Abstract
A small library of 2H-benzo[b][1,4] oxazine derivative was synthesized and their biological activity was tested on HepG2 cells under normoxic and hypoxic conditions. From preliminary screening, we found compound 10 and 11 specifically inhibit hypoxic cancer cell growth IC50 87 ± 1.8 μM and IC50 10 ± 3.7 μM while sparing ‘normoxic’ cells IC50 >600 M and >1 mM (not applicable), respectively. We tested the effect of 10 on MTT, clonogenic and hypoxia induced genes. The MTT correlates with clonogenic assays and most importantly compound 10 down regulates hypoxia induces genes (HIF-1α, P21 and VEGF) appropriately. We are in the process to explore the molecular mechanism of action of oxazine derivative compounds on hypoxia tumor cells.
Keywords: Hypoxia agent, Hypoxia inducing factor (HIF)-alpha, Oxazine, PET agent
Hypoxia is a universal hallmark of tumor cells in vivo. Within the tumor microenvironment, it contributes towards resistance to radiation and chemotherapy.1–3 The inability to treat hypoxic tumor cells effectively, represent opportunities for providing novel compounds and strategies for this unmet medical need.3
Mainly three types of strategies are used to treat the hypoxic tumor, the first strategy is (a) hypoxia-activated prodrugs- in this model, several drugs have entered the clinic; the most advanced is tirapazamine, which is in phase III clinical testing in a variety of solid tumor malignancies. However, tirapazamine lacks the ability for good tumor penetration, and has low in vivo potency at doses that are non-toxic (or have tolerable side-effects) to humans.4 We and others have previously investigated another promising hypoxia-activated prodrug, banoxantrone (AQ4 N).5–7 However, there remains some clinical issues with AQ4 N, like mucosal discoloration; however, there has been clinical activity documented in lymphomas. Other drugs belonging to this class of hypoxia agents Include 2-[-(2-bromoethyl)-2,4-dinitro-6-[[[2-phosphonooxy]ethyl]amino]carbonyl]aniline]-ethyl methanosulfonate (PR-1044).8 The other hypoxia-activated phosphoramidate DNA cross linking mustards like cyclophosphamide and ifosfamide have been approved by FDA for cancer treatment, however both compounds show low hypoxia selectivity and hence, are even cytotoxic to normal (and/or normoxic) cells.9
The second strategy is taking advantage of the (b) hypoxic tumor microenvironment. These include use of recombinant anaerobic bacterium (e.g., clostridium like C. novyi-NT) that are able to release enzymes in hypoxic conditions that convert 5-flourocytosine (5-FC) to 5-fluorouracil (5-FU), but this process is not practically feasible as anaerobic bacterium used in process.10
The third strategy is (c) bioreduction-in this process, hypoxia creates an environment conducive to reductive processes, which results in an important exploitable difference between normal and neoplastic tissues. This observation has leads extensive efforts design and develop bioreductive agents, quinones, nitro derivatives, and N-oxide containing heterocyclic's are either in clinical use or entering in clinical trials.11a Though above different strategies are used to treat hypoxic tumor cells but nothing has come out with great success. So, we hypothesized that hybrid compounds that possess reductive group and cytoxic group would be new generation of bioreductive compounds. Here, we report a novel approach to develop new generation of bioreductive compounds preferential toxic to hypoxic cells relative to their aerobic counter parts.
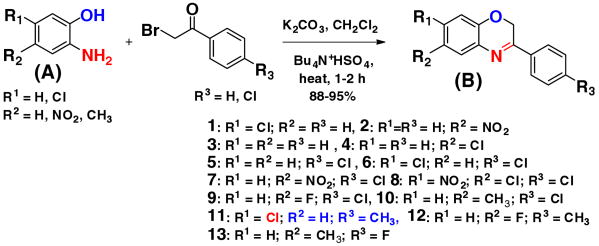
From literature study we found that dihydro 1,3 oxazine derivatives are potent antitumor agents.11b As oxazine moiety shows the antitumor activity, so we envisioned that if we introduce reductive group at any position of oxazine moiety (Fig. 1) that will create hybrid compounds containing both reductive group as well as cytotoxic group would be good precursor for novel hypoxia targeted compounds for cancer therapeutics.
Figure 1.
Synthesis of 2H-benzo [b][1,4] oxazine derivatives.
For proof of the concept, we synthesized a small library of 2H-benzo[b][1,4] oxazine derivatives (Fig. 1) and their cytotoxicity activity were tested against HepG2 hypoxic and normoxic cell. The 2H-benzo[b][1,4] oxazines were synthesized by modifying the existing methods.12 To a solution of 2-aminophenol (A) (0.001 mol) in dichloromethane (40 mL) aqueous potassium carbonate (20% w/v) and tetrabutylammonium hydrogen sulfate (0.0005 mol) was added and mixture was stirred for 2 h at room temperature. After 2 h, 2-bromo-4-chloroacetophenone (0.01 mol) in 20 mL dichloromethane was added drop-wise through a course of 15 min and the resultant mixture was refluxed till completion for 4–6 h. The organic layer was extracted with dichloromethane and dried over sodium sulfate evaporated in vacuum to give crude solid product. The solid was then recrystallized with hot ethanol to get pure yield 87–95% (B). New compounds are characterized by 1H, 13CNMR and HRMS (see the Supplementary data) and known compounds compared with existing analytical data's.
After synthesizing small oxazine library, their biological activity was tested in tumor cell lines under hypoxia and normoxic conditions. For this purpose, first we measured hypoxia inducible genes, after exposing cell to hypoxic conditions (Supplementary data (SI) Fig. 2 for VEGF) like EGF, VEGF and others, then we tested the efficacy of our compounds (a) on tumor hypoxia cells and (b) tumor normoxic cells and measure the effects of the compound 10 on hypoxia inducible genes (HIF-1α, P21 and VEGF).
First we evaluated the cytotoxicity of the compounds 1–10 (Table 1) on HepG2 cells by MTT assay13,14,19 (Supplementary data, Table 1 and Fig. 3). Out of 10 compounds, the compounds 3, 4, 6 and 7 is found to be significantly toxic to hypoxic exposure than to normoxic exposure. Compound 10 are minimally toxic to normoxic cells (SI-Fig. 3 and Table 1) and also less toxic to hypoxic cells. Other compounds 1, 2, 5, 8, and 9 are toxic to both normoxic and hypoxic cells (Supplementary data Fig. 3). Besides MTT assay, clonogenic assay under normoxic and hypoxic condition was performed for selected set of compounds to verify their effects.19
Table 1.
Inhibitory concentration values (MTT assay) in HepG2 cells
| Compound number | IC50 (normoxia) (μM) | IC50 (hypoxia) (μM) |
|---|---|---|
| 3 | 87 ± 1.3 | 10 ± 1.4 |
| 4 | 78 ± 1.2 | 30 ± 2.4 |
| 6 | 88 ± 2.4 | 28 ± 1.6 |
| 7 | 78 ± 1.4 | 20 ± 1.6 |
| 10 | >600 μM | 87 ± 1.8 |
| 11 | NA | 10 ± 3.7 |
| 12 | NA | 100 ± 2.2 |
| 13 | NA | NA |
The clonogenic assays15 (Fig. 4 in Supplementary data) correlate with the MTT results (Fig. 3 in Supplementary data). The colony count from the clonogenic assays show that both compounds 3 and 7 are minimally toxic to normoxic cells at concentrations ∼10 μM(compound 3–105% of colonies relative to controls and compound 7–110% relative to controls are alive). In hypoxic conditions, the cytotoxicity is significant for compound 3 and 7–71% and 28% of colonies are dead, respectively (p < 0.001, comparing this to control treated cells; also see Table 1). However, at 50 μM concentration of drug(s), there is slight cytotoxicity to normoxic cells (compound 3–12% and compound 7–11% of cells are dead compared with controls). In hypoxic conditions, virtually all the cells are dead (compound 3–98%; compound 7–83% dead; p < 0.00001, comparing this to control treated hypoxic conditions, (Fig. 4B in Supplementary data). Compound 10 is non-toxic to normoxic HepG2 cells up to concentrations ∼400 μM (Fig. 4C in Supplementary data).
However, as shown in (Fig. 4D Supplementary data), there is clear cytotoxicity in hypoxic cells. Despite the fact that the IC50 for 10 in hypoxic conditions remains high (87 ± 1.8 μM) this compound would be ideal for lead optimization. The absence of toxicity in normoxic cells is a crucial feature for these compounds and based on this premise, we will be able to lead optimize its potency in hypoxic conditions.
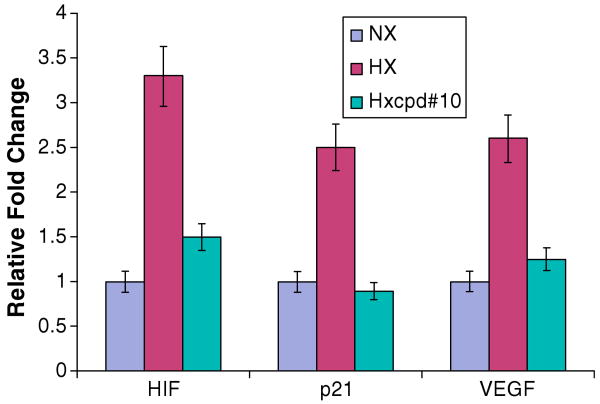
After getting our lead molecule 10, we tried to evaluate the effect of 10 on expression of hypoxic inducible genes. On treatment of sublethal dosage (20 μM) compound#10, it was found to down regulate expression of HIF-lα, P21, VEGF (Fig. 2). HepG2 cells were transiently exposed to hypoxic conditions and the inductions of hypoxic markers were confirmed by Real time PCR (Supplementary Fig. 1).16–19 Nx = Normoxic, Hx = Hypoxic and Hx compound = Hypoxic with compound.
Figure 2.
Quantitative PCR of hypoxia markers.
After having our lead compound 10 in hand, we further explored to study the structure–activity relationship of different functional groups in different positions and to synthesize more active compounds. We synthesized compound 11, 12, 13 (Fig. 1). The cytotoxicity activity of these compounds 11, 12 and 13 was tested on HepG2 tumor cells on both hypoxic and normoxic condition (Table 1 and Supplementary Fig. 3). To our surprise only by changing chloro and methyl group in different position in the phenyl group activity of compound 11 increased approximately nine times IC50 was obtained 10 μM scale (See SI-Fig. 3 and Table 1). Based on these results, we are in the process to explore the molecular mechanism of action of oxazine derivatives compound's on hypoxia tumor cells.
In conclusion, we synthesized a small library of 2H-benzo[b][1,4] oxazine derivatives and their cytotoxicity activity were tested against hypoxic and normoxic cell and most importantly showed that these oxazine derivative compounds indeed effects in hypoxia induced gene (HIF-1α, P21 and VEGF). It is feasible to develop 1,4-oxazine analogs for the purposes of bioreductive and oxidative biotransformation in hypoxic cells. In particular, in the context of cancer chemotherapy, this approach is likely to lead to active compounds. Alterations of the halogen positioning or other groups in the benzyl and/or phenyl ring can yield better, perhaps more potent compounds both as therapeutic and Positron Emission Tomography (PET) or SPECT imaging agents.
Supplementary Material
Acknowledgments
B.C.D. acknowledges Professor E.R. Stanley, Professor Susan B. Horwitz and Professor M. Donald Blaufox for their support and encouragement and AECOM start up funding. All authors are thankful to Dr. S. M. Mahalingam for his help in manuscript preparation.
Footnotes
Supplementary data: Supplementary data (biological and chemical experimental procedures, spectroscopic data, and NMR spectra for all new compounds) associated with this article can be found, in the online version, at doi:10.1016/j.bmcl.2009.05.110.
References and notes
- 1.Dewhirst MW, Cao Y, Moeller B. Nat Rev Cancer. 2008;8:425. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bristow RG, Hill RP. Nat Rev Cancer. 2008;8:180. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 3.Bache M, Kappler M, Said HM, Staab A, Vordermark D. Curr Med Chem. 2008;15:322. doi: 10.2174/092986708783497391. [DOI] [PubMed] [Google Scholar]
- 4.Marcu L, Olver I. Curr Clin Pharmacol. 2006;1:71. doi: 10.2174/157488406775268192. [DOI] [PubMed] [Google Scholar]
- 5.Rooney PH, Telfer C, McFadyen MC, Melvin WT, Murray GI. Curr Cancer Drug Targets. 2004;4:257. doi: 10.2174/1568009043333014. [DOI] [PubMed] [Google Scholar]
- 6.Albertella MR, Loadman PM, Jones PH, Phillips RM, Rampling R, Burnet N, Alcock C, Anthoney A, Vjaters E, Dunk CR, Harris PA, Wong A, Lalani AS, Twelves CJ. Clin Cancer Res. 2008;14:1096. doi: 10.1158/1078-0432.CCR-07-4020. [DOI] [PubMed] [Google Scholar]
- 7.Papadopoulos KP, Goel S, Beeram M, Wong A, Desai K, Haigentz M, Milian ML, Mani S, Tolcher A, Lalani AS, Sarantopoulos J. Clin Cancer Res. 2008;14:7110. doi: 10.1158/1078-0432.CCR-08-0483. [DOI] [PubMed] [Google Scholar]
- 8.Patterson AV, Ferry DM, Edmunds SJ, Gu Y, Singleton RS, Patel K, Pullen SM, Hicks KO, Syddall SP, Atwell GJ, Yang S, Denny WA, Wilson WR. Clin Cancer Res. 2007;13:3922. doi: 10.1158/1078-0432.CCR-07-0478. [DOI] [PubMed] [Google Scholar]
- 9.Duan JX, Jiao H, Kaizerman J, Stanton T, Evans JW, Lan L, Lorente G, Banica M, Jung D, Wang J, Ma H, Li X, Yang Z, Hoffman RM, Ammons WS, Hart CP, Matteucci M. J Med Chem. 2008;51:2412. doi: 10.1021/jm701028q. [DOI] [PubMed] [Google Scholar]
- 10.Brown NL, Lemoine NR. Methods Mol Med. 2004;90:451. doi: 10.1385/1-59259-429-8:451. [DOI] [PubMed] [Google Scholar]
- 11.(a) Cerecetto H, Gonzalez M, Lavaggi ML, Azqueta A, Cerain AL, Monge M. J Med Chem. 2005;48:21. doi: 10.1021/jm0492150. [DOI] [PubMed] [Google Scholar]; (b) Kuehne ME, Konopka EA. J Med Pharm Chem. 1962;51:257. doi: 10.1021/jm01237a005. [DOI] [PubMed] [Google Scholar]
- 12.Shridhar DR, Reddy CV, Sastry OP, Bansal OP, Rao PP. Synthesis. 1981:912. [Google Scholar]
- 13.Wang L, Gao J, Dai W, Lu L. J Biol Chem. 2008;283:25928. doi: 10.1074/jbc.M801326200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unruh A, Ressel A, Mohamed HG, Johnson RS, Nadrowitz R, Richter E, Katschinski DM, Wenger RH. Oncogene. 2003;22:3213. doi: 10.1038/sj.onc.1206385. [DOI] [PubMed] [Google Scholar]
- 15.Wu K, Wang C, D'Amico M, Lee RJ, Albanese C, Pestell RG, Mani S. Mol Cancer Ther. 2002;1:695. [PubMed] [Google Scholar]
- 16.Belaiba RS, Bonello S, Zahringer C, Schmidt S, Hess J, Kietzmann T, Gorlach A. Mol Biol Cell. 2007;18:4691. doi: 10.1091/mbc.E07-04-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori T, Sasaki J, Aoyama Y, Sera T. Nucleic Acids Symp Ser (Oxf) 2008:187. doi: 10.1093/nass/nrn095. [DOI] [PubMed] [Google Scholar]
- 18.Wang GL, Jiang BH, Rue EA, Semenza GL. Proc Natl Acad Sci USA. 1995;92:5510. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Cell culture and cytotoxicity assays: HepG2 cells (passage 2 from frozen stock) were cultured in RPMI 1640 containing 10% FBS. The tests were performed in 96-well plates with stock cells split equally into duplicate plates–one for normoxic and the other for hypoxic conditions. The drug(s) or vehicle (0.1% DMSO) was applied to the cells in log-exponential growth phase under normoxic conditions (5% CO2; 37 °C) for 48 h or under normoxic conditions (22 h) followed by 2 h hypoxic conditions (1% O2, 5% CO2, 94% N2 at 37 °C) then followed by reoxygenation for 24 h. For the hypoxia experiments, we used a glove chamber with two-way pressure value and one-way N2 inflow. Hypoxia conditions were met using methylene blue dye indicator (i.e., 0.015% methylene blue, 6% dextrose, 6 mM NaOH) which correlated with the O2 pressure in the glove box when measured continuously with a Clark-type electrode. At 48 h, a tetrazolium component (MTT) assay (Promega, Madison, WI; Cell Titer 96 Non-Radioactive Cell Proliferation Assay) was performed following an established protocol in the laboratory.; (b) HepG2 clonogenic assays: 3.5 × 103 per well were seeded in 6-well plates using RPMI1640 and 10% FBS. After 24 h of growth at 37 °C, the cells were exposed to vehicle or drug(s). After 10 days incubation with drug(s) or control, the wells were washed with PBS twice, then fixed in 10% acetic acid for 10 min, then stained with crystal violet for 10 min, and finally rinsed with distilled water at room temperature. The colonies (>50 μm likely representing >50 cells) were then visually counted in randomly chosen 1-mm × 1-mm grid repeated three times. Statistical analyses of the mean number of colonies were performed by using the Mann–Whitney t test, with significant differences established as P < 0.05 as previously published by our laboratory. For the hypoxia conditions, after 24 h growth in normoxic conditions as above, the vehicle or drug(s) treated cells were exposed to hypoxic environment (1% O2, 5% CO2, 94%N2 at 37 °C) for 5 h, followed by re-oxygenation (5% CO2; 37 °C) for the remaining 10 days.; (c) Quantitative RT-PCR: Total cellular RNA was extracted using TRIzol reagent (Invitrogen) according to manufacturer's instructions. Reverse transcription was first carried out to generate cDNA using total RNA with random oligodT primers (Invitrogen), dNTPs, and SuperScript III reverse transcriptase (Invitrogen). Real-time polymerase chain reaction (RT-PCR) was performed using the generated cDNA. Quantitative assessment of DNA amplification was carried out via FAM™ Dye and MGB probe using the PRISM 7700 Sequence Detector (Applied Biosystems, Foster City, CA) and specific primers for human VEGF(Hs00900054_m1)(amplicon length ∼60 bp)HIF-1(Hs00936372_m1) (∼72 bp), p21 (Hs01121168_m1)(∼72 bp) and β-actin (NM_001101.2; Human ACTB Endogenous Control Probe.(∼171 bp).Primers were synthesized by IDT Technologies (Coralville, IA).The following cycling parameters were used for the PCR: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. A final heating step up to 95 °C was performed to obtain melting curves of the final PCR products. The fluorescence threshold cycle value (Ct) was obtained for each curve and normalized to that obtained for the GAPDH housekeeping gene in the same sample to normalize for discrepancies in sample loading. The differences in Ct values between treated and control samples was then computed and exponentially multiplied to the base of 2 to obtain relative differences in expression levels. All experiments were carried out in duplicates and independently performed at least three times.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.