Abstract
Pituitary adenomas constitute the most frequent neuroendocrine pathology, comprising up to 15% of primary intracranial tumors. Current therapies for pituitary tumors include surgery and radiotherapy, as well as pharmacological approaches for some types. Although all of these approaches have shown a significant degree of success, they are not devoid of unwanted side effects, and in most cases do not offer a permanent cure. Gene therapy—the transfer of genetic material for therapeutic purposes—has undergone an explosive development in the last few years. Within this context, the development of gene therapy approaches for the treatment of pituitary tumors emerges as a promising area of research. We begin by presenting a brief account of the genesis of prolactinomas, with particular emphasis on how estradiol induces prolactinomas in animals. In so doing, we discuss the role of each of the recently discovered growth inhibitory and growth stimulatory substances and their interactions in estrogen action. We also evaluate the cell-cell communication that may govern these growth factor interactions and subsequently promote the growth and survival of prolactinomas. Current research efforts to implement gene therapy in pituitary tumors include the treatment of experimental prolactinomas or somatomammotropic tumors with adenoviral vector-mediated transfer of the suicide gene for the herpes simplex type 1 (HSV1) thymidine kinase, which converts the prodrug ganciclovir into a toxic metabolite. In some cases, the suicide transgene has been placed under the control of pituitary cell-type specific promoters, like the human prolactin or human growth hormone promoters. Also, regulatable adenoviral vector systems are being assessed in gene therapy approaches for experimental pituitary tumors. In a different type of approach, an adenoviral vector, encoding the human retinoblastoma suppressor oncogene, has been successfully used to rescue the phenotype of spontaneous pituitary tumors of the pars intermedia in mice.
We close the article by discussing the future of molecular therapies. We point out that although, gene therapy represents a key step in the development of molecular medicine, it has inherent limitations. As a consequence, it is our view that at some point, genetic therapies will have to move from exogenous gene transfer (i.e. gene therapy) to endogenous gene repair. This approach will call for radically new technologies, such as nanotechnology, whose present state of development is outlined.
Keywords: Gene therapy, viral vectors, pituitary tumors, estrogen, prolactinomas, neurosurgery, suicide gene therapy, combined therapy, nanotechnology
INTRODUCTION
Pituitary adenomas constitute the most frequent neuroendocrine pathology in humans, comprising up to 15% of primary intracranial tumors [Daniels and Martin, 1995]. Also, pituitary tumors represent the most prevalent pathology in old female rats [Burek, 1978]. Clinical manifestations of pituitary adenomas arise from overproduction of hormones in microadenomas or from mass effects in larger tumors.
There are five types of hormone-producing pituitary adenomas, which reflect the cell type that originated the tumor. These tumor types are prolactin (PRL)- and growth hormone (GH)-secreting tumors that cause reproductive abnormalities and the syndromes of acromegaly, respectively; corticotropin (ACTH)-producing tumors that cause Cushing’s disease; and gonadotropin- and thyrotropin-secreting tumors that cause abnormalities in their respective axes. Mass effects of enlarging tumors usually include visual field defects resulting from compression of the optic nerves, headaches, hypopituitarism and, rarely, invasion into the skull base causing multiple intracranial nerve palsies. Only exceptionally are pituitary tumors truly malignant with distant metastases.
Current therapies for pituitary tumors include surgery and radiotherapy, as well as pharmacological approaches for some types [Shimon and Melmed, 1998]. Transsphenoidal surgery has proven to be highly effective for microadenomas but not for macroadenomas [Melmed, 1997]. In both cases post-surgical complications, like the appearance of a new pituitary dysfunction, diabetes insipidus and sometimes fistula formation, develop even in patients operated on by specialist surgeons. Pharmacological therapies, with dopamine agonists like bromocriptine, cabergoline and quinagolide, have met with remarkable success in shrinking prolactinomas and reducing the hyperprolactinemia associated with them [Bevan et al., 1992]. Unfortunately, a significant rate of side effects like nausea, vomiting, postural hypotension, dizziness, headaches, and constipation is present in long-term treatments with these drugs [Webster et al., 1994]. In patients with GH-producing adenomas, somatostatin analogs and depot preparations of octreotide can lower the usual hypersomatotropinemia to levels compatible with acceptable long-term survival [Sheppard, 1994]. Treatment with octreotide of 27 newly diagnosed acromegalic patients for 6 months, followed by treatment with a long-acting octreotide preparation for additional 6 months, resulted in 79% of the patients having mean serum GH levels below 5 mU/liter, 53% having normal IGF-I levels, and 73% of the patients showing greater than 30% tumor shrinkage (Bevan, 2002). None of the aforementioned pharmacological treatments cure the tumors, which will usually recur if the medications are discontinued.
Radiotherapy is usually used as an adjunctive treatment after pituitary tumor surgery. Although long-term irradiation is often effective to prevent tumor regrowth, it usually causes complications, the most frequent (58 to 83%) of which is hypopituitarism (Sutton, 1985).
Other less frequent complications associated with conventional radiotherapy for pituitary adenomas are 2% optic chiasmal injury and 0.2% radiation brain necrosis (Sutton, 1985). Although rare, radiation-induced fibrosarcomas and osteosarcomas have been reported (Grigsby and Sheline, 1990).
In summary, although important advances have been made in the treatment of pituitary tumors, a fully satisfactory therapy is not yet available. In this context, gene therapy appears potentially useful as an alternative for the treatment of pituitary tumors. Although research efforts to apply this methodology to the hypophysis are relatively recent, promising experimental results, discussed later in this review, have already emerged.
GENESIS OF PITUITARY TUMORS
The genesis of pituitary tumors is multifactorial. Since PRL-secreting adenomas (prolactinomas), the more prevalent type of pituitary tumors, are the most studied regarding their etiology, we will focus our discussion on the genesis of this particular type of pituitary tumor.
Prolactinoma development involves clonal expansion. Some of the contributing genetic events include H-Ras mutation for invasive prolactinomas [Karga et al., 1992] and partial loss of chromosome 11, including the putative multiple endocrine neoplasia-1 (MEN-1) for non-invasive tumors [Herman et al., 1993]. However, development of tumors from a specific gene mutation has not been definitely proven. Prolactinomas have been linked to estrogen exposure in humans and animals. The mechanism by which estrogen increases mitogenesis in lactotropes has been well studied and is the primary focus of this section.
Pituitary tumors in experimental animals can be induced by estrogen. Most of these estrogen-induced pituitary tumors are PRL- or GH-secreting tumors [Sadoul et al., 1992; Ishibashi and Yamaji, 1985]. In both sexes of rats, long-term elevation of serum estrogen causes hyperplasia and/or adenomas [DeNicola et al., 1978; Wickland et al., 1981; Sarkar et al., 1983]. In Fischer-344 female rats maintenance of constant, elevated, systemic estradiol levels by subcutaneous implantation of 17β-estradiol-containing silastic capsules induces prolactinomas very rapidly; within 2 weeks of estrogen implantation [Wickland et al., 1981; Sarkar et al., 1982; Banerjee et al., 1994]. Subcutaneous implantation of 17β-estradiol-containing silastic capsules induces prolactinomas in either ovary intact or ovariectomized ACI rats [Shull et al., 1997]. Subcutaneous implantation of diethylstilbestrol (DES) causes pituitary hyperplasia and neoplasm in Fischer-344 rats [Lloyd, 1983]. In estrogen-sensitive rats, short-term administration of the steroid stimulates lactotropic cell proliferation and, in the long-term, treatment results in lactotropic cell hyperplasia.
Estrogen also appears to promote prolactinomas in humans. There are reports of the development of a prolactinoma in a male-to-female transsexual who received massive doses of estrogen [Gooren et al., 1987]. Some men with prolactinomas showed increased serum estradiol levels due to aromatized testosterone to estradiol [Prior et al., 1987]. There is evidence of growth of a microprolactinoma to a macroprolactinoma during estrogen therapy [Garcia and Kapcala, 1995]. Women taking oral estrogen contraceptive or hormonal replacement therapy showed higher prolactin levels [Carol et al, 1988; Fahy et al. 1992]. Women using oral contraceptive due to menstrual irregularities showed a 7- to 8-fold higher incidence of prolactinomas [Shy et al., 1983]. Data suggest that some women are more sensitive to the lactogenic effects of exogenous estrogen and, therefore, may be at greater risk for developing prolactinomas [Luciano et al., 1985]. During pregnancy, estrogen levels elevate and the number of PRL-secreting cells and serum prolactin content increases. These elevated levels of estrogen are associated with symptomatic pituitary tumor enlargement (>1 cm) in up to 30% of women with macroadenomas and may result in persistent hyperprolactinemia and postpartum amenorrhea or galactorrhea [Gomez et al., 1977]. However, the risk for development of significant clinical symptoms related to tumor expansion is less than 2% in pregnant women with microadenomas [Tonner and Schlechte, 1993]. There appears to be a close association between the use of oral estrogenic contraceptives and the onset of amenorrhea often accompanied by galactorrhea [Gomez et al., 1977; Sherman et al., 1978; Chang et al., 1977]. Hence, estrogen can be considered a risk factor for the development of prolactinomas in some laboratory animals and in a certain population of humans.
To understand how estrogen functions in cell proliferation and transformation, it is critical to understand the receptor mediation that occurs as a first step in the cascade that follows the introduction of estrogen into the cell. Steroid receptors and their related proteins are involved in a number of biological processes including reproduction, cellular differentiation, development, and metabolism. The transcriptional effects of estrogens are mediated by two closely related receptor isoforms, ERα and ERβ, each of which is encoded by a separate gene [Green et al., 1989; Kuiper, 1996; Mosselman et al., 1996]. The classical estrogen receptor is a typical steroid receptor consisting of a DNA-binding region, a hormone-binding domain, and two transactivation regions. One transactivation region is in the amino terminal portion of the receptor; the other is in the “hinge” region between the hormone- and DNA-binding domains. The importance of the two transcriptional activation regions in the receptor depends on cell type and the target gene. In the absence of estrogen, the receptors associate with binding proteins. This most likely serves to prevent inactive receptors from binding DNA. It has been reported that there are nearly 20,000 receptors per target cell [Katzenellenbogen and Gorski, 1975], and many of these may be spare receptors. This appears to be of major significance in lactotropes. It was recently demonstrated that only a small pool of estrogen receptors is required to regulate growth of these cells, while PRL synthesis requires activation of a larger number of estrogen receptors [Chun et al., 1998].
In the pituitary, ERα mRNA and protein have been demonstrated in normal and adenomatous lactotropes [Friend et al., 1994]. In addition, tumor-specific splice variants of ERα mRNA have been characterized in human prolactinoma specimens [Chaidarun et al., 1997]. The prolactin tumor tissue specimens also coexpressed ERβ, which has a high homology with ERα in the DNA- and ligand-binding domain, but encodes a distinct transcriptional activating function-1 (AF-1) domain [Enmark et al., 1997]. Recently, functional interactions between these receptors and receptor variants have been studied in vitro [Chaidarun et al., 1998]. It has been found that both ERα and ERβ isoforms mediate the mitogenic and hormonal regulatory effects of estradiol in prolactinomas. Additionally, tumor-specific alternative spliced ERα variants promote estradiol-regulated cell proliferation. Hence, coexpression and interaction of various ER isoforms may be important in the promotion of neoplastic lactotropic cell proliferation in the pituitary gland.
Steroids, acting through their receptors, can regulate the synthesis of growth factors and growth factor receptors [Lingham et al., 1988; DiAugustine et al., 1988]. There are a number of growth factors that are estrogen-dependent and function in lactotropic proliferation, differentiation, and/or transformation. The relatedness of these factors and the significance of each alone are not well understood. Some of these estrogen-regulated factors are epidermal growth factor (EGF), platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1) and IGF-2, transforming growth factor alpha (TGF-α), basic fibroblast growth factor (bFGF), interleukin-2 (IL-2) and IL-6, nerve growth factor (NGF), fibroblast growth factor-4 (FGF-4), and transforming growth factor-β (TGF-β). In particular, EGF, TGF-α, PDGF, IGF-1 and IGF-2 have been shown to stimulate lactotropic cell growth, and the roles of these growth factors in estradiol mitogenic action on lactotropes have been reviewed [Sarkar et al., 1998]. TGF-β-related peptides show stimulatory and inhibitory actions on lactotropes; and the TGF-β receptor knockout mice develop pituitary tumors [Hentges et al., 2000b; Shida et al., 1998]. Recently, pituitary tumor-transforming gene (PTTG1) has been isolated and shown to express in pituitary adenomas [Zhang et al., 1999]. This PTTG1 peptide has been shown to induce bFGF production from lactotropes and to mediate estradiol action on bFGF [Heaney et al., 1999]. It has been suggested that both PTTG1 and bFGF play key roles in pituitary tumorigenesis.
In addition to the growth factors, estrogen alters secretion of various hormones that regulate lactotropic cell function. Of these hormones, dopamine, galanin and PRL appear significant. Dopamine is a physiological regulator of PRL secretion. Estradiol administration reduces dopamine secretion, alters dopamine D2 receptor splicing in the pituitary [Ben-Jonathan and Hanasco, 2001] and causes a loss of dopamine neurons in the hypothalamus [Sarkar et al., 1982]. In dopamine D2 receptor knockout mice, prolactinomas develop spontaneously [Asa et al., 1999] suggesting that a loss of dopamine function promotes prolactinoma development and may be a part of estrogen tumorigenic action.
Prolactin may participate in dopamine-regulated prolactinomas, since either a lack of PRL receptor signaling or PRL gene-disruption in mice will result in reduced dopamine function and the development of prolactinomas [Schuff et al., 2002; Cruz-Soto et al., 2002]. Estrogen increases galanin production in lactotropes [Hsu et al., 1990], and targeted overexpression of galanin in the lactotropes of transgenic mice induces hyperprolactinemia and pituitary growth [Cai et al., 1999]. These data indicate that galanin production may also be critical during estrogen-induced pituitary tumorigenesis.
There is evidence suggesting that members of the steroid receptor family may be recipients and transducers of signals initiated at the cell membrane [Weigel, 1994]. Estrogen is known to stimulate the protein kinase C pathway and induce fos and jun expression. There are reports that stimulating constituents of the signaling pathway mimics the effects of estrogen without any of the hormone present [Beck et al., 1992; Aronica and Katzenellenbogen, 1991]. Ligand-independent activation of ER by cAMP has been shown to result from direct PKA or MAPK phosphorylation of ER isoforms [El-Tanani and Green, 1997; Chen et al., 1993]. A direct physical and functional interaction between Sma and MAD-related protein 3 (Smad3) and ERα by two-way crosstalk has been identified in which TGF-β signaling is suppressed by ER whereas ER-mediated transcriptional activation is enhanced by TGF-β signaling [Matsuda et al., 2001]. It has been proposed that bone morphogenic proteins (BMPs) and other TGF-β family peptides induce pituitary prolactinoma pathogenesis through Smad3 and ER receptor crosstalk [Páez-Pereda et al., 2003]. This indicates that there is a great deal of interaction between the estrogen receptors and other signal transduction pathways.
Estrogen-induced prolactinomas may also involve the regulation of cell-cell communication between lactotropes and other pituitary cells critical for cell growth, differentiation, and survival. Folliculostellate (FS) cells are known to be the macrophages of the anterior pituitary (AP). Schechter et al. [1988] demonstrated that estradiol activates FS cells as phagocytes during the onset of prolactinoma formation. They demonstrated that the vascular reorganization that occurs in response to estradiol is dependent upon activation of FS cells. This raises the interesting possibility that FS cells may be key regulators in the development and maintenance of estradiol-induced prolactinomas. The involvement of FS cells in angiogenesis is consistent with the finding that bFGF, a potent angiogenic factor, is secreted in large quantities from FS cells [Ferrara et al., 1987]. The production of bFGF is increased in response to estradiol [Baird et al., 1986]. These data indicate that FS cells may mediate the angiogenesis that follows estradiol exposure. In fact, it has been determined that a significant difference exists in the distribution of FS cells in low estrogen-responsive Sprague-Dawley and high-responsive Fischer-344 rats [Schechter and Weiner, 1991]. This difference allows for angiogenesis to occur in the blood vessels in adjacent meninges, resulting in systemic blood supply to the pituitary. Due to the recruitment of an arterial blood supply, the pituitary can escape the inhibitory influences of the hypothalamus. Therefore, estradiol induces tumors not only by increasing vascularization to the pituitary in these animals, but also by exerting its mitogenic effects on lactotropes unopposed by the inhibitory actions of dopamine.
Current data indicate that bFGF not only functions in angiogenic events but also stimulates lactotropic cell proliferation [Hentges et al., 2000b]. Of major interest is the finding that TGF-β3, produced by lactotropes, induces bFGF release [Hentges et al., 2000a]. In addition to TGF-β3, lactotropes secrete TGF-β1 [Sarkar et al., 1992]. This cytokine is a potent inhibitor of lactotropic proliferation and prolactin secretion both in vivo and in vitro [Hentges and Sarkar, 2001]. Inhibitory response to TGF-β1 is altered in AP cell lines, such as the PRL-secreting PR1 line and the GH- and PRL-secreting GH3 cell line. Both of these cell lines show low levels of TGF-β1 and its type II receptor, TGF-βRII mRNA [Sarkar et al., 1998]. It has also been shown that lactotropes, exposed to estradiol, express reduced levels of TGF-βRII and TGF-β1 and it has been proposed that the steroid increases TGF-β3 production and secretion from lactotropes. The secreted TGF-β3 is transported to the neighboring FS cells where it acts to induce the release of bFGF. The FS cell-derived bFGF stimulates lactotropic cell proliferation as lactotropes escape the effects of TGF-β1 growth inhibition and are activated by estradiol to express FGF receptors. It is also postulated that the loss of TGF-β1 growth inhibitory control may be a contributing factor for lactotropic cell transformation [Hentges and Sarkar, 2001].
Current data indicate TGF-β family related peptides play important role in normal and transformed lactotropic cell growth control by acting directly on the lactotropes and regulating cell-cell communication between FS cells and lactotropes critical for lactotropes’ proliferation and neovascularization for tumor survival. This is of future clinical significance. If pituitary tumorigenesis is indeed a stepwise process as it appears to be, it would be feasible to target the later events like TGF-β signaling. Indeed, TGF-βs have long been examined for their clinical relevance. However, there has been limited success due to the many actions of these factors. The tissue-targeted gene delivery to modulate pituitary production or action of TGF-β might prove to be beneficial for development of new gene therapy approaches for prolactinomas.
EXPERIMENTAL GENE THERAPY IN PITUITARY TUMORS
Studies with adenoviral and herpes-derived vectors demonstrated that these two approaches could be used to efficiently transfer different types of genes into normal rat AP cells in primary culture as well as in the corticotropic AtT20 and somatomammotropic GH3 tumor cell lines [Castro et al., 1997; Goya et al., 1998]. Interestingly, neoplastic AP cells were found to be more susceptible to viral vector-mediated transduction than were normal AP cells. More recently, a herpes simplex virus type-1 (HSV1)-derived vector was shown to be highly effective in vivo for gene transfer in rat pituitary prolactinomas [Bolognani et al., 2001].
A recombinant adenoviral (RAd) vector, RAdTK, harboring the HSV-1 thymidine kinase (TK) suicide gene under the control of the human cytomegalovirus (hCMV) promoter, was used to transfer the TK gene to GH3 and AtT20 cells. Incubation of RAdTK-treated GH3 and AtT20 cells with the prodrug ganciclovir (which after phosphorylation by viral TK becomes toxic) caused ample destruction of the cultures [Windeatt et al., 2000]. In the same study, estrogen/ sulpiride-induced rat prolactinomas were stereotaxically injected with the same RadTK. Subsequent injection of the host animals with two daily i.p. doses of 25 mg ganciclovir/kg for 7 days succeeded in partially reducing AP tumor size and serum PRL levels. Gene therapy, using adenoviral vectors harboring the TK gene under the control of specific AP hormone promoters, namely the human GH and glycoprotein hormone α-subunit promoters, was effective for the treatment of GH3 and α-subunit producing pituitary tumor cell lines in vitro, respectively [Lee et al, 1999]. An adenoviral vector, encoding the TK gene under the control of the human PRL promoter was also effective to induce apoptosis in GH3 cell cultures exposed to ganciclovir. It failed, however, to reduce the growth and PRL-secretory rate of estrogen-induced rat prolactinomas in vivo [Southgate et al., 2000]. Adenoviral vectors, harboring the TK gene under both a promiscuous (hCMV) and the hPRL promoter, showed expression of the transgene for up to 3 months in situ in the normal rat anterior pituitary [Southgate et al., 2001].
Nude mice carrying GH3 cell-grafted subcutaneous tumors were effectively treated with an adenoviral vector harboring the TK gene under the control of the hGH promoter [Lee et al., 1999]. More recently, a GH cell-type specific adenoviral vector was constructed in which a stuffer DNA fragment, flanked by two loxP sequences, was placed between the hGH promoter and the diphtheria toxin gene (GHp-loxP-DT). When the GH-producing cell line GH4 was co-transduced with this vector and with another one harboring the expression cassette for GHp-Cre, rapid destruction of the cultures occurred. Furthermore, subcutaneous GH4 tumors, generated in nude mice, involuted rapidly when topically injected with the above vectors in the tumor area [Lee and Jameson, 2002]. In vivo studies in rats have shown that intravascular administration of adenoviral vectors; harboring the β-galactosidase reporter gene under the control of the hGH or the glycoprotein hormone α-subunit promoters, fail to express the transgene at pituitary level. On the contrary, stereotaxic injection of these vectors into the pituitary of rats succeeded in selectively expressing the transgene in the appropriate AP cell populations [Lee et al., 2000].
Direct stereotaxic injection into the sheep pituitary of Ad vectors carrying the β-galactosidase gene under the control of either the hPRL or hCMV promoters led to high levels of transgene expression up to 7 days after surgery (Davis et al., 2002). Histologic examination of these pituitaries revealed varying degrees of inflammatory response, with periglandular fibrosis, lymphocytic infiltrate and venulitis in almost all cases.
Another type of gene therapy strategy for the treatment of pituitary cancer is that based on the transfer of a gene(s) with the ability to rescue the normal phenotype of tumor cells. This approach has been implemented in mice heterozygous for the retinoblastoma (RB) tumor suppressor gene (Rb+/− mice). Such mice develop and succumb to characteristic pituitary intermediate lobe melanotroph tumors [Hu et al., 1994]. Transduction of tumor melanotrophic cells with a recombinant adenoviral vector (rAd5.R.Rb) carrying the human RB cDNA under the control of its own promoter, showed a high level of efficiency both in vitro and in vivo [Riley et al., 1996]. Furthermore, intracranial delivery of this vector to mice carrying actively growing melanotrophic tumors significantly reduced tumor growth and prolonged animal survival. Melanotrophic tumor proliferative index and apoptotic rates were markedly lowered in the rAd5.R.Rb-treated animals, which also showed growth-inhibitory dopaminergic neuron reinnervation of melanotrophic cells. A point of concern regarding this study is that, although the authors claim an acceptable targeting of the mice’s pituitary gland with their transauricular injection method, they intrapituitarily injected up to 20 :l viral vector suspension per animal, a volume several-fold larger than that of a mouse pituitary gland [Riley et al., 1996].
REGULATABLE VECTOR SYSTEMS FOR THE TREATMENT OF PITUITARY TUMORS
Two central objectives in the development of viral vectors are the achievement of cell-type specificity for transgene delivery, and the design of vectors where, once the transgene is incorporated into the target cell, its expression can be regulated by small molecules. Cell-type specificity has already been achieved in pituitary tumor cells by placing transgenes under the control of cell-type specific promoters, like the GH, α-glycoprotein and PRL promoters for pituitary tumor cells, as noted previously.
Early inducible gene expression systems encountered limitations, such as pleiotropic effects of the inducer, basal leakiness, toxicity of inducing agents and a low level of expression. Nevertheless, nontoxic, tightly-regulated control of transgene expression has been achieved with a tetracycline (Tet) gene control system [Gossen and Bujard, 1992]. Two types of Tet gene control system have been devised. A Tet-Off system is one such system. It is composed of a Tet transactivator (a hybrid protein termed tTA, which contains the Tet and DNA binding domains of the E. coli Tet repressor fused to VP16, a HSV1 transactivator), which binds to E. coli-derived Tet-resistance operon regulatory elements embedded within a minimal CMV promoter (tetO/minCMV). Binding of tTA to this region recruits strong cellular transcription factors thus, inducing transcription of a transgene inserted downstream. When Tet is present, it binds to tTA causing the dissociation of tTA from the tetO/minCMV complex thereby, inhibiting transgene expression. The second type of gene control system is the Tet-On system. Here tTA is modified by four amino acids giving rise to the rtTA transactivator, which, by itself is inactive, but in the presence of the Tet analog doxycycline, binds to the tetO/minCMV region and induces high levels of transgene expression.
A number of Tet-regulatable viral systems have now been developed, including a Tet-off dual adenoviral system for the β-galactosidase reporter gene with the DNA sequence encoding the tTA transactivator placed under the control of the hPRL promoter. With this system, specific transgene expression in rodent lactotropic cells was achieved both in vitro and in vivo. Furthermore, this expression could be turned off by administration of doxycycline and then back on when the antibiotic was removed [Smith-Arica et al., 2001]. A similar dual regulatable adenoviral vector system, in this case expressing tyrosine hydroxylase (TH) under the control of the hCMV promoter, has been also constructed. As TH is the rate-limiting enzyme for dopamine synthesis, the growth of both lactotropic cell lines (GH3 and MMQ) and estrogen-induced rat prolactinomas was significantly inhibited when co-transduced with the two vector components of the regulatable TH vector system. The expression of TH could be turned off by doxycycline, and back on by the removal of the antibiotic [Williams et al., 2001].
Future application of regulatable gene delivery systems to neuroendocrine models would be of enormous interest considering the highly-regulated nature of neuroendocrine function.
POTENTIAL OF SUICIDE GENE THERAPY FOR THE TREATMENT OF HUMAN PITUITARY TUMORS
As indicated previously, the development of gene therapy approaches for the neuroendocrine system is still incipient. While some of these approaches have already generated a core of results that emerge as a promising area of research for the treatment of pituitary tumors, no clinical trials have yet been documented. We are aware of only one gene therapy study using human pituitary cells. In this study, primary cultures of human lactotroph adenoma cells were successfully transduced with an adenoviral vector harboring the cDNA for human TH. The transduced cells not only expressed immunocytochemically-detectable TH, but were also shown to release significant levels of L-DOPA and dopamine, which markedly reduced PRL release to the culture medium [Freese et al., 1996].
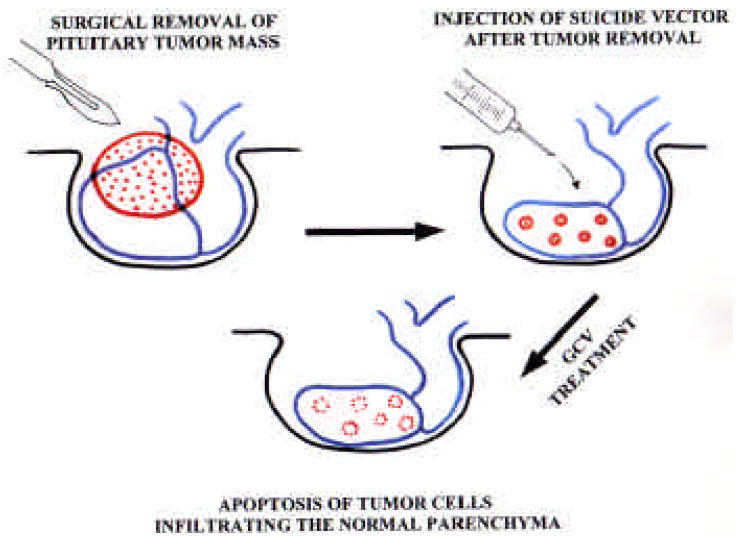
As already indicated, pituitary tumors seldom generate distant metastases. Although this feature allows the surgeon to spare normal pituitary tissue without the risk of tumor cell dissemination, neoplastic cells infiltrating the normal pituitary parenchyma may give rise to tumor regrowths. As mentioned before, pituitary tumor surgery is usually complemented with radiotherapy but, although this therapeutic combination is often effective to prevent tumor regrowth, it usually causes hypopituitarism due to excessive destruction of the normal pituitary parenchyma [Littley et al., 1991]. In this context, gene therapy appears to be a potentially superior alternative to radiotherapy for the treatment of pituitary tumors. In effect, after tumor removal, intrasurgical injection of an appropriate suicide vector into the spared pituitary parenchyma could achieve complete elimination of remaining tumor cells without affecting healthy cells (Fig. 1). This therapeutic combination for the treatment of pituitary tumors has already been proposed [Castro et al., 2001].
Fig. 1. Schematic representation of a possible application of suicide gene therapy to complement surgical treatment of pituitary tumors.
Initially the main tumor mass is removed, sparing the normal pituitary parenchyma. Subsequently, the surgeon injects in the pituitary tissue that surrounded the tumoral mass a suspension of an appropriate vector harboring the TK suicide transgene. A few days later the patient is treated with ganciclovir for a convenient period of time in order to eliminate those infiltrating tumor cells that escaped the scalpel.
As in other organs, adenoviral vectors have been shown to cause inflammation in the pituitary gland (see above), an adverse effect that makes it advisable to consider the use of less immunogenic viral vectors, like the adeno-associated viral vectors, for the treatment of pituitary tumors. It should also be pointed out that, although so far only the HSV1-TK-GCV suicide system has been used to implement destructive experimental gene therapy of pituitary tumors, other available toxic systems might offer advantages over the TK-GCV system. Thus, studies with recombinant Ad vectors harboring cDNAs for three toxic genes, namely those encoding the HSV1-TK, the E. coli cytosine deaminase and the deoxycytidine kinase, showed that cytosine deaminase had the highest efficacy to reduce the growth of different human lung carcinoma cell lines (Hoganson et al., 1996).
Antisense and dominant negative gene therapies may constitute alternative strategies for the treatment of pituitary tumors. Suppression of endogenous bFGF expression by means of an Ad vector carrying an antisense transgene for bFGF caused inhibition of the proliferation of human glioma cells. The same effect was achieved by means of an Ad vector carrying a transgene for a dominant negative FGF receptor (Aoki et al., 2002).
BEYOND GENE THERAPY: THE NANOTECHNOLOGICAL FRONTIER
The future of gene therapy is strongly linked to the evolution of gene transfer technology. The achievement of cell-type specificity for transgene delivery and regulatability in a single vector system is a reality that shows the impressive progress that viral vector technology is making. It should be pointed out, however, that despite ever-increasing refinements in gene vector technology, the progress in the therapeutic effectiveness of gene therapy may eventually reach a plateau. This is particularly true for the design of therapeutic strategies for chronic pathologies where a substantial number of important genes and gene networks are likely to deteriorate. It seems unlikely that exogenous gene transfer may be able to fully correct these multigenic derangements. At some point, genetic therapies will have to move from exogenous gene transfer (i.e. gene therapy) to endogenous gene repair. Certainly, the implementation of endogenous gene repair to cure or prevent complex pathologies like cancer will call for a quantum leap in technology, a transformation that observers from different venues of science and technology believe is about to happen; it is called the Nanotechnology Revolution [Drexler et al., 1991].
Nanotechnology may be defined as a technology based on the precise manipulation of individual atoms and molecules in order to build complex structures, specified at the atomic level. Living systems function on the principles of nanotechnology, since they can build up structures as complex as a human being, assembling them atom by atom. Although nanotechnology is still in its infancy, there is evidence that the seminal achievements that will lead to a mature technology are already taking place [Macilwain, 2000; Nanotech, 2001]. Nanotechnology is already attracting interest from physicists, engineers, chemists and biologists, a growing number of whom are getting involved in nanotechnological R&D [National Nanotechnology Initiative, 2002]. There is also a growing number of biomedical researchers who believe that one of the contributions of nanotechnology will be the development of nanomedicine, a medical specialty based on the use of intelligent nanoinstruments (nanobots) to cure the organism “from within.” Although no nanomedical applications are yet available, theoretical articles and books on nanomedicine are already being actively generated [Freitas, 1999].
It seems possible, therefore, to envisage a future therapeutic scenario where treatment of malignant tumors can be effected by means of, for instance, a single i.v. administration of a few million self-replicating, self destruction-enabled nanobots. These nanobots would first replicate within the patient in order to attain an appropriate working number. They would then survey the whole organism, searching for and destroying or repairing atypical cells. Subsequently, self-destruction and non-toxic elimination of nanobots would take place. This might prove to be the most effective and physiological method for prevention and/or treatment of cancer and other cellular pathologies. Time will tell whether this vision will remain just that, or will instead become a 21st-century reality.
Acknowledgments
The authors thank Ms. Yolanda Sosa for secretarial assistance with the manuscript and Ms. Stephanie Zimmerman for editorial assistance. This work was supported in part by grants to RGG from the Argentinian Research Council (CONICET) and the Argentinian Agency for the Promotion of Science and Technology (ANPCyT) to RG and NIH AA00220 to DKS.
References
- Aoki T, Kato S, Fox JC, Okamoto K, Morimatsu M, Shigemori M. Inhibition of autocrine fibroblast growth factor signaling by the adenovirus-mediated expression of an antisense transgene or a dominant negative receptor in human glioma cells. Int J Oncol. 2002;21:629–636. [PubMed] [Google Scholar]
- Aronica SM, Katzenellenbogen BS. Progesterone receptor regulation in uterine cells: stimulation by estrogen, cyclic adenosine 3,5-monophosphate and insulin-like growth factor I and suppression by antiestrogens and protein kinase inhibitors. Endocrinology. 1991;128:2045–2052. doi: 10.1210/endo-128-4-2045. [DOI] [PubMed] [Google Scholar]
- Asa SL, Kelly MA, Grandy DK, Low MJ. Pituitary lactotroph adenomas develop after prolonged lactotroph hyperpalsia in dopamine D2 receptor-deficient mice. Endocrinology. 1999;140:5348–5355. doi: 10.1210/endo.140.11.7118. [DOI] [PubMed] [Google Scholar]
- Baird A, Esch F, Mormede P, Ueno N, Ling N, Bohlen P, Ying SY, Wehrenberg WB, Guillemin R. Molecular characterization of fibroblast growth factor: distribution and biological activities in various tissues. In: Greep RO, editor. Recent Progress in Hormone Research. Academic Press; New York: 1986. pp. 143–205. [DOI] [PubMed] [Google Scholar]
- Banerjee SK, De A, Sarkar DK. Colocalization of prolactin and PCNA within anterior pituitary cells in estrogen treated rats: Evidence for the induction of lactotrope cell proliferation during estrogen induced pituitary tumors. Cancer Letter. 1994;83:139–14. doi: 10.1016/0304-3835(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Beck CA, Weigel NL, Edwards DP. Effects of hormone and cellular modulators of protein phosphorylation on transcriptional activity, DNA binding and phosphorylation of human progesterone receptors. Mol Endocrinol. 1992;6:607–610. doi: 10.1210/mend.6.4.1316549. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22:724–763. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- Bevan JS, Webster J, Burke CW, Scanlon MF. Dopamine agonists and pituitary tumor shrinkage. Endocr Rev. 1992;13:220–240. doi: 10.1210/edrv-13-2-220. [DOI] [PubMed] [Google Scholar]
- Bevan JS, Atkin SL, Atkinson AB, Bouloux PM, Hanna F, Harris PE, James RA, McConnell M, Roberts GA, Scanlon MF, Stewart PM, Teasdale E, Turner HE, Wass JA, Wardlaw JM. Primary medical therapy for acromegaly: an open, prospective, multicenter study of the effects of subcutaneous and intramuscular slow-release octreotide on growth hormone, insulin-like growth factor-I and tumor size. J Clin Endocrinol Metab. 2002;87:4554–4563. doi: 10.1210/jc.2001-012012. [DOI] [PubMed] [Google Scholar]
- Bolognani F, Albariño C, Romanowski V, Carri NG, Goya RG. In vitro and in vivo herpetic vector-mediated gene transfer in the pituitary gland: impact on hormone secretion. Eur J Endocrinol. 2001;145:497–503. doi: 10.1530/eje.0.1450497. [DOI] [PubMed] [Google Scholar]
- Burek JD. Pathology of aging rats. CRC Press; Boca Raton, FL: 1978. [Google Scholar]
- Cai A, Hayes DJ, Patel N, Hyde JH. Targeted overexpression of galanin in lactotropes of transgenic mice induces hyperprolactinemia and pituitary hyperplasia. Endocrinology. 1999;140:4955–4964. doi: 10.1210/endo.140.11.7120. [DOI] [PubMed] [Google Scholar]
- Carol N, Lauterbach H, Klinger G, Unger A, Michels W. Prolactin stimulation using the metoclopramide test in females taking oral contraceptives. Zentralbl Gynakol. 1988;110:1515–1521. [PubMed] [Google Scholar]
- Castro MG, Goya RG, Sosa YE, Rowe J, Larregina AT, Morelli AE, Lowenstein PR. Expression of transgenes in normal and neoplastic anterior pituitary cells using recombinant adenoviruses: long term expression, cell cycle dependency and effects on hormone secretion. Endocrinology. 1997;138:2184–2194. doi: 10.1210/endo.138.5.5134. [DOI] [PubMed] [Google Scholar]
- Castro MG, Southgate T, Lowenstein PR. Molecular therapy in a model of neuroendocrine disease: developing clinical gene therapy for pituitary tumours. TEM. 2001;12:58–64. doi: 10.1016/s1043-2760(00)00358-1. [DOI] [PubMed] [Google Scholar]
- Chaidarun SS, Klibanski A, Alexander JM. Tumor-specific expression of alternative spliced estrogen receptor messenger ribonucleic acid variants in human pituitary adenoma. J Clin Endocrinol Metab. 1997;82:1058–1165. doi: 10.1210/jcem.82.4.3864. [DOI] [PubMed] [Google Scholar]
- Chaidarun SS, Swearingen B, Alexander JM. Differential expression of estrogen receptor-β (ERβ) in human pituitary tumors: functional interactions with ERα and a tumor-specific splice variant. J Clin Endocrinol Metab. 1998;83:3308–3315. doi: 10.1210/jcem.83.9.5128. [DOI] [PubMed] [Google Scholar]
- Chang RJ, Keye WR, Young JR, Willson CB, Jaffe RB. Detection, evaluation and treatment of pituitary microadenomas in patients with galactorrhea and amenorrhea. Am J Obstet Gynecol. 1977;128:356–363. doi: 10.1016/0002-9378(77)90553-1. [DOI] [PubMed] [Google Scholar]
- Chen RH, Ebner R, Derynck R. Inactivation of the type II receptor pathways for the diverse TGF-β activities. Science. 1993;260:1335–1338. doi: 10.1126/science.8388126. [DOI] [PubMed] [Google Scholar]
- Chun T, Gregg D, Sarkar DK, Gorski J. Differential regulation by estrogens of growth and prolactin synthesis in pituitary cells suggests that only a small pool of estrogen receptors is required for growth. Proc Natl Acad Sci USA. 1998;95:2325–2330. doi: 10.1073/pnas.95.5.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Soto ME, Scheiber MD, Gregerson KA, Boivin GP, Horseman ND. Pituitary tumorigenesis in prolactin gene-disrupted mice. Endocrinology. 2002;143:4429–4436. doi: 10.1210/en.2002-220173. [DOI] [PubMed] [Google Scholar]
- Daniels GH, Martin JB. Harrison’s Principles of Internal Medicine . Neuroendocrine regulation and diseases of the anterior pituitary and hypothalamus. 1995. [Google Scholar]
- Davis JR, McMahon RF, Lowenstein PR, Castro MG, Lincoln GA, McNeilly AS. Adenovirus_mediated gene transfer in the ovine pituitary gland is associated with hypophysitis. J Endocrinol. 2002;173:265–271. doi: 10.1677/joe.0.1730265. [DOI] [PubMed] [Google Scholar]
- DeNicola AF, Von Lawzewifsch IS, Kaplan E, Libertum C. Biochemical and ultrastructural studies of estrogen-induced pituitary tumors in F344 rats. J Natl Cancer Inst. 1978;61:753–763. [PubMed] [Google Scholar]
- DiAugustine RP, Petrusz P, Bell GI, Brown CF, Korach KS, McLachlan JA, Teng CT. Influence of estrogens on mouse uterine epidermal growth factor precursor protein and messenger ribonucleic acid. Endocrinology. 1988;122:2355–2361. doi: 10.1210/endo-122-6-2355. [DOI] [PubMed] [Google Scholar]
- Drexler KE, Peterson C, Pergamit G. Unbounding the Future: The Nanotechnology Revolution. Willian Morrow; New York: 1991. [Google Scholar]
- El-Tanani MKK, Green CD. Two separate mechanisms for ligand-independent activation of the estrogen receptor. Mol Endocrinol. 1997;11:928–937. doi: 10.1210/mend.11.7.9939. [DOI] [PubMed] [Google Scholar]
- Enmark E, Pelto-huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjold M, Gustafson JA. Human estrogen receptor β-gene structure, chromosomal localization and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- Fahy UM, Foster PA, Torodo HW, Hartog M, Hull MG. The effect of combined estrogen/progesterone treatment in women with hyperprolactinemic amenorrhea. Gynecol Endocrinol. 1992;6:183–188. doi: 10.3109/09513599209015553. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Schweigerer L, Neufeld G, Mitchell R, Gospodarowicz D. Pituitary follicular cells produce basic fibroblast growth factor. Proc Natl Acad Sci USA. 1987;84:5773–5777. doi: 10.1073/pnas.84.16.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese A, During MJ, Davidson BL, Gennarelli TA, Kaplitt MG, Flamm ES, Snyder PJ. Transfection of human lactotroph adenoma cells with an adenovirus vector expressing tyrosine hydroxylase decreases prolactin release. J Clin Endocrinol Metab. 1996;81:2401–2404. doi: 10.1210/jcem.81.6.8964885. [DOI] [PubMed] [Google Scholar]
- Freitas RA., Jr . Nanomedicine. Vol. 1. Landes Bioscience; Palo Alto, CA: 1999. [Google Scholar]
- Friend KE, Chiou YK, Lopes MB, Laws ER, Jr, Hughes KM, Shupnik MA. Estrogen receptor expression in human pituitary: correlation with immunohistochemistry in normal tissue and immunohistochemistry and morphology in macroadenomas. J Clin Endocrinol Metab. 1994;78:1497–1504. doi: 10.1210/jcem.78.6.7515390. [DOI] [PubMed] [Google Scholar]
- Garcia MM, Kapcala LP. Growth of a microprolactinoma to a macroprolactinoma during estrogen therapy. J Endocrinol Invest. 1995;18:450–455. doi: 10.1007/BF03349744. [DOI] [PubMed] [Google Scholar]
- Gomez F, Reyes F, Fairman C. Nonpuerperal galactorrhea and hyperprolactinemia. Clinical findings, endocrine features and therapeutic responses in 56 cases. Am J Med. 1977;62:648–660. doi: 10.1016/0002-9343(77)90866-x. [DOI] [PubMed] [Google Scholar]
- Gooren L, Assies JG, Asscheman J, deSlegte AR, vanKessel H. Estrogen-induced prolactinoma in a man. J Clin Endocrinol Metab. 1987;66:444–446. doi: 10.1210/jcem-66-2-444. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline responsive promoters. Proc Natl Acad Sci USA. 1992;88:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goya RG, Rowe J, Sosa YE, Tomasec P, Lowenstein PR, Castro MG. Use of recombinant herpes simplex virus type I vectors for gene transfer into tumour and normal anterior pituitary cells. Molec Cell Endocrinol. 1998;139:199–207. doi: 10.1016/s0303-7207(98)00059-8. [DOI] [PubMed] [Google Scholar]
- Green S, Walter P, Kumar V, Bornert M, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to V-erb-A. Nature. 1989;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Grigsby PW, Sheline GE. Pituitary. In: Perez CA, Bray LA, editors. Principles and Practice of Radiation Oncology. Lippincott; Philadelphia: 1990. pp. 564–582. [Google Scholar]
- Heaney AP, Horwits GA, Wang Z, Singson R, Melmed S. Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinomas pathogenesis. Nature Med. 1999;5:1317–1322. doi: 10.1038/15275. [DOI] [PubMed] [Google Scholar]
- Hentges S, Sarkar DK. Transforming growth factor β regulation of estradiol-induced prolactinomas. Rec Prog Neuroendocrinol. 2001;22:340–363. doi: 10.1006/frne.2001.0220. [DOI] [PubMed] [Google Scholar]
- Hentges S, Boyadjieva N, Sarkar DK. Transforming growth factor-β3 stimulates lactotrope cell growth by increasing basic fibroblast growth factor from folliculo-stellate cells. Endocrinology. 2000a;141:859–867. doi: 10.1210/endo.141.3.7382. [DOI] [PubMed] [Google Scholar]
- Hentges S, Pastorcic M, De A, Boyadjieva N, Sarkar DK. Opposing actions of two transforming growth factor-β isoforms on pituitary lactotropic cell proliferation. Endocrinology. 2000b;141:1528–1535. doi: 10.1210/endo.141.4.7419. [DOI] [PubMed] [Google Scholar]
- Herman Y, Drazin NZ, Gonsky R, Melmed S. Molecular screening of pituitary adenomas for gene mutations and arrangements. J Clin Endocrinol Metab. 1993;77:50–55. doi: 10.1210/jcem.77.1.8100831. [DOI] [PubMed] [Google Scholar]
- Hoganson DK, Batra RK, Olsen JC, Boucher RC. Comparison of the effects of three different toxin genes and their levels of expression on cell growth and bystander effect in lung adenocarcinoma. Cancer Res. 1996;56:1315–1323. [PubMed] [Google Scholar]
- Hu N, Gutsmann A, Herbert DC, Bradley A, Lee WH, Lee EY. Heterozygous Rb-1 delta 20/+mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene. 1994;9:1021–1029. [PubMed] [Google Scholar]
- Hsu DW, El-Azouzi M, Black PM, Chin WW, Hedley-Whyte ET, Kaplan LM. Estrogen increases galanin immunoreactivity in hyperplastic prolactin-secreting cells in Fischer 344 rats. Endocrinology. 1990;126:3159–3167. doi: 10.1210/endo-126-6-3159. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Yamaji T. Functional heterogeneity of human prolactin-producing pituitary adenoma cells. In: MacLeod RM, Scapagnini U, Thorner MO, editors. Prolactin basic and clinical correlates. Springer-Verlag; New York: 1985. pp. 641–702. [Google Scholar]
- Karga HJ, Alexander JM, Hedley-Whyte ET, Klibanski A, Jameson JL. Ras mutation in human pituitary tumors. J Clin Endocrinol Metab. 1992;74:914919. doi: 10.1210/jcem.74.4.1312542. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Gorski J. Estrogen actions on synthesis of macromolecules in target cells. In: Litwack L, editor. Biochemical Actions of Hormones. Vol. 3. Academic Press; New York: 1975. pp. 187–243. [Google Scholar]
- Kuiper GGJM, Enmark E, Peltottuikko M, Nilsson S, Gustafsson JA. Cloning of a novel estrogen receptor expressed in rat prostrate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Thimmapaya B, Jameson JL. Stereotactic injection of adenoviral vectors that target gene expression to specific pituitary cell types: Implications for gene therapy. Neurosurgery. 2000;46:1461–1469. doi: 10.1097/00006123-200006000-00029. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Jameson JL. Cell-specific Cre-mediated activation of the diphtheria toxin gene in pituitary tumor cells: potential for cytotoxic gene therapy. Hum Gene Ther. 2002;13:533–542. doi: 10.1089/10430340252809829. [DOI] [PubMed] [Google Scholar]
- Lee EJ, anderson LM, Thimmapaya B, Jameson JL. Targeted expresion of toxic genes directed by pituitary hormone promoters: a potential strategy for adenovirus-mediated gene therapy of pituitary tumors. J Clin Endocrinol Metab. 1999;84:786–794. doi: 10.1210/jcem.84.2.5504. [DOI] [PubMed] [Google Scholar]
- Lingham RB, Stancel GM, Loose-Mitchell DS. Estrogen regulation of epidermal growth factor receptor messenger ribonucleic acid. Mol Endocrinol. 1988;2:230–235. doi: 10.1210/mend-2-3-230. [DOI] [PubMed] [Google Scholar]
- Littley MD, Shalet SM, Beardwell CG, Ahmed SR, Applegate G, Sutton ML. Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med. 1991;70:145–160. [PubMed] [Google Scholar]
- Lloyd RV. Estrogen-induced hyperplasia and neoplasia in the rat anterior pituitary gland. Am J Pathol. 1983;113:198–206. [PMC free article] [PubMed] [Google Scholar]
- Luciano AA, Sherman BM, Chapler FK, Hauser KS, Wallace RB. Hyperprolactinemia and contraception: a prospective study. Obstet Gynecol. 1985;65:506–510. [PubMed] [Google Scholar]
- Macilwain C. Nanotech thinks big. Nature. 2000;405:730–732. doi: 10.1038/35015778. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Yamamoto T, Muraguchi A, Saatcioglu F. Crosstalk between transforming growth factor-beta and estrogen receptor signaling through Smad3. J Biol Chem. 2001;276:42908–42914. doi: 10.1074/jbc.M105316200. [DOI] [PubMed] [Google Scholar]
- Melmed S. General aspects of the management of pituitary tumors by surgery or radiation therapy. In: DeGroot LJ, editor. Endocrinology. Saunders; Philadelphia: 1997. pp. 497–503. [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ERβ: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Nanotech. Sci Am. 2001;285(3):26–83. [Google Scholar]
- National Nanotechnology Initiative. http://www.nano.gov.
- Páez-Pereda M, Giacomini D, Refojo D, Nagashima AC, Hopfner U, Grübler Y, Chervin A, Goldberg V, Goya RG, Hentges ST, Low MJ, Holsboer F, Stalla GK, Arzt E. Involvement of bone morphogenetic protein 4 in prolactinoma pathogenesis: BMP-4 is overexpressed in pituitary prolactinomas and promotes cell growth through a Smad/estrogen receptor cross-talk. Proc Ntnl Acad Sci. 2003;100:1034–1039. doi: 10.1073/pnas.0237312100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior JC, Cox TA, Fairholm D, Kostashuk E, Nugent R. Testerone–related exacerbation of a prolactin-producing macroadenoma: possible role for estrogen. J Clin Endocrinol Metab. 1987;64:391–394. doi: 10.1210/jcem-64-2-391. [DOI] [PubMed] [Google Scholar]
- Riley DJ, Yu A, Lee WH. Adenovirus-mediated retinoblastoma gene therapy supresses spontaneous pituitary melanotroph tumors in Rb+/− mice. Nature Med. 1996;2:1316–1321. doi: 10.1038/nm1296-1316. [DOI] [PubMed] [Google Scholar]
- Sadoul JL, Thyss A, Freychet P. Invasive mixed growth hormone/prolactin secreting pituitary tumor: complete shrinking by octreotide withdrawal. Acta Endocrinol. 1992;126:179–183. doi: 10.1530/acta.0.1260179. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Gottschall PE, Meites J. Relation of the neuroendocrine system to development of prolactin secreting pituitary tumors. In: Meites J, editor. Neuroendocrinology of Aging. Plenum Press; New York: 1983. pp. 353–376. [Google Scholar]
- Sarkar DK, Gottschall PE, Meites J. Damage to hypothalamic dopaminergic neurons is associated with development of prolactin-secreting tumors. Science. 1982;218:684–686. doi: 10.1126/science.7134966. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Hentges ST, De A, Reddy RHR. Hormonal control of pituitary prolactin secreting tumors. Frontiers in Bioscience. 1998;3:934–943. doi: 10.2741/a334. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Kim KH, Minami S. Transforming growth factor-β1 mRNA and protein expression in the pituitary gland and its action on PRL secretion and lactotropic growth. Mol Endocrinol. 1992;6:1825–1833. doi: 10.1210/mend.6.11.1480172. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Pastorcic M, De A, Engel M, Moses H, Ghasemzadeh B. Role of transforming growth factor (TGF)-β type I and TGF-β type II receptors in the TGF-β1-regulated gene expression in pituitary prolactin-secreting lactotropes. Endocrinology. 1998;139:3620–3628. doi: 10.1210/endo.139.8.6135. [DOI] [PubMed] [Google Scholar]
- Schechter J, Ahmad N, Weiner R. Activation of anterior pituitary folliculo-stellate cells in the formation of estrogen-induced prolactin-secreting tumors. Neuroendocrinology. 1988;48:569–576. doi: 10.1159/000125064. [DOI] [PubMed] [Google Scholar]
- Schechter J, Weiner R. Changes in basic fibroblast growth factor coincident with estradiol induced hyperplasia of the anterior pituitaries of Fischer 344 and Sprague-Dawley rats. Endocrinology. 1991;129:2400–2408. doi: 10.1210/endo-129-5-2400. [DOI] [PubMed] [Google Scholar]
- Schuff KG, Hentges ST, Kelly MA, Binart N, Kelly PA, Iuvone PM, Asa SL, Low MJ. Lack of prolactin receptor signaling in mice results in lactotroph proliferation and prolactinomas by dopamine-dependent and -independent mechanism. J Clin Invest. 2002;110:973–981. doi: 10.1172/JCI15912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard MC. Aims of treatment and definition of cure. In: Wass JAH, editor. Treating Acromegaly- 100 Years On. Society for Endocrinology; Bristol: 1994. pp. 17–31. [Google Scholar]
- Sherman BM, Harris CE, Schlechte JN, Duello TM, Halmi S, VanGilder J, Chapler FK, Granner DK. Pathogenesis of prolactin-secreting pituitary adenomas. Lancet. 1978;11:1019–1021. doi: 10.1016/s0140-6736(78)92339-5. [DOI] [PubMed] [Google Scholar]
- Shida N, Ikeda H, Takashi Y, Oshima M, Taketo MM, Miyoshi I. Estrogen-induced tumorigenesis in the pituitary gland of TGF-β(+/−) knockout mice. Biochim Biophys Acta. 1998;140:79–83. doi: 10.1016/s0925-4439(98)00024-6. [DOI] [PubMed] [Google Scholar]
- Shimon I, Melmed S. Management of pituitary tumors. Annals Int Med. 1998;129:472–483. doi: 10.7326/0003-4819-129-6-199809150-00009. [DOI] [PubMed] [Google Scholar]
- Shull JD, Spady TJ, Snyder MC, Johansson SL, Pennington KL. Ovary-intact, but not ovariectomized female ACI rats treated with 17-fl estradiol rapidly develop mammary cacinoma. Carcinogenesis. 1997;18:1595–1602. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- Shy KK, McTiernan AM, Daling JR, Weiss NS. Oral contraceptive use and the occurrence of pituitary prolactinoma. J Am Med Assoc. 1983;249:2204–2207. [PubMed] [Google Scholar]
- Smith-Arica JR, Williams JC, Stone D, Smith J, Lowenstein PR, Castro MG. Switching on and off transgene expression within lactotrophic cells in the anterior pituitary gland in vivo. Endocrinology. 2001;142:2521–2532. doi: 10.1210/endo.142.6.8183. [DOI] [PubMed] [Google Scholar]
- Southgate TD, Stone D, Williams JC, Lowenstein PR, Castro MG. Long-term transgene expression within the anterior pituitary gland in situ: Impact on circulating hormone levels, cellular and antibody-mediated immune responses. Endocrinology. 2001;142:464–476. doi: 10.1210/endo.142.1.7898. [DOI] [PubMed] [Google Scholar]
- Southgate TD, Windeatt S, Smith-Arica J, Gerdes CA, Perone MJ, Morris I, Davis JR, Klatzmann D, Lowenstein PR, Castro MG. Transcriptional targeting to anterior pituitary lactotrophic cells using recombinant adenovirus vectors in vitro and in vivo in normal and estrogen/sulpiride-induced hyperplastic anterior pituitaries. Endocrinology. 2000;141:3493–3505. doi: 10.1210/endo.141.9.7639. [DOI] [PubMed] [Google Scholar]
- Sutton ML. Adult central nervous system. In: Easson EC, Pointon RCS, editors. The Radiotherapy of Malignant Disease. Springer; New York: 1985. pp. 215–236. [Google Scholar]
- Tonner D, Schlechte J. Contemporary therapy of prolactin-secreting adenomas. Am J Med Sci. 1993;306:395–397. doi: 10.1097/00000441-199312000-00008. [DOI] [PubMed] [Google Scholar]
- Webster J, Piscitelli G, Polli A, Ferrari CI, Ismail I, Scanlon MF. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. N Engl J Med. 1994;331:904–909. doi: 10.1056/NEJM199410063311403. [DOI] [PubMed] [Google Scholar]
- Weigel N. In: Steroid receptors and their regulation by phosphorylation. Khan S, Stancel G, editors. CRC Press; Boca Raton: 1994. pp. 1–12. [Google Scholar]
- Wickland SA, Werts N, Gorski Jr. A comparison of estrogen effect on uterine and pituitary growth and prolactin synthesis in F-344 and Holtzman rats. Endocrinology. 1981;109:1700–1707. doi: 10.1210/endo-109-5-1700. [DOI] [PubMed] [Google Scholar]
- Williams JC, Stone D, Smith-Arica JR, Morris ID, Lowenstein PR, Castro MG. Regulated adenovirus-mediated delivery of tyrosine hydroxylase suppresses growth of estrogen-induced pituitary prolactinomas. Mol Ther. 2001;4:593–602. doi: 10.1006/mthe.2001.0499. [DOI] [PubMed] [Google Scholar]
- Windeatt S, Southgate TD, Dewey RA, Bolognani F, Perone MJ, Larregina AT, Maleniak TC, Morris ID, Goya RG, Klatzmann D, Lowenstein PR, Castro MG. Adenovirus-mediated herpes simplex virus type-1 thymidine kinase gene therapy suppresses oestrogen-induced pituitary prolactinomas. J Clin Endocr Metab. 2000;85:1296–1305. doi: 10.1210/jcem.85.3.6482. [DOI] [PubMed] [Google Scholar]
- Zhang X, Horwitz GA, Prezant TR, Valentini A, Nakashima M, Bronstein MD, Melmed S. Structure, expression and function of human pituitary tumor-transforming gene (PTTG) Mol Endocrinol. 1999;13:156–166. doi: 10.1210/mend.13.1.0225. [DOI] [PubMed] [Google Scholar]