Abstract
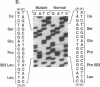
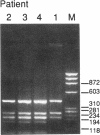
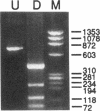
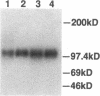
Variant von Willebrand disease designated as type I New York or type Malmö is characterized by enhanced ristocetin-induced platelet agglutination with normal von Willebrand factor multimeric distribution in plasma. We have studied four such patients belonging to three unrelated families and found in all of them a unique cytosine-to-thymine transition changing the codon for Pro503 (CCG) to Leu (CTG). In three patients the mutant allele also had a silent mutation in the codon for Ser500 (TCG-->TCA). Both nucleotide changes are present in the von Willebrand factor pseudogene; however, the characterization of distinctive markers where the gene and pseudogene differ, as well as the examination of amplified cDNA derived from platelet mRNA, confirmed that the abnormality occurs in the von Willebrand factor gene of the patients. Moreover, recombinant expression of the isolated glycoprotein Ib-binding domain of von Willebrand factor provided direct evidence that the Pro503-->Leu mutation is responsible for enhanced platelet reactivity to lower ristocetin concentrations. These results define a new structural element affecting the affinity of von Willebrand factor for glycoprotein Ib and establish the molecular basis of a variant form of von Willebrand disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma H., Dent J. A., Sugimoto M., Ruggeri Z. M., Ware J. Independent assembly and secretion of a dimeric adhesive domain of von Willebrand factor containing the glycoprotein Ib-binding site. J Biol Chem. 1991 Jul 5;266(19):12342–12347. [PubMed] [Google Scholar]
- Bahnak B. R., Lavergne J. M., Ferreira V., Kerbiriou-Nabias D., Meyer D. Comparison of the primary structure of the functional domains of human and porcine von Willebrand factor that mediate platelet adhesion. Biochem Biophys Res Commun. 1992 Jan 31;182(2):561–568. doi: 10.1016/0006-291x(92)91769-m. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Gene conversion: some implications for immunoglobulin genes. Cell. 1981 Jun;24(3):592–594. doi: 10.1016/0092-8674(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Bernardi F., Marchetti G., Bertagnolo V., Faggioli L., del Senno L. Two TaqI RFLPs in the human von Willebrand factor gene. Nucleic Acids Res. 1987 Feb 11;15(3):1347–1347. doi: 10.1093/nar/15.3.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt M. C., Ward C. M., Booth W. J., Castaldi P. A., Mazurov A. V., Andrews R. K. Identification of aspartic acid 514 through glutamic acid 542 as a glycoprotein Ib-IX complex receptor recognition sequence in von Willebrand factor. Mechanism of modulation of von Willebrand factor by ristocetin and botrocetin. Biochemistry. 1992 Nov 17;31(45):11144–11151. doi: 10.1021/bi00160a027. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney K. A., Nichols W. C., Bruck M. E., Bahou W. F., Shapiro A. D., Bowie E. J., Gralnick H. R., Ginsburg D. The molecular defect in type IIB von Willebrand disease. Identification of four potential missense mutations within the putative GpIb binding domain. J Clin Invest. 1991 Apr;87(4):1227–1233. doi: 10.1172/JCI115123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco L., Girolami A., Zimmerman T. S., Ruggeri Z. M. Interaction of purified type IIB von Willebrand factor with the platelet membrane glycoprotein Ib induces fibrinogen binding to the glycoprotein IIb/IIIa complex and initiates aggregation. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7424–7428. doi: 10.1073/pnas.82.21.7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco L., Mazzucato M., De Roia D., Casonato A., Federici A. B., Girolami A., Ruggeri Z. M. Distinct abnormalities in the interaction of purified types IIA and IIB von Willebrand factor with the two platelet binding sites, glycoprotein complexes Ib-IX and IIb-IIIa. J Clin Invest. 1990 Sep;86(3):785–792. doi: 10.1172/JCI114775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco L., Mazzuccato M., Grazia Del Ben M., Budde U., Federici A. B., Girolami A., Ruggeri Z. M. Type IIB von Willebrand factor with normal sialic acid content induces platelet aggregation in the absence of ristocetin. Role of platelet activation, fibrinogen, and two distinct membrane receptors. J Clin Invest. 1987 Aug;80(2):475–482. doi: 10.1172/JCI113095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnér M., Holmberg L., Kristoffersson A. C., Nilsson I. M. An HphI-polymorphism in exon 28 of the von Willebrand factor gene, and its frequency among patients with various forms of von Willebrand's disease. Br J Haematol. 1991 Jul;78(3):403–407. doi: 10.1111/j.1365-2141.1991.tb04455.x. [DOI] [PubMed] [Google Scholar]
- Fujimura Y., Titani K., Holland L. Z., Russell S. R., Roberts J. R., Elder J. H., Ruggeri Z. M., Zimmerman T. S. von Willebrand factor. A reduced and alkylated 52/48-kDa fragment beginning at amino acid residue 449 contains the domain interacting with platelet glycoprotein Ib. J Biol Chem. 1986 Jan 5;261(1):381–385. [PubMed] [Google Scholar]
- Fujimura Y., Usami Y., Titani K., Niinomi K., Nishio K., Takase T., Yoshioka A., Fukui H. Studies on anti-von Willebrand factor (vWF) monoclonal antibody NMC-4, which inhibits both ristocetin- and botrocetin-induced vWF binding to platelet glycoprotein Ib. Blood. 1991 Jan 1;77(1):113–120. [PubMed] [Google Scholar]
- Ginsburg D., Konkle B. A., Gill J. C., Montgomery R. R., Bockenstedt P. L., Johnson T. A., Yang A. Y. Molecular basis of human von Willebrand disease: analysis of platelet von Willebrand factor mRNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3723–3727. doi: 10.1073/pnas.86.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainick H. R., Williams S. B., McKeown L. P., Rick M. E., Maisonneuve P., Jenneau C., Sultan Y. Von Willebrand's disease with spontaneous platelet aggregation induced by an abnormal plasma von Willebrand factor. J Clin Invest. 1985 Oct;76(4):1522–1529. doi: 10.1172/JCI112132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Tanae A., Inoue H., Fujii-Kuriyama Y. Evidence for frequent gene conversion in the steroid 21-hydroxylase P-450(C21) gene: implications for steroid 21-hydroxylase deficiency. Am J Hum Genet. 1988 Jan;42(1):17–25. [PMC free article] [PubMed] [Google Scholar]
- Holmberg L., Berntorp E., Donnér M., Nilsson I. M. von Willebrand's disease characterized by increased ristocetin sensitivity and the presence of all von Willebrand factor multimers in plasma. Blood. 1986 Sep;68(3):668–672. [PubMed] [Google Scholar]
- Holmberg L., Nilsson I. M., Borge L., Gunnarsson M., Sjörin E. Platelet aggregation induced by 1-desamino-8-D-arginine vasopressin (DDAVP) in Type IIB von Willebrand's disease. N Engl J Med. 1983 Oct 6;309(14):816–821. doi: 10.1056/NEJM198310063091402. [DOI] [PubMed] [Google Scholar]
- Holmberg L., Nilsson I. M. von Willebrand's disease. Eur J Haematol. 1992 Mar;48(3):127–141. doi: 10.1111/j.1600-0609.1992.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Handa M., Kawano K., Kamata T., Murata M., Araki Y., Anbo H., Kawai Y., Watanabe K., Itagaki I. The role of von Willebrand factor and fibrinogen in platelet aggregation under varying shear stress. J Clin Invest. 1991 Apr;87(4):1234–1240. doi: 10.1172/JCI115124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Mancuso D. J., Tuley E. A., Westfield L. A., Lester-Mancuso T. L., Le Beau M. M., Sorace J. M., Sadler J. E. Human von Willebrand factor gene and pseudogene: structural analysis and differentiation by polymerase chain reaction. Biochemistry. 1991 Jan 8;30(1):253–269. doi: 10.1021/bi00215a036. [DOI] [PubMed] [Google Scholar]
- Mancuso D. J., Tuley E. A., Westfield L. A., Worrall N. K., Shelton-Inloes B. B., Sorace J. M., Alevy Y. G., Sadler J. E. Structure of the gene for human von Willebrand factor. J Biol Chem. 1989 Nov 25;264(33):19514–19527. [PubMed] [Google Scholar]
- Miller J. L., Castella A. Platelet-type von Willebrand's disease: characterization of a new bleeding disorder. Blood. 1982 Sep;60(3):790–794. [PubMed] [Google Scholar]
- Miller J. L., Cunningham D., Lyle V. A., Finch C. N. Mutation in the gene encoding the alpha chain of platelet glycoprotein Ib in platelet-type von Willebrand disease. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4761–4765. doi: 10.1073/pnas.88.11.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. L., Kupinski J. M., Castella A., Ruggeri Z. M. von Willebrand factor binds to platelets and induces aggregation in platelet-type but not type IIB von Willebrand disease. J Clin Invest. 1983 Nov;72(5):1532–1542. doi: 10.1172/JCI111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri H., Fujimura Y., Shima M., Yoshioka A., Houghten R. A., Ruggeri Z. M., Zimmerman T. S. Structure of the von Willebrand factor domain interacting with glycoprotein Ib. J Biol Chem. 1988 Dec 5;263(34):17901–17904. [PubMed] [Google Scholar]
- Mohri H., Yoshioka A., Zimmerman T. S., Ruggeri Z. M. Isolation of the von Willebrand factor domain interacting with platelet glycoprotein Ib, heparin, and collagen and characterization of its three distinct functional sites. J Biol Chem. 1989 Oct 15;264(29):17361–17367. [PubMed] [Google Scholar]
- Newman P. J., Gorski J., White G. C., 2nd, Gidwitz S., Cretney C. J., Aster R. H. Enzymatic amplification of platelet-specific messenger RNA using the polymerase chain reaction. J Clin Invest. 1988 Aug;82(2):739–743. doi: 10.1172/JCI113656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake I. R., Bowen D., Bignell P., Liddell M. B., Sadler J. E., Standen G., Bloom A. L. Family studies and prenatal diagnosis in severe von Willebrand disease by polymerase chain reaction amplification of a variable number tandem repeat region of the von Willebrand factor gene. Blood. 1990 Aug 1;76(3):555–561. [PubMed] [Google Scholar]
- Petes T. D., Hill C. W. Recombination between repeated genes in microorganisms. Annu Rev Genet. 1988;22:147–168. doi: 10.1146/annurev.ge.22.120188.001051. [DOI] [PubMed] [Google Scholar]
- Quadt R., Verweij C. L., de Vries C. J., Briët E., Pannekoek H. A polymorphic Xba I site within the human von Willebrand factor (vWF) gene identified by a vWF cDNA clone. Nucleic Acids Res. 1986 Sep 11;14(17):7139–7139. doi: 10.1093/nar/14.17.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randi A. M., Rabinowitz I., Mancuso D. J., Mannucci P. M., Sadler J. E. Molecular basis of von Willebrand disease type IIB. Candidate mutations cluster in one disulfide loop between proposed platelet glycoprotein Ib binding sequences. J Clin Invest. 1991 Apr;87(4):1220–1226. doi: 10.1172/JCI115122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribba A. S., Lavergne J. M., Bahnak B. R., Derlon A., Piétu G., Meyer D. Duplication of a methionine within the glycoprotein Ib binding domain of von Willebrand factor detected by denaturing gradient gel electrophoresis in a patient with type IIB von Willebrand disease. Blood. 1991 Oct 1;78(7):1738–1743. [PubMed] [Google Scholar]
- Ruggeri Z. M., De Marco L., Gatti L., Bader R., Montgomery R. R. Platelets have more than one binding site for von Willebrand factor. J Clin Invest. 1983 Jul;72(1):1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., Pareti F. I., Mannucci P. M., Ciavarella N., Zimmerman T. S. Heightened interaction between platelets and factor VIII/von Willebrand factor in a new subtype of von Willebrand's disease. N Engl J Med. 1980 May 8;302(19):1047–1051. doi: 10.1056/NEJM198005083021902. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Ware J. The structure and function of von Willebrand factor. Thromb Haemost. 1992 Jun 1;67(6):594–599. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. Variant von Willebrand's disease: characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets. J Clin Invest. 1980 Jun;65(6):1318–1325. doi: 10.1172/JCI109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba H. I., Saba S. R., Dent J., Ruggeri Z. M., Zimmerman T. S. Type IIB Tampa: a variant of von Willebrand disease with chronic thrombocytopenia, circulating platelet aggregates, and spontaneous platelet aggregation. Blood. 1985 Aug;66(2):282–286. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scott J. P., Montgomery R. R., Retzinger G. S. Dimeric ristocetin flocculates proteins, binds to platelets, and mediates von Willebrand factor-dependent agglutination of platelets. J Biol Chem. 1991 May 5;266(13):8149–8155. [PubMed] [Google Scholar]
- Sugimoto M., Mohri H., McClintock R. A., Ruggeri Z. M. Identification of discontinuous von Willebrand factor sequences involved in complex formation with botrocetin. A model for the regulation of von Willebrand factor binding to platelet glycoprotein Ib. J Biol Chem. 1991 Sep 25;266(27):18172–18178. [PubMed] [Google Scholar]
- Urabe K., Kimura A., Harada F., Iwanaga T., Sasazuki T. Gene conversion in steroid 21-hydroxylase genes. Am J Hum Genet. 1990 Jun;46(6):1178–1186. [PMC free article] [PubMed] [Google Scholar]
- Ware J., Dent J. A., Azuma H., Sugimoto M., Kyrle P. A., Yoshioka A., Ruggeri Z. M. Identification of a point mutation in type IIB von Willebrand disease illustrating the regulation of von Willebrand factor affinity for the platelet membrane glycoprotein Ib-IX receptor. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2946–2950. doi: 10.1073/pnas.88.7.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. J., Meyer D., Rabinowitz R., Pietu G., Girma J. P., Vicic W. J., Rogers J. Pseudo-von Willebrand's disease. An intrinsic platelet defect with aggregation by unmodified human factor VIII/von Willebrand factor and enhanced adsorption of its high-molecular-weight multimers. N Engl J Med. 1982 Feb 11;306(6):326–333. doi: 10.1056/NEJM198202113060603. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Sussman I. I. A new von Willebrand variant (type I, New York): increased ristocetin-induced platelet aggregation and plasma von Willebrand factor containing the full range of multimers. Blood. 1986 Jul;68(1):149–156. [PubMed] [Google Scholar]
- Weiss H. J., Turitto V. T., Baumgartner H. R. Effect of shear rate on platelet interaction with subendothelium in citrated and native blood. I. Shear rate--dependent decrease of adhesion in von Willebrand's disease and the Bernard-Soulier syndrome. J Lab Clin Med. 1978 Nov;92(5):750–764. [PubMed] [Google Scholar]
- Zimmerman T. S., Ratnoff O. D., Littell A. S. Detection of carriers of classic hemophilia using an immunologic assay for antihemophilic factor (factor 8(). J Clin Invest. 1971 Jan;50(1):255–258. doi: 10.1172/JCI106481. [DOI] [PMC free article] [PubMed] [Google Scholar]