Abstract
Objective:
Epilepsy neurosurgery is a treatment option for children with refractory epilepsy. Our aim was to determine if outcomes improved over time.
Methods:
Pediatric epilepsy surgery patients operated in the first 11 years (1986–1997; pre-1997) were compared with the second 11 years (1998–2008; post-1997) for differences in presurgical and postsurgical variables.
Results:
Despite similarities in seizure frequency, age at seizure onset, and age at surgery, the post-1997 series had more lobar/focal and fewer multilobar resections, and more patients with tuberous sclerosis complex and fewer cases of nonspecific gliosis compared with the pre-1997 group. Fewer cases had intracranial EEG studies in the post-1997 (0.8%) compared with the pre-1997 group (9%). Compared with the pre-1997 group, the post-1997 series had more seizure-free patients at 0.5 (83%, +16%), 1 (81%, +18%), 2 (77%, +19%), and 5 (74%, +29%) years, and more seizure-free patients were on medications at 0.5 (97%, +6%), 1 (88%, +9%), and 2 (76%, +29%), but not 5 (64%, +8%) years after surgery. There were fewer complications and reoperations in the post-1997 series compared with the pre-1997 group. Logistic regression identified post-1997 series and less aggressive medication withdrawal as the main predictors of becoming seizure-free 2 years after surgery.
Conclusions:
Improved technology and surgical procedures along with changes in clinical practice were likely factors linked with enhanced and sustained seizure-free outcomes in the post-1997 series. These findings support the general concept that clearer identification of lesions and complete resection are linked with better outcomes in pediatric epilepsy surgery patients.
GLOSSARY
- AED
= antiepileptic drug;
- FDG
= fluorodeoxyglucose;
- HIPAA
= Health Insurance Portability and Accountability Act;
- IRB
= institutional review board;
- SEGA
= subependymal giant cell astrocytoma;
- TSC
= tuberous sclerosis complex;
- UCLA
= University of California, Los Angeles.

e–Pub ahead of print

CME
Surgery for children with refractory epilepsy has become an important treatment option over the past 30 years. Initially, most patients were adolescents with focal lesions involving the temporal lobe similar to adult epilepsy surgery.1,2 With modern neuroimaging (e.g., MRI SPECT and fluorodeoxyglucose [FDG]–PET), the number of surgical centers expanded, as did etiologies and types of operations. Today, pediatric epilepsy surgery has evolved to include extratemporal operations and cerebral hemispherectomy for children of all ages. Etiologies range from cortical dysplasia, tumors, and perinatal strokes to rarer syndromes such as hemimegalencephaly, tuberous sclerosis complex (TSC), Rasmussen encephalitis, Sturge-Weber syndrome, and hypothalamic hamartomas.3 Many children are treated because they are at risk for epileptic encephalopathies.4
Studies report favorable outcomes on surgical cohorts from single centers, groups of centers focused for an age category, and patients with similar etiologies and procedures.3,5–13 The aim of this study was to determine if outcomes improved over time.
METHODS
Patient cohorts.
The initial cohort consisted of all patients who underwent epilepsy neurosurgery at the UCLA Pediatric Epilepsy Surgery Program from January 1986 to December 2008 (n = 580). Patients had pharmacoresistant epilepsy, defined as persistent unprovoked seizures after adequate trials of 2 or more antiepileptic drugs (AED).14 Excluded were patients who had craniotomy without cortical resection (biopsy only; n = 4), diagnostic intracranial electrodes without resection (n = 4), and multiple subpial transections without cortical excision (n = 1), leaving a final cohort of 571.
Study design.
Patients were separated into 2 groups based on the date of surgery. The pre-1997 group were patients operated from January 1986 to December 1997 (n = 192). The post-1997 group included patients operated from January 1998 to December 2008 (n = 379). The 1997 to 1998 transition was chosen because it was the midpoint of the series and a previous publication summarized our epilepsy surgical experience from that era.6 The findings of that study altered our approach in the post-1997 period. Specifically, we strove to use multimodality neuroimaging to enhance identification of epileptogenic lesions, advocated for complete surgical resection of the lesion, and altered postsurgical medication management. Patients were further subclassified by their operative procedure into those undergoing palliative (corpus callosotomy and vagus nerve stimulators; n = 146) and resection operations (n = 425). Additional details are available in e-Methods on the Neurology® Web site at www.neurology.org.
Standard protocol approvals, registrations, and patient consents.
This research was approved by the University of California, Los Angeles (UCLA) institutional review board (IRB), and since enactment of Health Insurance Portability and Accountability Act (HIPAA), patients or families have signed research informed consents and HIPAA authorizations. Prior to enactment of HIPAA, this study was considered by UCLA's IRB to be exempt from requiring research informed consent. This study is not a clinical trial, and it is not registered in any public registry.
Statistical analyses.
The pre-1997 and post-1997 groups were compared for differences in clinical variables, surgical procedures, and postsurgical outcomes. StatView 5 (SAS Institute, Inc., Cary, NC) was used for statistical analysis. Univariate statistical tests included Student t test, analysis of variance, and χ2. Multivariate tests included logistic regression and log-linear analysis. All tests were 2-tailed and the threshold for significance was set a priori at p < 0.05. Univariate statistical analysis did not include adjustments for multiple comparisons.
RESULTS
General comparisons.
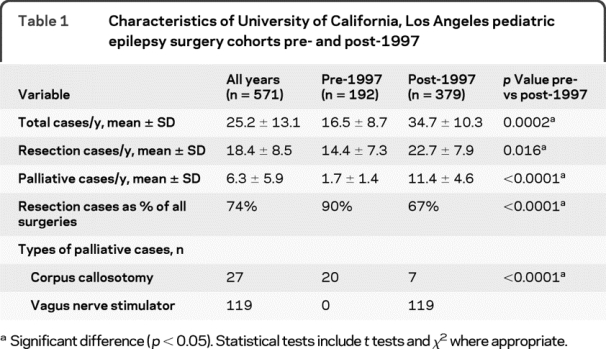
The average cases per year and the proportion of palliative operations increased in the post-1997 series compared with the pre-1997 group (table 1). For the post-1997 series, there was a 58% increase in resection and a 570% increase in palliative cases per year (p < 0.016) compared with the pre-1997 group. In the pre-1997 group, palliative operations were 10% of all pediatric epilepsy surgery procedures, and consisted of corpus callosotomy. In the post-1997 series, palliative operations were 33% of all surgical cases, and fewer corpus callosotomy procedures were performed. Vagus nerve stimulation, which was approved by the Food and Drug Administration in 1997, comprised 90% (119/126) of palliative procedures in the post-1997 series.
Table 1 Characteristics of University of California, Los Angeles pediatric epilepsy surgery cohorts pre- and post-1997
Comparisons of resection cases.
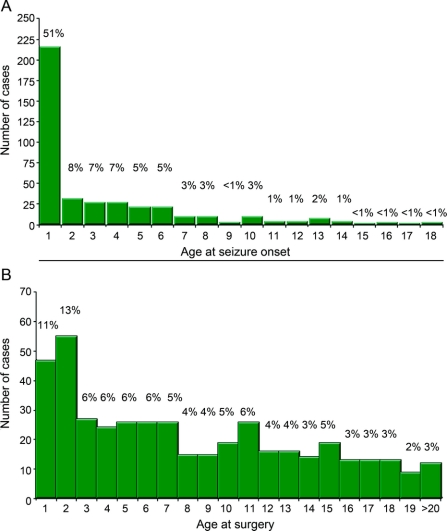
For UCLA pediatric patients undergoing resections, age at seizure onset was 1 year or less in 51% of cases, which is similar to findings from other pediatric epilepsy surgical centers (figure, A).3 However, 24% of resection patients had their operation by age 2 years, which is younger than reported by many centers (figure, B).
Figure Frequency histogram
Age at seizure onset (A) and age at surgery (B) for resection cases from 1986 to 2008 for University of California, Los Angeles's pediatric epilepsy surgery patients. Percentages are shown above each bar.
There were no differences in most presurgical clinical characteristics comparing the pre- and post-1997 groups (table 2). Age at seizure onset, age at surgery, epilepsy duration, gender, side of resection, percentage of patients with a history of infantile spasms, and the percentage of patients with daily or more seizures were similar comparing the pre- with the post-1997 group (table 2; p > 0.12). Overall, 80% of patients underwent hemispherectomy or extratemporal operations compared with 20% with temporal lobe resections, which is greater than reported from many pediatric epilepsy surgery centers.3,7,8,11 There were no differences in the ratio of temporal vs extratemporal operations comparing the pre- and post-1997 groups (p = 0.377).
Table 2 Characteristics of resection cases, University of California, Los Angeles pediatric series pre- and post-1997
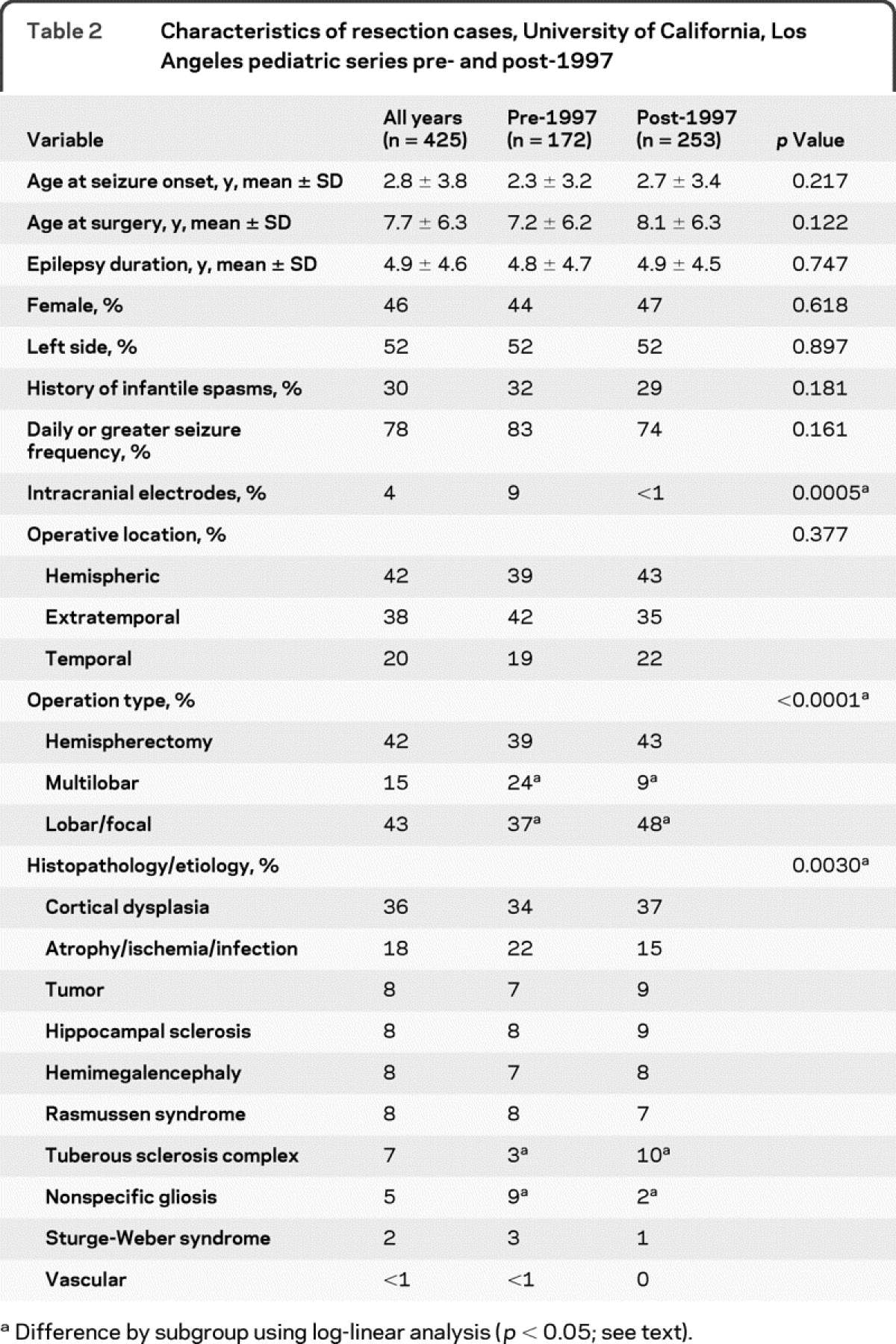
Within the UCLA cohort, differences were noted in types of operations, etiologies, and use of intracranial electrodes comparing the pre- and post-1997 groups. Compared with the pre-1997 group, patients in the post-1997 series had proportionally more lobar/focal (+11%; p = 0.001; log-linear analysis) and fewer multilobar resections (−15%; table 2; p = 0.002). Using log-linear analysis, there was a higher proportion of patients with TSC (+7%; p = 0.024), and fewer cases of nonspecific gliosis (−7%; p = 0.002) in the post-1997 compared with pre-1997 groups. Other etiologies were not different between the 2 series (p > 0.05). Overall, diagnostic intracranial EEG studies (phase II) were performed in 4% of UCLA pediatric epilepsy surgery patients (table 2). Intracranial electrodes were used in 15 patients in the pre-1997 group, compared with 2 patients in the post-1997 series (p = 0.0005).
Seizure freedom after resection.
The percentage of patients with outcome data (reporting rate) decreased with longer follow-up durations for both the pre-1997 and post-1997 groups (table 3). Compared with the pre-1997 group, the post-1997 series had better reporting rates at 0.5 and 1 year (p < 0.012), and similar reporting rates at 2 and 5 years after surgery (p > 0.21).
Table 3 Seizure outcome of University of California, Los Angeles pediatric epilepsy surgery patients pre- and post-1997
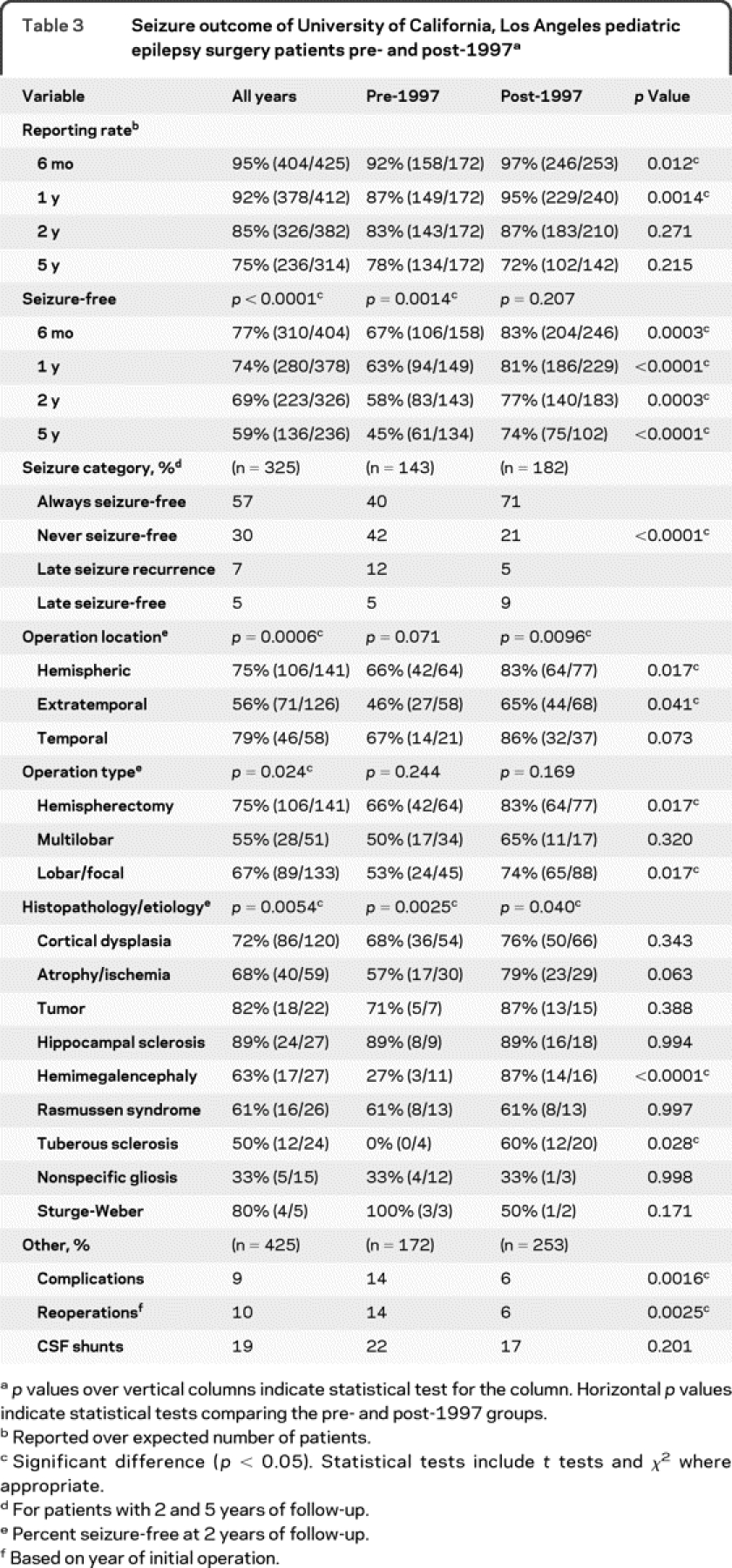
The percentage of patients seizure-free was greater in the post-1997 series compared with the pre-1997 group at 0.5 (+16%), 1 (+18%), 2 (+19%), and 5 (+29%) years after surgery (p < 0.0003). In the pre-1997 group, the percentage of patients seizure-free decreased from 67% at 0.5 years to 45% at 5 years after surgery (−22%; p = 0.0014). In the post-1997 series, the percentage of patients seizure-free was 83% at 0.5 years that decreased to 74% at 5 years after surgery (−9%; p = 0.207).
There was less variability in seizure category in the post-1997 series. In patients with at least 2 years of follow-up, more patients were always seizure-free (+31%), fewer patients were never seizure-free (−21%) or had late recurrence of seizures (−7%) in the post-1997 series compared with the pre-1997 group (p < 0.0001). Likewise, more patients became seizure-free after initial failure after surgery in the post-1997 series compared with the pre-1997 group (+4%).
The percentage of patients seizure-free 2 years after surgery was different by location, type of operation, and etiology (table 3). For all patients, those who had nonhemispheric extratemporal operations were less likely to be seizure-free (56%) compared with cases undergoing hemispherectomy (75%) and temporal (79%) resections (p = 0.0006). Similarly, patients who had multilobar operations were less likely to be seizure-free (55%) compared with patients undergoing hemispherectomy and lobar/focal (67%) resections (p = 0.024). By etiology, more patients with hippocampal sclerosis and tumors were seizure-free compared with those with hemimegalencephaly, Rasmussen syndrome, and TSC (p = 0.0054). Compared with the pre-1997 group, the post-1997 series showed that more patients were seizure-free who had hemispherectomy, extratemporal resections, and lobar/focal operations (p < 0.041), but not temporal or multilobar resections (p > 0.07). Likewise, compared with the pre-1997 group, the post-1997 series had a higher percentage of patients seizure-free with hemimegalencephaly (p < 0.0001) and TSC (p = 0.028). Other etiologies showed no differences in seizure-free outcomes comparing the pre- with the post-1997 groups.
Using outcomes at 2 years postsurgery, the percentage of patients seizure-free for both the pre- and post-1997 groups did not correlate with age at seizure onset (p = 0.93), age at surgery (p = 0.61), epilepsy duration (p = 0.62), gender (p = 0.69), side of resection (p = 0.66), history of infantile spasms (p = 0.80), and presurgery seizure frequency (p = 0.48).
AEDs after surgery.
Medication use after surgery was different comparing the pre- and post-1997 groups (table 4). Although all patients met criteria for medically refractory epilepsy, 5 patients (1%) were not using AEDs at the time of surgery (parent preference).
Table 4 Antiepileptic drug use, University of California, Los Angeles pediatric cohort: pre- and post-1997
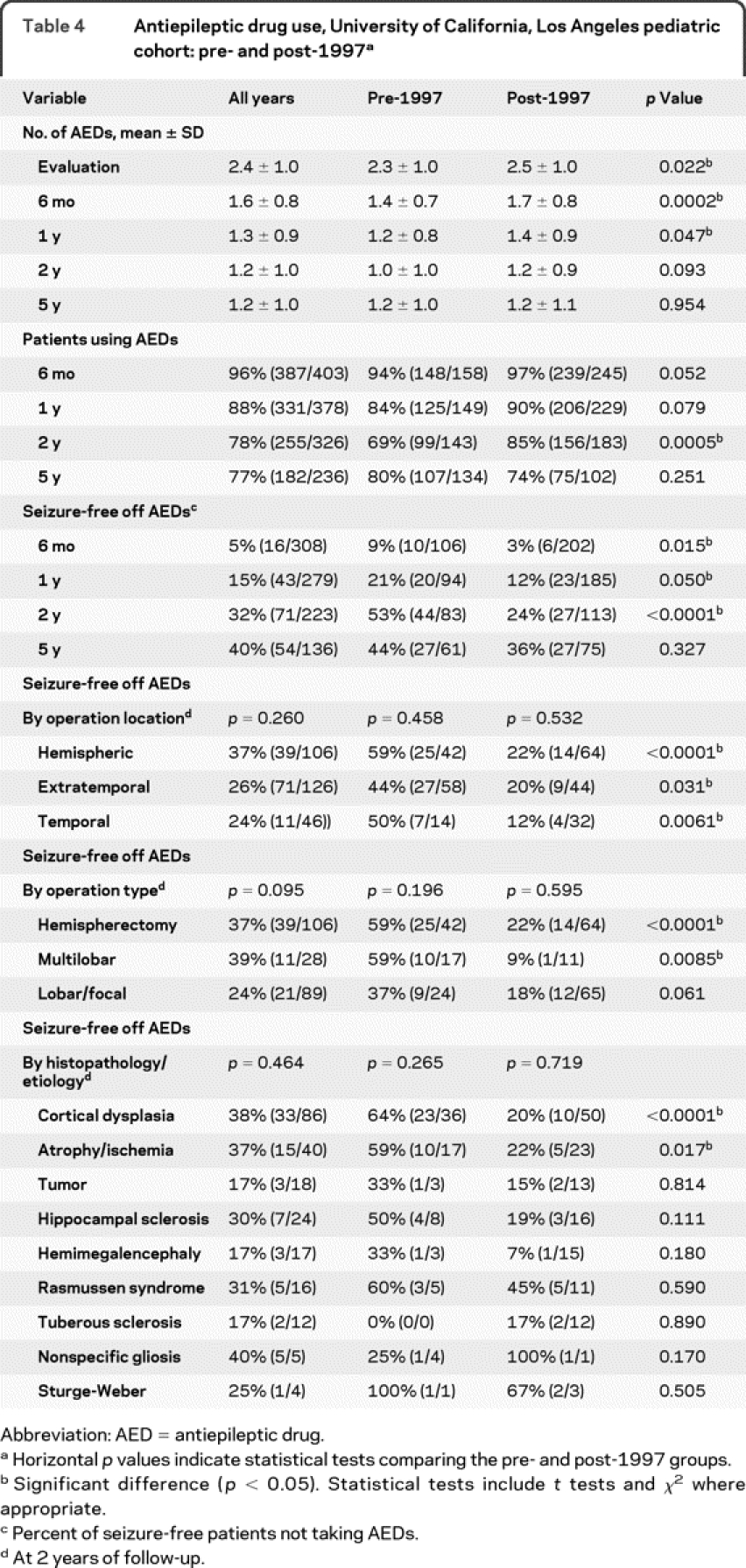
Overall, the number of AEDs per patient decreased at all time points after surgery (table 4). The proportion of patients using AEDs decreased from 96% at 0.5 year to 77% at 5 years after surgery (table 4). The withdrawal of AEDs was faster in the pre-1997 group, with 31% not taking AEDs at 2 years of follow-up, compared with 15% of patients in the post-1997 series (p = 0.0005).
Similar findings were seen in the use of AEDs in seizure-free patients after surgery. Compared with the pre-1997 group, the post-1997 series had more seizure-free patients taking AEDs at 0.5 (97%; +6%), 1 (88%; +9%), and 2 (76%; +29%), but not 5 (64% +8%) years after surgery. In the post-1997 group, more seizure-free patients were taking AEDs at 2 years postsurgery for cases undergoing hemispherectomy (+37%), extratemporal (+22%), temporal (+38%), and multilobar (+50%) resections. In addition, more seizure-free patients with cortical dysplasia (+44%) and atrophic etiologies (+37%) were taking AEDs comparing the post-1997 group with the pre-1997 series.
Complications and reoperations.
For the entire series, serious and permanent complications were identified in 39 (9.2%) patients. The complication rate was less in the post-1997 compared with the pre-1997 group (table 3; p = 0.0016). There are 11 (2.6%) known deaths. Two deaths occurred during surgery (both in the pre-1997 group) as previously reported.6 There were 9 long-term deaths involving accidents, status epilepticus, and sudden unexplained death in epilepsy (8 in the pre- and 1 in the post-1997 group). The mean (±SD) time from operation until late death was 6.2 ± 4.4 years (range 2–14 years). Five of the late deaths occurred more than 5 years after surgery. There were 6 operative-related intracranial bleeds that required a return to surgery for evacuation (all in the pre-1997 group), 12 infections requiring long-term IV antibiotics (8 in the pre- and 4 in the post-1997 groups), and 4 cases of unanticipated neurologic deficits (cranial nerve palsies, increased motor and language deficits; all in the post-1997 group). In addition, one patient had a posterior cerebral artery infarct on the same side as the hemispherectomy (without increased neurologic compromise), 2 patients had tumor recurrence after an initial epilepsy operation, and 1 patient with TSC developed a subependymal giant cell astrocytoma (SEGA) after epilepsy surgery (all in the post-1997 group).
Reoperations for epilepsy surgery occurred in 42 (9.8%) patients. The mean (±SD) time from first to last operation was 3.1 ± 3.9 years (range 6 days–14 years). Most of the reoperations (29/42; 69%) occurred less than 3 years after the first operation. Most had reoperations to convert a previous multilobar resection into cerebral hemispherectomy (n = 17; 13 in the pre- and 4 in the post-1997 groups). Others had completion of hemispheric disconnection after an unsuccessful first operation (n = 10; 6 in the pre- and 4 in the post-1997 groups), further resection involving lobar/focal operations (n = 9; 5 in the pre- and 4 in the post-1997 groups), multistage reoperations for patients with TSC (n = 3; all post-1997 group), and recurrent tumor (n = 2) or SEGA (n = 1; all post-1997 group). The percentage of patients with reoperations was greater in the pre-1997 group compared with the post-1997 series (p = 0.0025).
CSF shunts were necessary in 79 (18.6%) patients in this series. Most CSF shunts were in patients undergoing cerebral hemispherectomy (39.5%; n = 70/177) with fewer patients needing shunts with multilobar (5%; 3/64) and lobar/focal (7%; 6/84) resections. The use of CSF shunts was similar in the pre- and post-1997 groups (p = 0.201). For hemispherectomy patients, the need for CSF shunts was greater in the pre-1997 group (47%; 32/68) compared with the post-1997 series (34%; 37/109; p = 0.05).
Multivariate analysis.
Logistic regression analysis was performed using pre- and post-1997 groups, operation location, operation type, etiology, and use of AEDs at 2 years postsurgery as independent variables in a model with seizure-free cases at 2 years postsurgery as the dependent variable. The period of evaluation (pre- vs post-1997, p = 0.0001) and AED use at 2 years (p < 0.0001) were associated with greater percentage of patients with seizure-free outcomes, but not operation type, location, or etiology (p > 0.081). In other words, better postsurgical seizure-free outcomes were linked with having surgery after 1997 and with less aggressive withdrawal of AEDs after surgery.
DISCUSSION
Despite similarities in age at seizure onset, age at surgery, epilepsy duration, and other presurgical clinical variables, this study identified differences in presurgical and postsurgical clinical variables comparing the first 11 years (pre-1997) with the second 11 years (post-1997) of our program. Compared with the pre-1997 group, the post-1997 series showed more resection and palliative operations per year, more lobar/focal and fewer extratemporal and multilobar resections, more patients with TSC and fewer cases with nonspecific gliosis, fewer patients with diagnostic intracranial electrode studies, a higher rate of patients seizure-free at all measured time points after surgery, a lower proportion of seizure-free patients not taking AEDs at 0.5, 1, 2, and 5 years postsurgery, and fewer operative complications and reoperations. Logistical regression identified the period of surgery (pre- vs post-1997) and AED use after surgery as the most important predictors of becoming seizure-free. Taken together, these results indicate that over time there were sustained improvements in pediatric epilepsy surgery patients at the UCLA program.
The improvement in surgical outcome for pediatric epilepsy surgery patients was likely due to multiple overlapping and interacting factors, not a single reason. These factors would include better presurgical noninvasive technology to identify epileptogenic lesions, improved selection of potential surgical candidates, and our conscious decision to completely remove the lesion at surgery and alter postoperative AED management after 1997. For example, the use of stronger MRI magnets with better software, thinner slice FDG-PET scans, and incorporation of FDG-PET/MRI coregistration, MSI, and fMRI into the presurgical evaluation process likely improved the identification of the epileptogenic zone and important functional cortex.15–19 Better neuroimaging technologies and experience in using them probably explain the decrease in patients with nonspecific gliosis in the post-1997 series. Improved neuroimaging probably also explains the increase in the percent of patients with focal/lobar operations compared with multilobar resections after 1997.17,20 Likewise, changes in practice, such as not reducing AEDs so quickly after surgery in seizure-free patients, were associated with better outcomes. Improved surgical procedures, such as for cerebral hemispherectomy, were likely related to better outcomes, reduced complications, and reoperations in the post-1997 group.21–23
We also learned over time the importance of performing complete resections in pediatric epilepsy surgery patients.24,25 Before 1997, we often restricted our cortical excisions to prevent neurologic deficits such as performing multilobar temporal-occipital-parietal resections in patients with mostly posterior MRI findings and an incomplete hemiparesis. However, we found in the pre-1997 group that a significant number of patients with incomplete operations were not seizure-free with longer follow-ups and needed additional surgery often 2 or more years after the initial operation. Since 1997, we have altered our approach and advocated for complete resections with the initial operation, especially in young children at risk for epileptic encephalopathy, even if that means removing the motor-sensory cortex and other partially functional cortex.26 More complete resections might explain the better and persistent seizure control at 2 and 5 years of follow-up in the post-1997 series.17,26 The number of reoperations, and eventual seizure-free patients off medications with longer follow-up durations, are similar between our series and the literature.10,27–30
Phase II intracranial EEG studies were used at a lower rate than usually reported. The ILAE survey of 20 pediatric epilepsy surgery centers involving 543 children found that intracranial electrodes were used in 27% of patients.3 Furthermore, 9% to 73% of pediatric patients were reported to use intracranial electrodes in previous surgical cohorts.5,8,9,11–13 Our lower rate of intracranial electrode implantation is probably attributable to our approach of using multiple noninvasive technologies and intraoperative ECoG to identify the zone of cortical abnormality likely responsible for epileptogenesis. This is a different approach than targeting areas of EEG ictal onsets for resection.31,32
Our study from a single center achieved high reporting rates for seizure outcome. However, the reader should note that our series has a higher proportion of younger patients, more cases of hemispherectomy, and fewer cases of temporal lobe resections compared with other cohorts from pediatric epilepsy surgery centers.3,7,8,11–13,28 Thus, comparisons of outcomes may or may not be similar when other centers report their long-term findings.
The reader should be aware of the inherent limitations of our study. For example, this was a retrospective analysis. As such, we can only infer cause and effect from our findings. Prospective multicenter studies will be necessary to determine if the presurgical evaluation, surgical approach, completeness of resection, and AED use after surgery is linked with the best postsurgical seizure-free outcomes.33 Likewise, we did not assess cognitive and developmental outcomes.22,34,35 Over the 22 years we have found it more difficult to obtain approval of these studies from insurance companies. Thus, it is possible that some patients were cognitively improved despite not being seizure-free after surgery. Finally, we have 5-year outcome data on a proportion of patients in the post-1997 series. As we have learned from our analysis, we will need to follow this cohort to determine if the findings related to seizure control, late deaths, and reoperations remain valid.
This study is pertinent for the practicing neurologist because it indicates that with improved technologies and greater clinical experience including management of postoperative medications a significant proportion of pediatric patients can expect to become seizure-free after surgery. This finding emphasizes the conclusion of the ILAE Sub-Commission on Pediatric Epilepsy Surgery that all children with therapy-resistant epilepsy of unknown etiology should be referred to an experienced center for diagnostic evaluation and surgical consideration.4 These children are at risk for epileptic encephalopathy, and some may be candidates for cortical resections with a high chance of becoming seizure-free if an experienced surgical team can identify a surgically treatable etiology and remove it.22,34,35 Even if not a resection candidate, these children may be offered alternate treatments, including palliative operations.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Gary W. Mathern.
ACKNOWLEDGMENT
The authors acknowledge the contribution of the following individuals over the 22 years of the program: Drs. Alan Shewmon, Harry Chugani, Youseff Comair, Warwick Peacock, Christopher C. Giza, and Susan Koh.
DISCLOSURE
Dr. Hemb has received research support from the Brazilian government (CAPES 2503-08-5). Dr. Velasco has received research support from the Brazilian government (CNPq 201031/2008-6). M.S. Parnes reports no disclosures. Dr. Wu serves on the Professional Advisory Board for the Tuberous Sclerosis Alliance and receives/has received research support from Novartis and the NIH/NINDS (K23 NS051637 [PI]). Dr. Lerner receives research support from the Thrasher Research Fund. Dr. Matsumoto estimates that greater than 60% of her time is spent reading routine EEGs and extended video EEG telemetry monitoring, along with seeing epilepsy and other general neurology patients in clinic. S. Yudovin reports no disclosures. Dr. Shields serves on scientific advisory boards and as a consultant for and receives research support from Lundbeck Inc. (formerly Ovation Pharmaceuticals) and Questcor and appeared before an FDA advisory panel on behalf of Lundbeck Inc. (formerly Ovation Pharmaceuticals). Dr. Sankar serves on scientific advisory boards for and has received funding for travel from Ortho-McNeil-Janssen Pharmaceuticals, Inc., NeuroTherapeutics Pharma, Inc., and Valeant Pharmaceuticals International; receives royalties from the publication of Pediatric Neurology, 3rd ed. (Demos Publishing, 2008); serves on speakers' bureaus for and has received speaker honoraria from Ortho-McNeil-Janssen Pharmaceuticals, Inc., Valeant Pharmaceuticals International, UCB, Eisai Inc., GlaxoSmithKline, and Cyberonics, Inc.; and has received research support from Valeant Pharmaceuticals International, Marinus Pharmaceuticals, Inc., the NIH (NINDS NS046516 ([PI], NINDS NS045911 [Co-PI], NINDS NS059505 (Co-I), and NINDS MH079933 [Co-I]), and the Epilepsy Foundation of America. Dr. Salamon receives research support from the American Society of Neuroradiology Research Foundation. Dr. Vinters serves on the editorial boards of the Journal of Neuroscience Research, Neuropathology & Applied Neurobiology, Korean Journal of Pathology, and Neuropathology (journal of the Japanese Society of Neuropathology); and holds stock in Minnesota Mining & Manufacturing, Teva Pharmaceutical Industries Ltd., GlaxoSmithKline, and Pfizer Inc. Dr. Mathern serves on the editorial boards of Neurology®, Surgical Neurology, and Epilepsy Research and on the Data Management Committee of Neuropace, Inc.; and receives research support from the NIH (RO1 NS 38992 [PI]).
Supplementary Material
Address correspondence and reprint requests to Dr. Gary W. Mathern, Reed Neurological Research Center, 710 Westwood Plaza, Room 2123, Los Angeles, CA 90095-1769 gmathern@ucla.edu
Editorial, page 1756
Supplemental data at www.neurology.org
e-Pub ahead of print on April 28, 2010, at www.neurology.org.
Study funding: Supported by NIH grant R01 NS38992. M.H. was supported by CAPES (2503-08-5). T.R.V. was supported by CNPq (201031/2008-6). H.V.V. was supported in part by the Dalyit S. and Flaine Sarkaria Chair in Diagnostic Medicine.
Disclosure: Author disclosures are provided at the end of the article.
Received July 28, 2009. Accepted in final form February 4, 2010.
REFERENCES
- 1.Davidson S, Falconer MA. Outcome of surgery in 40 children with temporal lobe epilepsy. Lancet 1975;1:1260–1263. [DOI] [PubMed] [Google Scholar]
- 2.Goldring S. Pediatric epilepsy surgery. Epilepsia 1987;28 suppl 1:S82–102. [DOI] [PubMed] [Google Scholar]
- 3.Harvey AS, Cross JH, Shinnar S, Mathern BW. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia 2008;49:146–155. [DOI] [PubMed] [Google Scholar]
- 4.Cross JH, Jayakar P, Nordli D, et al. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia 2006;47:952–959. [DOI] [PubMed] [Google Scholar]
- 5.Kim SK, Wang KC, Hwang YS, et al. Epilepsy surgery in children: outcomes and complications. J Neurosurg Pediatr 2008;1:277–283. [DOI] [PubMed] [Google Scholar]
- 6.Mathern GW, Giza CC, Yudovin S, et al. Postoperative seizure control and antiepileptic drug use in pediatric epilepsy surgery patients: the UCLA experience, 1986–1997. Epilepsia 1999;40:1740–1749. [DOI] [PubMed] [Google Scholar]
- 7.Munari C, Lo Russo G, Minotti L, et al. Presurgical strategies and epilepsy surgery in children: comparison of literature and personal experiences. Childs Nerv Syst 1999;15:149–157. [DOI] [PubMed] [Google Scholar]
- 8.Paolicchi JM, Jayakar P, Dean P, et al. Predictors of outcome in pediatric epilepsy surgery. Neurology 2000;54:642–647. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair DB, Aronyk KE, Snyder TJ, et al. Pediatric epilepsy surgery at the University of Alberta: 1988–2000. Pediatr Neurol 2003;29:302–311. [DOI] [PubMed] [Google Scholar]
- 10.Steinbok P, Gan PY, Connolly MB, et al. Epilepsy surgery in the first 3 years of life: a Canadian survey. Epilepsia 2009;50:1442–1449. [DOI] [PubMed] [Google Scholar]
- 11.Terra-Bustamante VC, Fernandes RM, Inuzuka LM, et al. Surgically amenable epilepsies in children and adolescents: clinical, imaging, electrophysiological, and post-surgical outcome data. Childs Nerv Syst 2005;21:546–551. [DOI] [PubMed] [Google Scholar]
- 12.Van Oijen M, De Waal H, Van Rijen PC, et al. Resective epilepsy surgery in childhood: the Dutch experience 1992–2002. Eur J Paediatr Neurol 2006;10:114–123. [DOI] [PubMed] [Google Scholar]
- 13.Wyllie E, Comair YG, Kotagal P, et al. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol 1998;44:740–748. [DOI] [PubMed] [Google Scholar]
- 14.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia Epub 2009 Nov 3. [DOI] [PubMed]
- 15.Cepeda C, Andre VM, Flores-Hernandez J, et al. Pediatric cortical dysplasia: correlations between neuroimaging, electrophysiology and location of cytomegalic neurons and balloon cells and glutamate/GABA synaptic circuits. Dev Neurosci 2005;27:59–76. [DOI] [PubMed] [Google Scholar]
- 16.Chandra PS, Salamon N, Huang J, et al. FDG-PET/MRI coregistration and diffusion-tensor imaging distinguish epileptogenic tubers and cortex in patients with tuberous sclerosis complex: a preliminary report. Epilepsia 2006;47:1543–1549. [DOI] [PubMed] [Google Scholar]
- 17.Lerner JT, Salamon N, Hauptman JS, et al. Assessment and surgical outcomes for mild type I and severe type II cortical dysplasia: a critical review and the UCLA experience. Epilepsia 2009;50:1310–1335. [DOI] [PubMed] [Google Scholar]
- 18.Vinters HV. Histopathology of brain tissue from patients with infantile spasms. Int Rev Neurobiol 2002;49:63–76. [DOI] [PubMed] [Google Scholar]
- 19.Wu JY, Sutherling WW, Koh S, et al. Magnetic source imaging localizes epileptogenic zone in children with tuberous sclerosis complex. Neurology 2006;66:1270–1272. [DOI] [PubMed] [Google Scholar]
- 20.Salamon N, Kung J, Shaw SJ, et al. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology 2008;71:1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook SW, Nguyen ST, Hu B, et al. Cerebral hemispherectomy in pediatric patients with epilepsy: comparison of three techniques by pathological substrate in 115 patients. J Neurosurg 2004;100(2 suppl pediatrics):125–141. [DOI] [PubMed]
- 22.Jonas R, Nguyen S, Hu B, et al. Cerebral hemispherectomy: hospital course, seizure, developmental, language, and motor outcomes. Neurology 2004;62:1712–1721. [DOI] [PubMed] [Google Scholar]
- 23.Heller AC, Padilla RV, Mamelak AN. Complications of epilepsy surgery in the first 8 years after neurosurgical training. Surg Neurol 2009;71:631–637, discussion 637. [DOI] [PubMed]
- 24.Mathern GW. Challenges in the surgical treatment of epilepsy patients with cortical dysplasia. Epilepsia 2009;50 suppl 9:45–50. [DOI] [PubMed] [Google Scholar]
- 25.Mathern GW. Epilepsy surgery patients with cortical dysplasia: present and future therapeutic challenges. Neurology 2009;72:206–207. [DOI] [PubMed] [Google Scholar]
- 26.Krsek P, Maton B, Jayakar P, et al. Incomplete resection of focal cortical dysplasia is the main predictor of poor postsurgical outcome. Neurology 2009;72:217–223. [DOI] [PubMed] [Google Scholar]
- 27.Lachhwani DK, Loddenkemper T, Holland KD, et al. Discontinuation of medications after successful epilepsy surgery in children. Pediatr Neurol 2008;38:340–344. [DOI] [PubMed] [Google Scholar]
- 28.Gilliam F, Wyllie E, Kashden J, et al. Epilepsy surgery outcome: comprehensive assessment in children. Neurology 1997;48:1368–1374. [DOI] [PubMed] [Google Scholar]
- 29.Hoppe C, Poepel A, Sassen R, Elger CE. Discontinuation of anticonvulsant medication after epilepsy surgery in children. Epilepsia 2006;47:580–583. [DOI] [PubMed] [Google Scholar]
- 30.Sinclair DB, Jurasek L, Wheatley M, et al. Discontinuation of antiepileptic drugs after pediatric epilepsy surgery. Pediatr Neurol 2007;37:200–202. [DOI] [PubMed] [Google Scholar]
- 31.Adelson PD, Peacock WJ, Chugani HT, et al. Temporal and extended temporal resections for the treatment of intractable seizures in early childhood. Pediatr Neurosurg 1992;18:169–178. [DOI] [PubMed] [Google Scholar]
- 32.Shields WD, Shewmon DA, Chugani HT, Peacock WJ. Treatment of infantile spasms: medical or surgical? Epilepsia 1992;33 suppl 4:S26–31. [DOI] [PubMed] [Google Scholar]
- 33.Berg AT, Mathern GW, Bronen RA, et al. Frequency, prognosis and surgical treatment of structural abnormalities seen with magnetic resonance imaging in childhood epilepsy. Brain 2009;132:2785–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asarnow RF, LoPresti C, Guthrie D, et al. Developmental outcomes in children receiving resection surgery for medically intractable infantile spasms. Dev Med Child Neurol 1997;39:430–440. [DOI] [PubMed] [Google Scholar]
- 35.Jonas R, Asarnow RF, LoPresti C, et al. Surgery for symptomatic infant-onset epileptic encephalopathy with and without infantile spasms. Neurology 2005;64:746–750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.