Abstract
The transcriptional response to hypoxia is primarily mediated by two hypoxia-inducible factors – HIF-1α and HIF-2α. While these proteins are highly homologous, increasing evidence suggests they have unique transcriptional targets and differential impact on tumor growth. Furthermore, non-transcriptional effects of the HIF-α subunits, including effects on the Notch and c-Myc pathways, contribute to their distinct functions. HIF-2α transcriptional targets include genes involved in erythropoiesis, angiogenesis, metastasis, and proliferation. Therefore, HIF-2α contributes significantly to both normal physiology as well as tumorigenesis. Here we summarize the function of HIF-2α during development as well as its contribution to pathologic conditions such as tumors and vascular disease.
Keywords: hypoxia, erythropoiesis, development, renal carcinoma, neuroblastoma
Introduction
Cells, tissues, and organisms experience reduced oxygen (O2) tension, or “hypoxia,” under both physiologic and pathologic conditions.1 During the rapid cell proliferation associated with early embryonic development, physiologic hypoxia stimulates the development of the hematopoietic and circulatory systems.2 Rapidly growing tumors also experience hypoxia due to insufficient and defective vascularization.3 The hypoxia-inducible factors (HIFs) mediate the adaptive response to decreased O2 availability at the cellular and organismal level. HIFs are heterodimeric proteins belonging to the basic helix-loop-helix (bHLH)/Per-Arnt-Sim (PAS) domain family of transcription factors.4 Under normoxic conditions the HIF-α subunits are hydroxylated on key proline residues, which allows for recognition by the von Hippel-Lindau (pVHL) tumor suppressor, the substrate recognition component of an E3 ubiquitin ligase complex that targets HIF-α for proteosomal degradation.5-9 In hypoxic conditions, these hydroxylation events are inhibited allowing the α subunits to enter the nucleus and bind the stable β subunit or aryl hydrocarbon receptor nuclear translocator (ARNT), forming a functional transcriptional complex. Among HIF transcriptional targets are genes involved in glycolysis, angiogenesis, erythropoiesis, cell death and differentiation.10 HIF-1α and HIF-2α are the most extensively studied of the three HIF-α isoforms. While these proteins share significant sequence homology, they have unique tissue distributions, embryonic deletion phenotypes and effects on tumorigenesis.
Tissue Distribution
HIF-2α is also known as endothelial PAS domain protein 1 (EPAS1), HIF-like factor (HLF), HIF-related factor (HRF) or member of PAS superfamily 2 (MOP2).11-14 While it was initially detected in endothelial cells, HIF-2α is expressed in the parenchyma and interstitial cells of multiple organs.11,15,16 HIF-2α stabilization can be observed by immunohistochemistry in renal interstitial cells, hepatocytes, epithelial cells of the duodenum, cardiomyocytes, and astrocytes in rodents exposed to systemic hypoxia.16 While HIF-2α is stabilized at higher O2 tensions than HIF-1α in vitro, HIF-2α was not detected under normoxic conditions in the organs examined.16-18 Accumulation of HIF-2α is predominantly due to post-translational regulation as mRNA levels are not significantly induced under hypoxia.16 Interestingly, HIF-2α mRNA levels are particularly high in tissues that are important for the systemic delivery of O2, for example the lung, heart and endothelium.11,16 In the lung, HIF-2α protein is stabilized in type II pneumocytes and pulmonary endothelial cells in response to hypoxia while HIF-1α is not detectable.16,19 HIF-2α transcript levels are also developmentally regulated in the lung, with an induction in mRNA levels occurring during the late stages of fetal development.19 Neural crest derivatives, which synthesize hormones important in modulating vascular tone and cardiac output, also express HIF-2α.15 In particular, HIF-2α is highly expressed in the organ of Zuckerkandl, an important site for catecholamine synthesis during embryonic development. Analysis of HIF-α staining patterns in ischemic myocardium showed that acute induction of both α subunits occurs primarily in the region surrounding the infarcted tissue, including cardiomyoctes, endothelial cells, and macrophages.20 With time HIF-2α also becomes detectable in more distant areas of myocardium. These distinct spatio-temporal patterns of HIF expression suggest that the two α subunits may have unique and complementary roles in the adaptive response to tissue hypoxia.
Transcriptional targets
Both HIF-α subunits dimerize with ARNT and recognize identical DNA sequences (5′-CGTG-3′) found within the promoters or enhancers of target genes, termed hypoxia-response elements (HREs). Despite these similarities, expression profiling and functional studies have revealed that the HIF-α subunits regulate both shared and unique target genes.21-25 For instance, HIF-1α uniquely stimulates the expression glycolytic enzymes, such as phosphoglycerate kinase (PGK) and lactate dehydrogenase (LDH-A), carbonic anydrase-9 (CA IX), and the pro-apoptotic gene BNIP-3.21,23,24,26 By contrast, the embryonic transcription factor Oct-4 (Pou5f1, Oct-3/4), CYCLIN D1, TWIST1, transforming growth factor-α (TGF-α), and erythropoietin (EPO) are upregulated under hypoxia in a HIF-2α-dependent manner.22,24,27-31 A third group of genes, including vascular endothelial growth factor (VEGF), adrenomedullin (ADM) and glucose transporter 1 (GLUT-1) are regulated by both α subunits.18,21
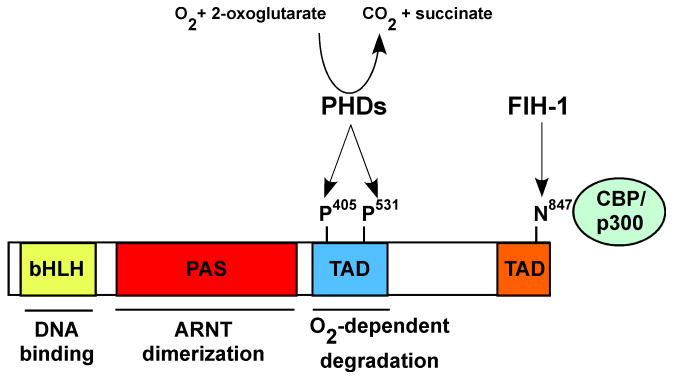
The HIF-α subunits display the highest degree of sequence homology in the bHLH (85%), PAS-A (68%) and PAS-B (73%) domains (Figure 1).11 HIF-α subunits also contain two transcriptional activation domains (TADs) termed the N-terminal (N-TAD) and C-terminal (C-TAD).32 The C-TAD recruits the transcriptional co-activators Creb binding protein CBP/p300 in an O2-dependent fashion. During normoxic conditions, a conserved asparigine residue in the C-TAD is hydroxylated by the factor inhibiting HIF-1α (FIH1), preventing interaction with CBP/p300.33,34 Domain-swapping and chromatin immunoprecipitation studies have shown that selective HIF target gene activation is not based on the unique DNA binding abilities of these factors. Both HIF-α subunits can bind to the endogenous HREs of hypoxia-responsive genes; however, binding is not sufficient for target gene activation.35,36 In contrast, C-terminal regions of each protein are required for specificity. For select targets, the N-TAD is sufficient to confer target gene specificity and replacement of the HIF-2α N-TAD with the analogous region of HIF-1α switches the specificity of these proteins.36 In contrast, targets such as prolyl hydroxylase-3 (PHD3) require additional regions of the HIF-2α protein to be induced.35
Figure 1.
Structure of HIF-2α including the bHLH DNA binding domain, PAS domain and transactivation domains (TADs). Prolyl hydroxylases (PHDs) hydroxylate proline residues 405 and 531 in the oxygen-dependent degradation (ODD) of HIF-2α under normoxic conditions, targeting it for degradation by the proteosome. In addition, hydroxylation of asparagine 847 in the C-terminal TAD by factor-inhibiting HIF (FIH-1) inhibits interaction with the co-activators CBP/p300.
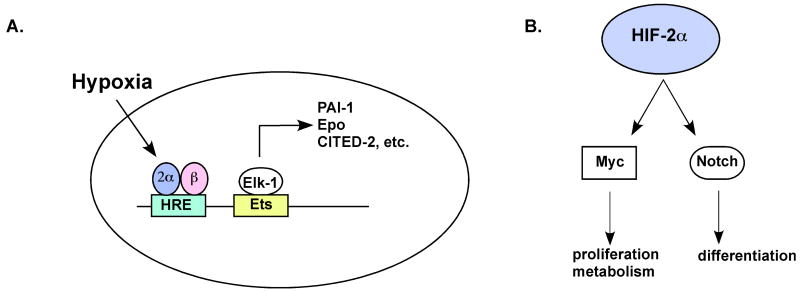
Cooperation with other transcription factors may also be required for maximal and cell type specific upregulation of HIF target genes (Figure 2). Multiple Ets family transcription factors can cooperate with HIF-2α for hypoxic gene induction.36-38 For example, Ets-1 and HIF-2α synergistically activate the expression of VEGF receptor-2 (VEGFR-2 or Flk-1).37 Interestingly, physical interaction between HIF-2α and Ets-1 is mediated by the carboxy-terminus of HIF-2α. Another Ets family transcription factor, Elk-1, cooperates with HIF-2α to activate the target genes CITED-2, EPO, insulin-like growth factor binding protein-3 (IGFBP3), and PAI-1.36,38 Several other genes preferentially regulated by HIF-2α in MCF7 breast cancer cells exhibit Ets binding sites adjacent to HREs, suggesting this may be a common mechanism for regulating target gene selectivity by HIF-2α.38 The NF-κB essential modulator (NEMO) was also identified as a HIF-2α-interacting protein through yeast-two hybrid screening. NEMO physically interacts with HIF-2α specifically, but not HIF-1α, and enhances HIF-2α transcriptional activity in reporter assays. However, the target gene selectivity and requirement for NEMO at endogenous HIF-2α responsive genes has not been explored.
Figure 2.
A. Transcription-dependent functions. Ets family transcription factors have been shown to cooperate specifically with HIF-2α to activate select target genes (e.g. PAI-1, EPO, CITED-2). B. Transcription-independent activities. HIF-2α regulates the activity of transcription factors such as Notch and c-Myc, thereby influencing cell proliferation, metabolism and differentiation.
Loss of Function Phenotypes
Targeted disruption of HIF-2α leads to embryonic lethality between E9.5 and E13.5, with divergent phenotypes depending on the genetic background of the mice (Table 1). Tian H. et. al found that mice lacking HIF-2α died at mid-gestation with bradycardia and reduced levels of noradrenaline, suggesting that HIF-2α regulates embryonic catecholamine synthesis in response to physiologic hypoxia.15 Indeed, HIF-2α is expressed in neural crest-derivatives that synthesize catecholamines and tyrosine hydroxylase, a critical enzyme in catecholamine synthesis that is hypoxia inducible.39 A fraction of these embryos could be rescued to birth by exogenous administration of D,L-threo-3,4-dihydroxyphenylserine (DOPS), a metabolic intermediate that can be directly converted to noradrenaline.15 Nevertheless, homozygous pups died soon after birth. This is due to impaired fetal lung maturation, leading to perinatal lethality and respiratory distress syndrome (RDS). 19 HIF-2α mediated upregulation of VEGF appears to be required for stimulating surfactant production by type II pneumocytes of the lung. Inhibition of VEGF receptor signaling using neutralizing anti-Flk1 antibodies recapitulated the lung phenotype of HIF-2α deficient mice and intra-amniotic VEGF administration improved lung function and survival of preterm mice. In an independent HIF-2α knockout model generated by Peng J et. al, vascular defects in the yolk sac and embryos of mutant mice were observed.40 HIF-2α also appears to play a role in regulating blood vessel dynamics during pathologic conditions. Heterozygosity for HIF-2α can be protective against pulmonary hypertension and right ventricular hypertrophy induced by chronic hypoxia. This effect may be mediated by hypoxic upregulation of endothelin-1 (ET-1), which causes pulmonary vasoconstriction and remodeling of pulmonary vasculature. Mice heterozygous for HIF-2α did not upregulate ET-1 or increase circulating levels of catecholomines in response to hypoxia.41
Table 1.
Summary of HIF-2α knockout phenotypes.
| Study | Phenotype |
|---|---|
| Tian H, et. al, 1998 | Bradycardia, reduced noradrenaline, partial rescue with metabolic intermediate |
| Peng J, et. al, 2000 | Vascular defects in yolk sac and embryo |
| Compernolle V, et. al, 2002 | Reduced surfactant production, respiratory distress syndrome |
| Scortegagna M, et. al, 2003 | Pancytopenia, hepatic steatosis, cardiac hypertrophy, retinopathy, increase oxidative stress |
| Gruber M, et. al, 2007 | Global postnatal deletion, anemia rescued by exogenous Epo |
By crossing mice in different genetic backgrounds, Scortegagna M. et. al were able to obtain a small fraction of viable Hif-2α-null adult mice. These mice exhibit multiorgan dysfunction, including pancytopenia, hepatic steatosis, cardiac hypertrophy and retinopathy, associated with increased oxidative stress. This study suggested a novel role for HIF-2α in regulating the expression of antioxidant enzymes such as catalase, superoxide dismutase (SOD), and glutathione peroxidase.42 Bone marrow transplant experiments demonstrated that the hematopoietic defect in these mice is non-cell autonomous.43 Reconstitution of lethally irradiated wild-type recipients with hematopoietic stem cells from Hif-2α-/- mice corrected the pancytopenia, suggesting that the phenotype was mediated by the microenvironment of the cells. Hif-2α-/- mice had inappropriately low levels of renal erythropoietin (Epo) mRNA and failed to upregulate Epo in response to intermittent hypoxia, suggesting that decreased Epo production was responsible for defective hematopoiesis. Notably, administration of exogenous Epo corrected the pancytopenic phenotype.44
Given these disparate phenotypes for global HIF-2α deletion, the differential requirements for the α subunits during development remained unclear. As a genetic test of the ability of HIF-2α to compensate for HIF-1α function, we “knocked-in” the HIF-2α coding sequence into the HIF-1α locus, thereby expanding HIF-2α expression to all tissues in which HIF-1α is normally expressed.45 In addition to demonstrating that the HIFs are not redundant proteins, as expanded HIF-2α expression was unable to rescue lack of HIF-1α, this study revealed a gain-of-function phenotype for HIF-2α knock-in, due to its unique ability to regulate Oct-4 and Tgf-α. 29 Increased Oct-4 expression in mice homozygous for the HIF-2α knock-in allele led to early embryonic lethality. Tumors derived from homozygous HIF-2α knock-in embryonic stem (ES) cells displayed increased growth and angiogenesis in xenograft assays.29,45 These tumors were also characterized by an increased fraction of undifferentiated cells, which could be reversed by knockdown of Oct-4.
Using a conditional deletion system, we studied the function of HIF-2α postnatally independent of the confounding requirements for HIF-2α during development. Acute deletion of HIF-2α using a tamoxifen-inducible ubiquitously expressed Cre leads to anemia associated with decreased levels of circulating Epo, demonstrating that HIF-2α is the physiologic regulator of Epo production in adult mice.31 Deletion of HIF-2α also impaired stress-induced Epo synthesis and erythropoiesis. Prior to the onset of renal Epo production, the liver is the major source of systemic Epo in neonatal mice. Consistent with the global deletion of HIF-2α, hepatocyte specific deletion using an albumin-Cre reveals that in neonatal mice hepatic Epo production is dependent on HIF-2α activity. Moreover, HIF-2α binds the endogenous Epo 3′ enhancer HRE in hepatocytes.31,46 Local synthesis of Epo by astrocytes in the central nervous system also appears to be regulated by HIF-2α and may be important for survival of neurons during periods of ischemia.47
O2 transport by red blood cells is mediated by reversible binding to iron present in the hemoglobin molecule. Therefore, organisms must coordinate erthyropoiesis with iron availability. HIFs regulate the expression of several genes involved in iron homeostasis, such as transferrin.48 Iron regulatory proteins (IRPs), on the other hand, control iron metabolism through post-transcriptional regulation of select mRNAs. IRPs regulate translation of these mRNAs by binding to iron response elements (IREs) present in untranslated regions (UTRs) of the transcript.49 A conserved IRE was recently identified in the 5′UTR of HIF-2α.50 This element promotes HIF-2α protein translation under conditions of increased iron availability, thereby coupling erythropoiesis with iron homeostasis.
Role in Tumorigenesis
HIF-2α is frequently expressed in solid tumors. In particular, the role of HIF-2α in the pathogenesis of renal clear cell carcinoma (RCC) has been most extensively studied. Loss of VHL is associated with the autosomal dominant hereditary cancer syndrome von Hippel-Lindau disease, in which patients inherit one mutant allele of VHL through the germline. Somatic mutation, deletion or epigenetic silencing of the remaining allele leads to the formation of highly vascular tumors in specific tissues, including hemangioblastomas of the central nervous system, renal clear cell carcinomas, and pheochromocytomas.51 Loss of pVHL function is also associated with the majority (80-90%) of sporadic renal clear cell carcinoma.52 In the absence of pVHL, the HIF-α subunits accumulate, leading to a constitutive hypoxia-like pattern of gene expression. Examination of kidneys from patients with VHL renal disease suggested that HIF activation in renal epithelium precedes the development of overt malignancy.24,53 HIF-2α staining was absent in early lesions, but frequently observed in overt carcinomas and cell lines derived from renal tumors.
Restoration of pVHL protein in VHL-null renal carcinoma cell lines inhibits tumor growth in xenograft models 54,55. Given the highly vascular lesions associated with VHL disease, it was hypothesized that dysregulated HIF activity stimulated the growth of VHL-deficient tumors. Surprisingly, expression of a normoxically stable HIF-1α protein further inhibited xenograft growth in pVHL-rescued tumors while expression of stabilized HIF-2α promoted it, demonstrating that suppression of HIF-2α activity is critical for tumor suppression by pVHL.54,55 Elimination of HIF-2α by shRNA-mediated knockdown similarly inhibited the growth of VHL-null renal cell lines in xenograft experiments.56 These studies were amongst the first to suggest differential abilities of the HIF-α subunits to promote tumor growth and prompted investigation into the differences between the α subunits.
VHL disease is characterized by striking genotype-phenotype correlations, with select point mutations conferring increased risk of renal carcinoma in patients.52 Introduction of pVHL mutations (Y98N, Y112N, W117R, R167Q) that are associated with a high risk of renal carcinoma (type 2B) into VHL-deficient cell lines leads to increased normoxic HIF-1α and HIF-2α stabilization compared to wild-type VHL.57,58 Cells harboring these mutations also display increased proliferation in serum-depleted media and growth in orthotopic xenograft assays, which can be reversed by shRNA-mediated knockdown of HIF-2α.57 The R167Q mutation, in particular, uniquely disrupts regulation of HIF-2α in embryonic stem cells.58 In contrast, type 2A mutations (Y98H, Y112H, A149T, T157I), which have a lower risk for renal tumors, display reduced levels of normoxic HIF-α accumulation and growth in xenograft assays compared to type 2B mutants.57 In contrast to tumor-associated VHL mutations, the R200W mutation in pVHL leads to Chuvash polycythemia, a heritable disease characterized by elevated levels of serum Epo and VEGF. Importantly, mice homozygous for the R200W mutation recapitulate the human disease and display upregulation of HIF-2α-preferential target genes in multiple tissues.59
HIF-2α may also play a role in renal carcinomas caused by mutations in the tumor suppressor tuberous sclerosis-2 (Tsc-2). Cell lines and primary tumors derived from the Eker rat, a model of spontaneous renal carcinoma caused by heterozygous mutation in Tsc-2, display increased levels of HIF-2α protein. High levels of VEGF expression and increased vascularization are features of these tumors as well.60
CYCLIN D1 and TGF-α represent two HIF-2α-regulated genes that could mediate these pro-tumorigenic properties in renal tumors.24,27,28,61 Activation of an autocrine signaling loop through TGF-α mediated stimulation of the epidermal growth factor receptor (EGFR) has been proposed to drive serum-independent growth of renal carcinoma cells.28,62 Indeed, silencing of EGFR in VHL-deficient RCC cell lines impairs proliferation in the absence of serum and growth in xenograft assays.61 Given the challenge of developing drugs that target transcription factors, identifying signaling pathways activated downstream of HIF-2α will be critical for developing therapeutics.
An apparent paradox of constitutive HIF activation in VHL-deficient renal tumors is the ability of FIH-1 to inhibit recruitment of the co-activators CBP/p300 by hydroxylating the C-TAD. Dissection of the relative contributions of the C-TAD and N-TAD of HIF-2α to target gene activation in RCC lines indicates that the C-TAD is largely dispensable for transcriptional activation by HIF-2α.63 Nevertheless, deletion of either the C-TAD or N-TAD impairs tumor formation in nude mice suggesting that subtle changes in gene expression and transcription-independent functions of HIF-2α mediated by these domains could contribute to tumor growth. The HIF-2α C-TAD also appears to be resistant to the effects of FIH-1 in transcriptional activation.
Transcription independent functions of the HIF-α subunits also contribute to their differential activity (Figure 2B). HIF-1α functionally antagonizes c-Myc transcriptional activity under hypoxia, leading to upregulation of the cyclin-dependent kinase inhibitor p21 and cell-cycle arrest.64 In contrast, HIF-2α promotes cell cycle progression by enhancing c-Myc-mediated expression of Cyclin D2 and E2F1 in multiple cell types.65 HIF-2α also enhances c-Myc and Ras induced transformation of primary fibroblasts. Increased expression of c-Myc target genes can also be observed in human tumor samples that solely express HIF-2α.66 HIF-2α-only expressing tumors also exhibit increased proliferation compared to tumors expressing both α subunits. Both HIFs have also been shown to regulate the expression of Mxi1, which inhibits c-Myc activity by competing for Max.67,68 Furthermore, it has been shown that HIF-1α targets c-Myc for proteosomal degradation independently of Mxi1.67 Decreased c-Myc activity in VHL-deficient RCC lines was correlated with reduced mitochondrial DNA content and O2 consumption.
The HIFs can also enhance stabilization of the Notch intracellular domain and expression of Notch target genes, such as Hes1 and Hey2.69 Enhanced Notch signaling under hypoxia blocks myogenic and neuronal differentiation. It will be interesting to compare the relative contributions of the two α subunits in enhancing Notch activity and determine whether this function contributes to tumorigenesis.
In addition to cell intrinsic responses to hypoxia, changes in the tumor microenvironment can significantly impact tumor growth and metastasis. Infiltrating immune cells influence angiogenesis and metastasis by secreting chemokines and matrix-remodeling enzymes.70 HIF-2α is highly expressed in tumor-associated macrophages of human breast carcinomas and correlates with increased micro-vessel density and high tumor grade.71,72 HIF-2α also upregulates several genes that are key mediators of metastasis, including CXCR4, LOX, and TWIST1.30,73,74 The chemokine receptor CXCR4 is a HIF-2α-inducible gene in renal clear cell carcinoma and high levels of expression correlate with reduced patient survival. Lysyl oxidase (LOX) is critical for metastasis of breast cancer xenografts. High levels of LOX expression are correlated with poor metastasis-free and overall survival rates in human breast and head/neck cancers. Recently, a RNAi screen in C. elegans to identify genes in the HIF pathway isolated hlh-8, a homologue of human TWIST1. TWIST1 is a bHLH transcription factor that promotes epithelial-mesenchymal transition and is required for metastasis in murine breast cancer models.75 Subsequent analysis in human tumor cell lines showed that TWIST1 is uniquely regulated by HIF-2α, although direct binding of HIF-2α to TWIST1 regulatory elements has yet to be demonstrated.
A pro-tumorigenic function for HIF-2α has also been demonstrated in neuroblastoma, a childhood malignancy derived from sympathetic neural precursors. Hypoxia alters the phenotypic properties of these tumors, promoting de-differentiation toward a neural crest-like state.76 HIF-2α stabilization can be detected in well-vascularized regions of neuroblastoma patient samples and at higher O2 tensions (5%) in neuroblastoma cell lines than HIF-1α.17 The temporal pattern of HIF-α subunit accumulation is also varied in these cells. HIF-1α is stabilized acutely, while HIF-2α protein remains stabilized over longer periods of hypoxia. Transient knockdown of HIF-2α inhibits growth of neuroblastoma xenografts in nude mice. In addition, high levels of HIF-2α expression in patient samples correlate with advanced clinical stage and poor prognosis.
In contrast to human neuroblastomas, overexpression of Hif-2α in rodent glioma cells has been shown to reduce tumor growth through increased apoptosis, despite enhanced vascularization.77 Teratomas derived from Hif-2α-/- ES cells were also observed to grow faster than control teratomas. To date, this is the only study suggesting that HIF-2α can function as a tumor suppressor and there is a conflicting report on the role of HIF-2α in apoptosis. A previous study of Hif-2α-/- ES cells suggested that hypoxia-induced accumulation of p53 and apoptosis are HIF-1α–dependent and that unlike HIF-1α, deletion of Hif-2α did not protect against hypoxia-induced apoptosis.78 Furthermore, the pro-apoptotic gene BNIP-3 is positively regulated by HIF-1α and can be repressed by HIF-2α in certain cell types23 Collectively, these studies suggest that HIF-2α has cell type-dependent effects on tumor growth and its role in apoptosis is unresolved.
Renal clear cell carcinomas are unique tumors in that the majority of cases have mutations in VHL, resulting in sustained HIF-α accumulation, independent of O2-tension. HIF-1α is ubiquitously expressed and mediates multiple adaptive responses to hypoxia that may inhibit tumor growth when activated constitutively. For instance, HIF-1α inhibits proliferation by antagonizing c-Myc transcriptional activity and reprograms the metabolism of cells to reduce anabolic processes through the upregulation of pyruvate dehydrogenase kinase-1 (Pdk1).79 Reduced growth of Vhl-deficient fibrosarcomas and teratocarcinomas also suggests that HIF-1α can inhibit tumor growth.80,81 HIF-2α, on the other hand, promotes c-Myc activity and expression of cyclin D1, both of which can promote tumor growth (Figure 3). HIF-2α is also stabilized at higher O2 levels (5%) and over prolonged time periods in neuroblastomas. Thus, tissues in which HIF-2α is preferentially stabilized at higher O2 levels may show stronger dependence of this α subunit during tumorigenesis. However, to clearly discern the functional significance of the individual α subunits on tumorigenesis it will be important to use conditional alleles to individually knock-out these genes in mouse models of disease, particularly RCCs and neuroblastomas. An important prerequisite for these studies will be the development of appropriate mouse models of renal tumorigenesis. Conditional deletion of Vhl in the kidney leads to cyst formation, but not frank carcinoma.82 Moreover, the two subunits may also behave differently depending upon whether they are deleted in the tumors themselves or in cells within the tumor microenvironment, such as macrophages or endothelial cells.
Figure 3.
The HIF-α subunits may mediate different adaptive responses to hypoxia. HIF-2α appears to be stabilized at higher O2 levels and displays fewer growth inhibitory properties than HIF-1α Moreover, prolonged accumulation of HIF-2α suggests it could be important for adaptation to chronic hypoxia.
Conclusions
While HIF-1α was the first HIF-α subunit to be identified and has been most extensively studied, recent examination of HIF-2α in the context of both development and tumorigenesis demonstrates significant differences in the biological function of these two proteins. Loss of function studies suggest that the contribution of HIF-2α activity to diseases such as pulmonary hypertension, polycythemia and anemia will be interesting to investigate further. In addition, both HIF-α subunits regulate genes involved in tumor angiogenesis, metastasis, and differentiation, but appear to have cell type specific effects on tumor progression. Given the importance of hypoxia in solid tumors, identification of small molecule inhibitors of the HIF-pathway has been a growing area of interest and several promising compounds have been identified83-85 Understanding the differences between the α subunits and their relative contributions to different tumor types will be important for appropriately testing these compounds for use in cancer therapy.
Abbreviations
- Adm
adrenomedullin
- ARNT
aryl hydrocarbon nuclear translocator
- CA IX
carbonic anhydrase-9
- EPAS1
endothelial PAS domain protein 1
- Epo
erythropoietin
- GLUT-1
glucose-transporter 1
- HIFs
Hypoxia-inducible factors
- HLF
HIF-like factor
- HRE
hypoxia response element
- HRF
HIF-related factor
- IRE
iron response element
- IRP
iron response protein
- LDH-A
lactate dehydrogenase-A
- LOX
lysyl oxidase
- PAS
Per-ARNT-Sim
- PDK1
pyruvate dehydrogenase kinase-1
- PGK
phosphoglycerate kinase
- pVHL
von Hippel-Lindau
- RCC
renal clear cell
- RDS
respiratory distress syndrome
- TGF-α
transforming growth factor-α
- TAD
transactivation domain
- UTR
untranslated region
- VEGF
vascular endothelial growth factor
References
- 1.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 2.Covello KL, Simon MC. HIFs, hypoxia, and vascular development. Curr Top Dev Biol. 2004;62:37–54. doi: 10.1016/S0070-2153(04)62002-3. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 4.Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–61. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 5.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 6.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 8.Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A. 2001;98:9630–5. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–40. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 10.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 11.Tian H, Mcknight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 12.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A. 1997;94:4273–8. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flamme I, Frohlich T, von Reutern M, Kappel A, Damert A, Risau W. HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor-1 alpha and developmentally expressed in blood vessels. Mech Dev. 1997;63:51–60. doi: 10.1016/s0925-4773(97)00674-6. [DOI] [PubMed] [Google Scholar]
- 14.Hogenesch JB, Chan WK, Jackiw VH, Brown RC, Gu YZ, Pray-Grant M, et al. Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway. J Biol Chem. 1997;272:8581–93. doi: 10.1074/jbc.272.13.8581. [DOI] [PubMed] [Google Scholar]
- 15.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–4. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. Faseb J. 2003;17:271–3. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 17.Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–23. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, et al. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood. 1998;92:2260–8. [PubMed] [Google Scholar]
- 19.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–10. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 20.Jurgensen JS, Rosenberger C, Wiesener MS, Warnecke C, Horstrup JH, Grafe M, et al. Persistent induction of HIF-1alpha and -2alpha in cardiomyocytes and stromal cells of ischemic myocardium. Faseb J. 2004;18:1415–7. doi: 10.1096/fj.04-1605fje. [DOI] [PubMed] [Google Scholar]
- 21.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–74. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, et al. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. Faseb J. 2004;18:1462–4. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- 23.Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005;65:3299–306. doi: 10.1158/0008-5472.CAN-04-4130. [DOI] [PubMed] [Google Scholar]
- 24.Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–86. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol Cell Biol. 2006;26:3514–26. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grabmaier K, AdW MC, Verhaegh GW, Schalken JA, Oosterwijk E. Strict regulation of CAIX(G250/MN) by HIF-1alpha in clear cell renal cell carcinoma. Oncogene. 2004;23:5624–31. doi: 10.1038/sj.onc.1207764. [DOI] [PubMed] [Google Scholar]
- 27.Baba M, Hirai S, Yamada-Okabe H, Hamada K, Tabuchi H, Kobayashi K, et al. Loss of von Hippel-Lindau protein causes cell density dependent deregulation of CyclinD1 expression through hypoxia-inducible factor. Oncogene. 2003;22:2728–38. doi: 10.1038/sj.onc.1206373. [DOI] [PubMed] [Google Scholar]
- 28.Gunaratnam L, Morley M, Franovic A, de Paulsen N, Mekhail K, Parolin DA, et al. Hypoxia inducible factor activates the transforming growth factor-alpha/epidermal growth factor receptor growth stimulatory pathway in VHL(-/-) renal cell carcinoma cells. J Biol Chem. 2003;278:44966–74. doi: 10.1074/jbc.M305502200. [DOI] [PubMed] [Google Scholar]
- 29.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–70. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gort EH, van Haaften G, Verlaan I, Groot AJ, Plasterk RH, Shvarts A, et al. The TWIST1 oncogene is a direct target of hypoxia-inducible factor-2alpha. Oncogene. 2007 doi: 10.1038/sj.onc.1210795. [DOI] [PubMed] [Google Scholar]
- 31.Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A. 2007;104:2301–6. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Rourke JF, Tian YM, Ratcliffe PJ, Pugh CW. Oxygen-regulated and transactivating domains in endothelial PAS protein 1: comparison with hypoxia-inducible factor-1alpha. J Biol Chem. 1999;274:2060–71. doi: 10.1074/jbc.274.4.2060. [DOI] [PubMed] [Google Scholar]
- 33.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–71. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–86. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau KW, Tian YM, Raval RR, Ratcliffe PJ, Pugh CW. Target gene selectivity of hypoxia-inducible factor-alpha in renal cancer cells is conveyed by post-DNA-binding mechanisms. Br J Cancer. 2007;96:1284–92. doi: 10.1038/sj.bjc.6603675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu CJ, Sataur A, Wang L, Chen H, Simon MC. The N-Terminal Transactivation Domain Confers Target Gene Specificity of Hypoxia Inducible Factors HIF-1{alpha} and HIF-2{alpha} Mol Biol Cell. 2007 doi: 10.1091/mbc.E06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T, et al. Cooperative interaction of hypoxia-inducible factor-2alpha (HIF-2alpha) and Ets-1 in the transcriptional activation of vascular endothelial growth factor receptor-2 (Flk-1) J Biol Chem. 2003;278:7520–30. doi: 10.1074/jbc.M211298200. [DOI] [PubMed] [Google Scholar]
- 38.Aprelikova O, Wood M, Tackett S, Chandramouli GV, Barrett JC. Role of ETS transcription factors in the hypoxia-inducible factor-2 target gene selection. Cancer Res. 2006;66:5641–7. doi: 10.1158/0008-5472.CAN-05-3345. [DOI] [PubMed] [Google Scholar]
- 39.Norris ML, Millhorn DE. Hypoxia-induced protein binding to O2-responsive sequences on the tyrosine hydroxylase gene. J Biol Chem. 1995;270:23774–9. doi: 10.1074/jbc.270.40.23774. [DOI] [PubMed] [Google Scholar]
- 40.Peng J, Zhang L, Drysdale L, Fong GH. The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc Natl Acad Sci U S A. 2000;97:8386–91. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, et al. Heterozygous deficiency of hypoxia-inducible factor-2alpha protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest. 2003;111:1519–27. doi: 10.1172/JCI15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet. 2003;35:331–40. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 43.Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood. 2003;102:1634–40. doi: 10.1182/blood-2003-02-0448. [DOI] [PubMed] [Google Scholar]
- 44.Scortegagna M, Ding K, Zhang Q, Oktay Y, Bennett MJ, Bennett M, et al. HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood. 2005;105:3133–40. doi: 10.1182/blood-2004-05-1695. [DOI] [PubMed] [Google Scholar]
- 45.Covello KL, Simon MC, Keith B. Targeted replacement of hypoxia-inducible factor-1alpha by a hypoxia-inducible factor-2alpha knock-in allele promotes tumor growth. Cancer Res. 2005;65:2277–86. doi: 10.1158/0008-5472.CAN-04-3246. [DOI] [PubMed] [Google Scholar]
- 46.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–77. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci. 2006;26:9471–81. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rolfs A, Kvietikova I, Gassmann M, Wenger RH. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem. 1997;272:20055–62. doi: 10.1074/jbc.272.32.20055. [DOI] [PubMed] [Google Scholar]
- 49.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–97. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez M, Galy B, Muckenthaler MU, Hentze MW. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nat Struct Mol Biol. 2007;14:420–6. doi: 10.1038/nsmb1222. [DOI] [PubMed] [Google Scholar]
- 51.Kaelin WG., Jr Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2:673–82. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- 52.Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 53.Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM, et al. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell. 2002;1:459–68. doi: 10.1016/s1535-6108(02)00071-5. [DOI] [PubMed] [Google Scholar]
- 54.Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD. The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002;1:247–55. doi: 10.1016/s1535-6108(02)00044-2. [DOI] [PubMed] [Google Scholar]
- 55.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–46. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 56.Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1:E83. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li L, Zhang L, Zhang X, Yan Q, Minamishima YA, Olumi AF, et al. Hypoxia-inducible factor linked to differential kidney cancer risk seen with type 2A and type 2B VHL mutations. Mol Cell Biol. 2007;27:5381–92. doi: 10.1128/MCB.00282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rathmell WK, Hickey MM, Bezman NA, Chmielecki CA, Carraway NC, Simon MC. In vitro and in vivo models analyzing von Hippel-Lindau disease-specific mutations. Cancer Res. 2004;64:8595–603. doi: 10.1158/0008-5472.CAN-04-1430. [DOI] [PubMed] [Google Scholar]
- 59.Hickey M, Rathmell WK, Lam JC, Bezman NA, Simon MC. von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2a signaling and splenic erythropoiesis. J Clin Invest. 2007 doi: 10.1172/JCI32614. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu MY, Poellinger L, Walker CL. Up-regulation of hypoxia-inducible factor 2alpha in renal cell carcinoma associated with loss of Tsc-2 tumor suppressor gene. Cancer Res. 2003;63:2675–80. [PubMed] [Google Scholar]
- 61.Smith K, Gunaratnam L, Morley M, Franovic A, Mekhail K, Lee S. Silencing of epidermal growth factor receptor suppresses hypoxia-inducible factor-2-driven VHL-/- renal cancer. Cancer Res. 2005;65:5221–30. doi: 10.1158/0008-5472.CAN-05-0169. [DOI] [PubMed] [Google Scholar]
- 62.de Paulsen N, Brychzy A, Fournier MC, Klausner RD, Gnarra JR, Pause A, et al. Role of transforming growth factor-alpha in von Hippel--Lindau (VHL)(-/-) clear cell renal carcinoma cell proliferation: a possible mechanism coupling VHL tumor suppressor inactivation and tumorigenesis. Proc Natl Acad Sci U S A. 2001;98:1387–92. doi: 10.1073/pnas.031587498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan Q, Bartz S, Mao M, Li L, Kaelin WG., Jr The hypoxia-inducible factor 2alpha N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Mol Cell Biol. 2007;27:2092–102. doi: 10.1128/MCB.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. Embo J. 2004;23:1949–56. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–47. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gordan J. manuscript in preparation.
- 67.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–20. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 68.Corn PG, Ricci MS, Scata KA, Arsham AM, Simon MC, Dicker DT, et al. Mxi1 is induced by hypoxia in a HIF-1-dependent manner and protects cells from c-Myc-induced apoptosis. Cancer Biol Ther. 2005;4:1285–94. doi: 10.4161/cbt.4.11.2299. [DOI] [PubMed] [Google Scholar]
- 69.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–28. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–21. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, et al. Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in Human breast cancer. Cancer Res. 2002;62:1326–9. [PubMed] [Google Scholar]
- 73.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–11. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 74.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–6. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 75.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 76.Jogi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, et al. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci U S A. 2002;99:7021–6. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Acker T, Diez-Juan A, Aragones J, Tjwa M, Brusselmans K, Moons L, et al. Genetic evidence for a tumor suppressor role of HIF-2alpha. Cancer Cell. 2005;8:131–41. doi: 10.1016/j.ccr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Brusselmans K, Bono F, Maxwell P, Dor Y, Dewerchin M, Collen D, et al. Hypoxia-inducible factor-2alpha (HIF-2alpha) is involved in the apoptotic response to hypoglycemia but not to hypoxia. J Biol Chem. 2001;276:39192–6. doi: 10.1074/jbc.C100428200. [DOI] [PubMed] [Google Scholar]
- 79.Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–13. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mack FA, Rathmell WK, Arsham AM, Gnarra J, Keith B, Simon MC. Loss of pVHL is sufficient to cause HIF dysregulation in primary cells but does not promote tumor growth. Cancer Cell. 2003;3:75–88. doi: 10.1016/s1535-6108(02)00240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mack FA, Patel JH, Biju MP, Haase VH, Simon MC. Decreased growth of Vhl-/- fibrosarcomas is associated with elevated levels of cyclin kinase inhibitors p21 and p27. Mol Cell Biol. 2005;25:4565–78. doi: 10.1128/MCB.25.11.4565-4578.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rankin EB, Tomaszewski JE, Haase VH. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res. 2006;66:2576–83. doi: 10.1158/0008-5472.CAN-05-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Melillo G. Targeting hypoxia cell signaling for cancer therapy. Cancer Metastasis Rev. 2007;26:341–52. doi: 10.1007/s10555-007-9059-x. [DOI] [PubMed] [Google Scholar]
- 84.Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66:6264–70. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- 85.Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell. 2004;6:33–43. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]