpc, i.e., at the same time at which Sry and Sox9 have reached their maximal levels in the male gonad. It represses the expression of Sox9, and along with other female markers leads to the development of the ovary. If Foxl2 is inactivated (red X), Sox9 is turned on, possibly by a putative “M” factor (see text), and somatic cells begin to differentiate along the testis pathway, resulting in a sex reversal that is only partial in mice. XX germ cells follow an independent pathway, characterized by the colonization of the gonad and entry into meiosis before somatic cell sex reversal is complete. Though sex choice of the germ cell lineage is resistant to the individual loss of Foxl2 in mice, this is at least partly due to the persistent action of other ovarian somatic genes, including Rspo1 and Wnt4, The nature and functional relevance of inductive interactions among distinct female cell lineages during early ovary differentiation is still poorly understood. Hammered blue lines indicate antagonistic interactions.
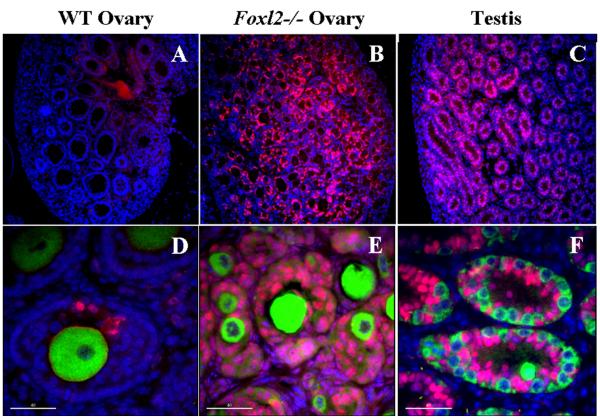
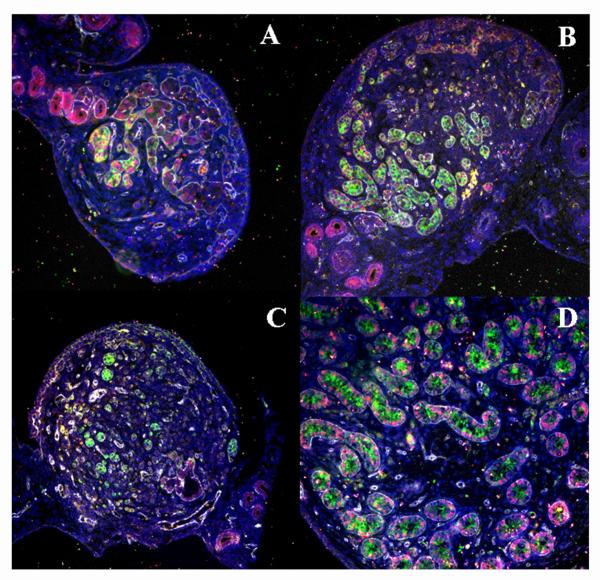
Figure 4.
Immunohistochemistry showing expression of SOX9 in Foxl2 −/− adult ovary (B and E) relative to a prepubertal wildtype ovary (A and D) and a prepubertal wildtype testis (C and F). SOX9 expression (pink) is virtually absent in wildtype ovary (A) and follicles (D), whereas it is strongly expressed in testis (C) and testis tubules (F). Note a few support cells that express SOX9 in wildtype ovary (B); thus, partial sex reversal does occur in occasional wildtype follicles (and provides an excellent control for staining conditions). However, in Foxl2 −/− ovary (B), SOX9 floods the gonad and fetal ovigerous cords are not fragmented into individual follicles. Oocytes (stained with Hsp90, green) are present in wildtype ovaries and well-preserved in Foxl2-null counterparts. Spermatogonia in newborn testes are smaller than oocytes but also stain for Hsp90. Nuclear counterstaining with DAPI (blue). Bar: 40 μm.
Partial sex reversal has been seen in mice lacking Wnt4 or Rspo1. In ovaries lacking either, Sox9 is upregulated at the time of sex determination only transiently (12.5 dpc), and is more sharply and stably reactivated at birth or at the end of fetal life (about 18 dpc), respectively. These results are consistent with an early though (transiently) redundant anti-testis action. In addition, embryofetal ovaries lacking Wnt4 or Rspo1 are unique in that they produce high levels of testosterone, reflecting an early sex reversal of the steroidogenic stroma. This leads to partial masculinization of genitalia, contrasting with the female genitalia and absent steroidogenesis observed in XX mice lacking Foxl2. Thus, in spite of some conspicuous differences, each of several genes, required for the development of particular lineages, is sufficient to repress Sox9 ordinarily, and the testis pathway can begin to take over in the absence of any of them –though the full effect is considerably delayed compared to the timing of primary sex determination by the direct action of Sry or Sox9.
In fact, from a series of results that initially seemed surprising, the loss of any of a plethora of early or late-acting female genes, such as Foxl2 or the estrogen receptors, induce ovary-to-testis sex reversal in one or multiple somatic cell lineages at various time-points after birth (e.g., Crumeyrolle-Arias and Aschheim, 1981; Taketo-Hosotani et al, 1985; Guigon et al, 2005; Couse et al, 1999). In addition to representing a case of cell fate choice with alternative commitments, sex determination seems to assign opposed degrees of commitment to homologous cell lineages. In this sense, the mechanism of sex determination could itself be sexually dimorphic.
This might explain why testis tubules apparently never form ovarian follicle-like alternatives though the structures can form together and stay more or less intermingled in true hermaphrodites. Nevertheless, partial de-repression of female genes has been reported in neoplastic conditions in the testis (Kalfa et al, 2008, Ottolenghi et al, 2007b), and a slight upregulation of Rspo1 and Wnt4 was recently reported in conditional knockout mice lacking both Sox9 and Sox8 (Barrionuevo et al, 2009). Although Rspo1 and Wnt4 are involved in female sex determination, they are expressed at high levels in bipotential gonads as well, and maintain low levels of expression in normal testes. Therefore, they do not represent unambiguous markers of male-to-female sex reversal, and a case of dedifferentiation of somatic cells (rather than transdifferentiation of male into female sex) cannot be ruled out. This would thus be similar to the model of conditional inactivation of the Wilms’ tumor gene, Wt1, which also involved loss of Sox9 and Sox8 expression during fetal life without sex reversal (Gao et al., 2006).
Future studies should reveal whether specific female markers like Foxl2 are also derepressed in non-tumoral postembryonic testes that are deficient for male sex determining genes. This would imply that secondary sex reversal can occur in testes as well as ovaries; and in both cases, the event would follow the disruption of sex determining genes, which thus function after the time of sex determination and perhaps throughout life in both sexes. But even if that is so, molecular anomalies suggestive of sex reversal have not been associated with any histologically detectable reprogramming in the testes when male sex determining genes were ablated. This contrasts with the ability of follicle cells to redifferentiate toward a testis tubule morphology at any time during ovary differentiation and maturation (see above and Ottolenghi et al, 2007b). Nevertheless, any such cases of molecular secondary male-to-female sex reversal may assist in the study of infertility associated with very little specific testis morphological anomaly or dysfunction. Dysfunction may include the so-called testicular dysgenesis syndrome that has embryo-fetal origin and shows complicated phenotypic expression (reviewed by Wohlfahrt-Veje C et al, 2009).
The dimorphism of the relative susceptibility to sex reversal between sexes (the much greater frequency of morphological reprogramming in ovaries than testes) is possibly related to the presence or absence of stem cells in the corresponding adult cell lineages. Indeed, it is well known that stem cells exist among pregranulosa cells, which retain the ability to replicate throughout life, but stem cells are not found among adult Sertoli cells (Kossowska-Tomaszczuk et al, 2009). There are thus provocative findings that link Foxl2 and stem cell-ness of gonadal somatic cells, with clear implications for female reproduction. Nevertheless, all results on Foxl2 thus far were obtained in animal models, and it is not possible to predict whether they will apply to humans. For example, following our work in mice, a sharp dichotomy in expression pattern between FOXL2 and SOX9 was documented in humans as well (Hersmus et al., 2008 PMID: 18348162), but it remains to be seen if any mutations involving FOXL2 can lead to 46,XX sex reversal in patients.
III. Sex commitment and reversal in lineages in mice vs. higher mammals
Ablation of single genes involved in gonadal sex determination has apparently far more penetrant effects in higher mammals than in rodents in the three cases studied thus far. For Rspo1, its absence leads to nearly complete sex reversal in humans (Parma et al, 2006), rather than the partial reversal in mice (Tomizuka et al, 2008, Chassot, et al., 2008). A case of 46,XX maleness associated with a mutation in WNT4 has now unequivocally been reported in humans (Mandel et al, 2008), which again contrasts with the much milder mouse phenotype (Vainio et al., 1998). And for Foxl2, the partial sex reversal in the mouse knockout (Ottolenghi et al., 2005) contrasts with the sometimes nearly complete phenotypic sex reversal that is observed when Foxl2 transcription is blocked by a naturally occurring deletion in goats (Pailhoux et al, 2001).
Sex reversal caused becomes histologically complete, however, in the case where both Foxl2 and Wnt4 are ablated (Ottolenghi et al, 2007a). Fig. 5 illustrates the subtle partial effect in newborn Wnt4−/− mice if even one Foxl2 allele remains active, and the overt resultant “ovotestis” that forms when both genes are completely gone. Laminin 1 immunostaining delineates structural features; the molecular indicator of sex reversal is the SOX9 protein, whereas a marker consistent with both primary and secondary sex reversal is a much earlier expression of AMH in the XX gonad (panels A and B) than seen in newborn ovaries (panel C). The Wnt4−/−;Foxl2−/− double knockout is the only instance that has been extensively explored; but the sum of current data suggest that compound knockout of several lineage-specific “master genes” is required for complete sex reversal in the murine model. However, even in this double knockout model, as noted above, sex reversal is not as pronounced and does not occur as early as in transgenic mice expressing Sry or Sox9 (see also next section).
Figure 5.
Immunohistochemical detection of SOX9 (pink), AMH (green) and Laminin1 (“Lama1”) (white) in newborn Wnt4−/−;Foxl2−/− double knockout ovary showing testis tubules and extensive Sox9 expression (A-B) that are absent in newborn Wnt4−/−;Foxl2+/− knockout ovary (C) and present in a control testis (D). Large tubular structures that are located near the hilum of the dysgenetic ovaries and express SOX9 but not AMH (A-C) correspond to the “rete” and directly communicate with male-like internal genitalia. Nuclei are counterstained with DAPI (blue).
In any case, because sex reversal in mice requires combined inactivations of genes that are individually able to produce sex reversal when they are ablated in higher mammals, it is conceivable that female sex determining pathways may be more integrated and interdependent in higher mammals than mice. In particular, one needs to reconcile the observation that the anti-testis action of the same gene (Foxl2) that operates like an upstream regulator of female sex in goats (complete sex reversal phenotype in PIS goats lacking Foxl2 expression), is not at the top of a hierarchy in mice, and can be replaced by at least one other gene pathway (Rspo1) (Chassot et al, 2008). This suggests that the PIS condition in goats may result from self-aggravating feedback interactions that would connect early ovarian genes. These interactions would be missing or would occur at later time-points in mice, but would be facilitated if multiple lineage pathways are affected, as in the Wnt4−/−;Foxl2−/− animals.
This hypothesis may help to resolve another apparent contradiction between observations that are otherwise equally well founded. On the one hand several unrelated ovarian genes are redundantly involved in antagonizing male sex determination in mice (see above) and likely in goats as well (where RSPO1 and FOXL2 are expressed in partly distinct cell populations, Kocer et al., 2008). On the other hand, long-standing genetic evidence, from recessive cases of XX sex reversal in humans and other mammals, indicates that single loci can apparently alter the entire mechanism of engagement of the bipotential gonad into female sex determination (Mc Elreavey et al., 1993). In addition, mutations associated with XY sex reversal may be interpreted as indicating primary antitestis effects by a single ovarian gene in mice – although alternative interpretations are possible (Eicher and Washburn, 1986). Thus, the autonomy of distinct ovarian pathways is still consistent with primary XX sex reversal-like phenotypes in higher mammals, and could extend to mice without involving any additional upstream regulator. It would rather involve some alteration of regulatory connections among the known ovarian genes or other ovarian genes operating at the same level of the hierarchy.
Similar feedback interactions may regulate male sex determining genes downstream of Sry. They could account for the effects of single-gene mutations that all can lead to complete sex reversal when many other male sex genes are normal.
Alternative mechanisms to account for complete sex reversal cannot be excluded, but we suggest that regulatory interactions (e.g., involving DNA targets of relevant transcription factors) are best suited to account for the fast evolutionary divergence that is observed across mammals. Overall, although certain mutations in some mammalian species simulate the effect of a single master regulator of female sex, there is no obvious need, nor any conclusive evidence, for such a single gene.
IV. The importance of timing in the race for determination of somatic sex commitment
We have suggested an updated version of classical models for gonadal sex determination (Fig. 3), in which Sry/Sox9 have a “head start” in the bipotential gonad if they are present. If they are not, Foxl2, Rspo1/Wnt4 and other female genes are derepressed and turn on further ovary development. To account for the “re-activation” of the testis pathway if Foxl2 or other ovarian genes are absent in XX individuals, we have followed and modified the traditional suggestion that the process of stroma mobilization at the time of follicle formation may be homologous to testis tubule formation, and may thus predispose the ovary to sex reversal (Ottolenghi et al, 2007b). As for the arguments favoring this notion, we have recently used genome-wide expression profiling to provide molecular evidence for a differential timing of activation of male-like genes in testes (early) vs ovaries (late) that is the inverse of the timing of entry into meiosis (Garcia-Ortiz et al, 2009 in press). But perhaps the most striking recent argument for similarity between early testis and peri-postnatal ovary development is provided by the association of mutations involving steroidogenic factor 1 (SF1/NR5A1) with both premature ovarian failure in 46,XX individuals and with complete sex reversal in 46,XY individuals (Laurenço et al, 2009). Thus, a gene long known to be associated with early testis differentiation also affects later events of ovary differentiation or maturation in humans.
Mechanistically, at least some testis genes (including SF1/NR5A1) are thus activated to support follicle formation or later features of folliculogenesis in the ovary; but during normal ovary development, the function of these genes is apparently “confined” within limits compatible with ovary development. If the safeguard mechanism fails, the male pathway can be derepressed in full, notably including the expression of male-specific Sox9, and sex reversal ensues.
An unresolved critical question is whether there are multiple sequential male-like activities antagonized by distinct ovarian genes in female gonads, or whether there is a single male-like pathway that is activated more-or-less late during development in different species (or under different conditions) and is antagonized by a single female mechanism. The former possibility is more or less implicit in most current models, whereas the latter was termed the “M” hypothesis (Ottolenghi et al, 2005, 2007a, 2007b).
The M hypothesis implies that depending on context, and notably in available mouse models, male-like genes can start to be expressed so late that they only produce partial secondary sex reversal in XX gonads lacking female determining genes. By contrast, in higher mammals, male-like genes are clearly able to produce earlier forms of complete or nearly complete sex reversal (See above, Section III). The M hypothesis also requires that female genes be expressed throughout female reproductive life, i.e., as long as folliculogenesis and other testis formation-like processes such as stroma mobilization continue. Genes expressed both before and after birth, such as Foxl2, are the best candidates for such an action, although other genes are clearly involved before birth (Rspo1 and Wnt4) or after birth (aromatase, estrogen receptors, and others). In this framework, lifelong expression of FOXL2, as well as its involvement in premature ovarian failure in women, suggests that the mechanisms of maintenance of female sex determination may participate in the mechanisms that regulate menopause (Ottolenghi et al., 2005).
Alternatively, it is possible that one or multiple distinct male-like activities as well as additional female determining genes may act before Rspo1, Wnt4 and Foxl2 (again corresponding to question marks in Fig.1). However, this possibility would not explain why in humans, the phenotypes of 46,XX sex reversed patients that harbor mutations in Rspo1 or in other putative ovarian genes are more clearly “primary” than is observed in mice, but they are systematically less pronounced than is seen when Sry or Sox9 has directly been activated in XX individuals (by natural chromosomal rearrangements or transgenic constructs; Sinclair et al, 1990; Bishop et al, 2000; Vidal et al, 2001; Koopman et al, 1991).
What can we infer from these results about the mechanisms of sex determination, i.e., the antagonistic gene interactions underlying the sex fate choices? In male embryos, it seems reasonable that Sry would induce the active suppression of female-like genes such as meiotic genes, as well as the activation of male-like genes such as SF1/NR5A1; but, in females, can the transient embryonic suppression of male-like genes be the effect of female sex determination pathways? Any such mechanism would confer a risk that such early-acting, default female sex determining genes might interfere with Sry in XY individuals. Thus, it is reasonable to suggest that other, sex non-specific processes (acting in the bipotential gonad) keep male-like genes from early expression in all individuals, as this would be particularly important in prospective females. Male-like gene expression would also be repressed by (other?) bipotential gonad genes, and it would be released in females at late timepoints when “definitive” female sex determining genes are already active. This was in fact the “M” gene hypothesis (cf. Fig. 3), stating that M (male-like) activity in females follows an “Od/Z” (female determining activity), which itself follows Sry (Ottolenghi et al., 2005).
A prediction of the M gene hypothesis is that female-to-male sex reversal due to the loss of female genes would always occur later than female-to-male sex reversal associated with gain of male gene activity. Also, the inferred later timing of activation of male-like genes, coupled to the fact that several autonomous anti-testis pathways appear to be involved in female sex determination, would imply that sex reversal may be less complete following the loss of female genes than by gain of male-like activity. This is consistent with all available observations (see above).
We have suggested that the yin and yang of sex determination may involve direct competition between Foxl2 and Sox9. It is a close race, because Foxl2 begins to be expressed in female bipotential gonad within 24 hr of the initiation of Sox9 in the male, and in mice lacking Foxl2, testis markers are already sharply activated by E13 (and more sharply activated if Wnt4 is also ablated, data not shown). Furthermore, in XY ovotestes obtained either in natural mouse mutants or in transgenic mice, a slow-motion version of the sex determination race is apparent: somatic cells expressing Sox9 or Foxl2, but not both, appear to push toward alternative states while they stay intermingled for a relatively long period of time (Ottolenghi et al, 2007a; Wilhelm et al, 2009).
In particular, the implication that testis genes remain accessible to transcription in ovaries and can thus be expressed in the absence of Foxl2 is paralleled in experiments in which a Foxl2 transgene under a controllable heat shock promoter was activated at E15 (Ottolenghi et al, 2007a). When activated in the testis the transgene was able to disorganize testis tubule formation, inhibit the transcription of most testis marker genes and induce a series of ovary markers to expression levels comparable to those in the ovary. Furthermore when the transgene was activated in the ovary, it augmented the synthesis of many ovary markers about 2-fold! Markers included germ cell as well as somatic cell genes (Ottolenghi et al, 2007a; Garcia et al, 2009 in press).
Notably, a very similar mechanism to that discussed here for somatic sex determination is also topical for germ cell sex determination, with a race envisaged between male and female factors -- though those factors are incompletely identified (Kocer et al, 2009).
V. Open questions for the next phase of investigations
The current level of information offers a glimpse of the overall course of gonadal differentiation and the genes involved in sex determination and the maintenance of reproductive capacity. However, no one would deny that the knowledge of the increasing cast of gene characters in this lifelong drama far exceeds our knowledge of the plot. Here are some open questions for the coming phase of studies.
At the initiation of the processes, the hit-and-run action of Sry in the testis, and the events at the chromatin level accompanying the commitment to one or another fate are not understood. A probing set of relevant possible analyses focusing on Sox9 upregulation are suggested by Sekido and Lovell-Badge (2009). The corresponding mechanisms of reciprocal shutdown of Sox9 and Foxl2 in testis vs. ovary remain completely unknown. Nature indeed seems to have increased the mystery by making the regulation of transcription of these genes especially complex: the Sry promoter, though cloned some years ago, has proven to be unusually refractory to analysis, and for FOXL2 (in BPES) (Crisponi et al, 2004) as for SOX9, clinically pathogenetic translocations that inactivate their expression have implicated putative control elements hundreds of kb to a megabase from the transcription start sites. Future analyses of the expression of these genes, and of higher order chromatin changes associated with the establishment or lability of sex determination, remain an open field of inquiry that one expects will explain the first steps in the implementation of sexual dimorphism.
-
To understand the course of sex determination, the traditional search for “top genes in a hierarchy” seems to have diminishing relevance. Instead, to understand the regulatory network involved, it may be more productive to focus on feedback interactions among known genes and any candidate genes with early sexually dimorphic phenotypes, even if they are partial. This is particularly true in mice, where XX sex reversal occurs systematically much later and in a more cell type-autonomous fashion than in larger mammals, so that individual pathways and their interactions can be temporally distinguished.
In addition, conditional knock-out experiments may be required to test the M hypothesis. Perhaps other important tests will include 1) showing distinct regulatory mechanisms that “bipotential gonad” and “ovary” determinant genes employ to silence male genes, possibly stronger or more diverse for the latter; 2) showing the requirement for “ovary” determinants not only during early ovary differentiation, but also at later time-points; and 3) throughly assessing the degree of differentiation attained by XX germ cells that undergo sex reversal by loss of ovarian genes.
It may be especially productive to focus on distinguishing pathways active in the formation of the bipotential gonad (sex non-specific) from female specific pathways (as has been done in Drosophila). The distinction is particularly important, because we have noted the possibility that two different mechanisms of suppression could help to stabilize sex determination: an earlier mechanism (associated with the bipotential gonad) that can be relatively easily overridden by the alternative male sex pathway, and a more “definitive” second mechanism associated with true femaleness (Fig. 3).
One intriguing developmental feature that may warrant targeted investigation, as mentioned above, is the relation between commitment and reversibility and its relation to morphological sex reversal and stem cell-ness in sex determination. A particularly interesting approach may be to produce long-lived stem cells from Sertoli cells while preserving their sexual differentiation, but without neoplastic transformation or induction of the putative female somatic stem cell marker, Foxl2 (see above).Similarly, expression of female-specific markers such as Foxl2 in testes of infertile males may help to discriminate sex-determinination-related pathology even in the absence of morphological sex reversal. This might have important consequencens for the study of the mechanisms underlying the testicular dysgenesis syndrome (reviewed by Wohlfahrt-Veje C et al, 2009).
Overall, we encourage the current trend toward producing mouse models of mutations in candidate genes for a (relatively!) early role on ovarian sexual differentiation. This would complement the extensive knowledge that has accumulated on early testis differentiation by this approach. Mouse work on the ovary had traditionally focused on postnatal maturation, i.e., follicle dynamics; but the latter, as we have seen, may depend on the continuous action of early ovary-differentiation genes. Additional analyses that would be useful for the study of both ovary and testis differentiation will include the use of “induced pluripotent stem cell” (iPS) technology as well as direct transdifferentiation assays, as recently developed in other systems (Zhou et al., 2008, and references therein).
The mechanism of follicle formation/histogenesis remains an enigma, but there are starting points for investigation. They include the study of the nature, establishment, and maintenance of the corticomedullary gradient, identified as the spatial embodiment of the regulation of follicle development for life (e.g., Byskov et al., 1997).
Concurrently, and of most interest for all those concerned with reproductive capacity and dysfunction, current technology can sustain a search for additional follicle-quiescence genes -- possibly related to sex determination -- and their mode of action.
Acknowledgment
We thank Chris Ottolenghi (Université Paris Descartes, Paris) for insightful suggestions and for the immunohistochemistry.
This research was supported by the Intramural Research Program of the National Institute on Aging, NIH.
References
- Barrionuevo F, Georg I, Scherthan H, Lécureuil C, Guillou F, Wegner M, Scherer G. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol. 2009;15:301–312. doi: 10.1016/j.ydbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Bishop CE, Withworth DJ, Qin Y, Agoulnik AI, Agoulnik IU, Harrison WR, Behringer RR, Overbeek PA. A transgenic insertion upstream of Sox9 is associated with dominant XX sex reversal in the mouse. Nat Genet. 2000;26:490–494. doi: 10.1038/82652. [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–521. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS. Role of mammalian Y chromosome in sex determination. Philos Trans R Soc Lond B Biol Sci. 1988;322:63–72. doi: 10.1098/rstb.1988.0114. [DOI] [PubMed] [Google Scholar]
- Bysgov AG, Guoliang X, Andersen CY. The cortex-medulla oocyte growth pattern is organized during fetal life: an in-vitro study of the mouse ovary. Mol Hum Reprod. 1997;3:795–800. doi: 10.1093/molehr/3.9.795. [DOI] [PubMed] [Google Scholar]
- Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- Choi Y, Ballow DJ, Xin Y, Rajkovic A. Lim homeobox gene, LHX8, is Essentials for Mouse oocyte differentiation and survival. Biol Reprod. 2008:79-442–449. doi: 10.1095/biolreprod.108.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Luigi B, Zelante L, Nagaraja R, Porcu S, Ristaldi M Serafina, Martella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159–166. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- Crisponi L, Uda M, Deiana M, Loi A, Nagaraja R, Chiappe F, Schlessinger D, Cao A, Pilia G. FOXL2 inactivation by a traslocation 171 kb away: analysis of 500kb of chromosome 3 for candidate long-range regulatory sequences. Genomics. 2004;83:757–764. doi: 10.1016/j.ygeno.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Crumeyrolle-Arias M, Ashheim P. Post-hypophysectomy ovarian senescence and its relation to the spontaneous structural changes in the ovary of intact aged rats. Gerontology. 1981;27:58–71. doi: 10.1159/000212450. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Washburn LL. Genetic control of primary sex determination im mice. Annu Rev Genet. 1986;20:327–360. doi: 10.1146/annurev.ge.20.120186.001551. [DOI] [PubMed] [Google Scholar]
- Gao F, Maiti S, Alam N, Zhang Z, Deng JM, Behringer RR, Lécureuil C, Guillou F, Huff V. The Wilms tumor gene, Wt1, is required for Sox9 expression and maintenance of tubular architecture in the developing testis. Proc Natl Acad Sci USA. 2006;103:11987–11992. doi: 10.1073/pnas.0600994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ortiz JE, Pelosi E, Omari S, Nedorezov T, Piao Y, Karmazin J, Uda M, Cao A, Cole S, Forabosco A, Schlessinger D, Ottolenghi C. Foxl2 functions in sex determination and histogenesis throughout mouse ovary development. BMC. 2009 doi: 10.1186/1471-213X-9-36. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow PN, Darling SM. Genetics of sex determination in man and mouse. Development. 1988;102:251–258. doi: 10.1242/dev.102.2.251. [DOI] [PubMed] [Google Scholar]
- Guigon CJ, Coudouel N, Mazaud-Guittot S, Forest MG, Magre S. Follicular cells aquire sertoli cell characteristics after oocyte loss. Endocrinology. 2005;146:2992–3004. doi: 10.1210/en.2005-0045. [DOI] [PubMed] [Google Scholar]
- Guigon CJ, Magre S. Contribution of germ cells to the differentiation and maturation of the ovary: insights from models of germ cell depletion. Biol Reprod. 2006;74:450–458. doi: 10.1095/biolreprod.105.047134. [DOI] [PubMed] [Google Scholar]
- Hersmus R, Kalfa N, de Leeuw B, Stoop H, Oosterhuis JW, de Krijger R, Wolffenbuttel KP, Drop SL, Veitia RA, Fellous M, Jaubert F, Looijenga LH. FOXL2 and SOX9 as parameters of female and male gonadal differentiation in patients with various forms of disorders of sex development (DSD) J Pathol. 2008;215:31–38. doi: 10.1002/path.2335. [DOI] [PubMed] [Google Scholar]
- Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- Jost A. A new look at the mechanisms controlling sex differentiation in mammals. Johns Hopkins Med J. 1972;130:38–53. [PubMed] [Google Scholar]
- Kalfa N, Fellous M, Boizet-Bonhoure B, Patte C, Duvillard P, Pienkowski C, Jaubert F, Ecochard A, Sultan C. Aberrant expression of ovary determining gene FOXL2 in the testis and juvenile granulosa cell tumor in children. J Urol. 2008;180:1810–1813. doi: 10.1016/j.juro.2008.03.097. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kobayashi A, Sekido R, DiNapoli R, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4:e187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocer A, Reichmann J, Best D, Adams IR. Germ cell determination in mammals. Mol Hum Reprod. 2009;15:205–213. doi: 10.1093/molehr/gap008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Kocer A, Pinheiro I, Pannetier M, Renault L, Parma P, Radi O, Kim KA, Camerino G, Pailhoux E. R-spondin1 and FOXL2 act into two distinct cellular types during goat ovarian differentiation. BMC Dev. Biol. 2008;8:36. doi: 10.1186/1471-213X-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossowska-Tomaszczuk K, De Geyter C, De Geyter M, Martin I, Holzgreve W, Scherberich A, Zhang H. Multipotency of luteinizing granulosa cells collected from mature ovarian follicles. Stem Cells. 2009;27:210–219. doi: 10.1634/stemcells.2008-0233. [DOI] [PubMed] [Google Scholar]
- Laurenço D, Brauner R, Lin L, De Perdigo A, Weryha G, Muresan M, Boudjenah R, Guerra-Junior G, Maciel-Guerra AT, Achermann JC, McElreavey K, Bashamboo A. Mutations in NR5A1 associated with ovarian insufficiency. N Engl J Med. 2009;360:1200–1210. doi: 10.1056/NEJMoa0806228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler KA, Zarkower D, Koopman P. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early acting gene in vertebrate ovarian development. Endocrinology. 2003;144:3237–3243. doi: 10.1210/en.2002-0095. [DOI] [PubMed] [Google Scholar]
- Lyet L, Louis F, Forest MG, Josso N, Behringer RR, Vigier B. Ontogeny of reproductive abnormalities induced by deregulation of anti-mullerian hormone expression in transgenic mice. Biol Reprod. 1995;52:444–454. doi: 10.1095/biolreprod52.2.444. [DOI] [PubMed] [Google Scholar]
- Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of β-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel H, Shemer R, Borochowitz ZU, Okopnik M, Knopf C, Indelman M, Drugan A, Tiosano D, Gershoni-Baruch R, Choder M, Sprecher E. SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am J Hum Genet. 2008;82:39–47. doi: 10.1016/j.ajhg.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElreavey K, Vilain E, Abbas N, Herskowitz I, Fellous M. A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc Natl Acad Sci USA. 1993;90:3386–3372. doi: 10.1073/pnas.90.8.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren A. Sex determination in mammals. Trends Genet. 1988;4:153–157. doi: 10.1016/0168-9525(88)90020-0. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Omari S, Garcia-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D. Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet. 2005;14:2053–2062. doi: 10.1093/hmg/ddi210. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Noderezov T, Cao A, Forabosco A, Schlessinger D. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet. 2007a;16:1795–2804. doi: 10.1093/hmg/ddm235. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Uda M, Crisponi L, Omari S, Cao A, Forabosco A, Schlessinger D. Determination and stability of sex. Bioessays. 2007b;29:15–25. doi: 10.1002/bies.20515. [DOI] [PubMed] [Google Scholar]
- Pailhoux E, Vigier B, Chaffaux S, Servel N, Taurit S, Furet JP, Fellous M, Grosclaude S, Cribiu EP, Cotinot C, Vailman D. A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat Genet. 2001;29:453–458. doi: 10.1038/ng769. [DOI] [PubMed] [Google Scholar]
- Park SY, Jameson JL. Transcriptional regulation of gonadal development and differentiation. Endocrinology. 2005;146:1035–1042. doi: 10.1210/en.2004-1454. [DOI] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G. R-spondin1 is essential in sex detemination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–1309. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine helix-winged transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet. 2009;25:19–29. doi: 10.1016/j.tig.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Taketo-Hosotani T, Merchant-Larios H, Thau RB, Koide SS. Testicular cell differentiation in fetal mouse ovaries following transplantation into adult male mice. J Exp Zool. 1985;236:229–237. doi: 10.1002/jez.1402360213. [DOI] [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T, Kakitani M. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet. 2008;17:1278–1291. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- Uda M, Ottolenghi C, Crisponi L, Garcia-Ortiz JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13:1171–1181. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Vidal VPI, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Koopman P. The making of maleness: towards an integrated view of male sexual development. Nat Rev Genet. 2006;7:620–631. doi: 10.1038/nrg1903. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Washburn LL, Truong V, Fellous M, Eicher EM, Koopman P. Antagonism of the testis- and ovary-determining pathways during ovotestis development in mice. Mech Dev. 2009 doi: 10.1016/j.mod.2009.02.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfahrt-Veje C, Main KM, Skakkebæk NE. Testicular dysgenesis syndrome; fetal origins of adult reproductive problems. Clin Endocrinol (Oxf) 2009 doi: 10.1111/j.1365-2265.2009.03545.x. in press. [DOI] [PubMed] [Google Scholar]
- Yao HH. The pathway of femaleness: current knowledge on embryonic development of the ovary. Mol Cell Endocrinol. 2005;230:87–93. doi: 10.1016/j.mce.2004.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]