Abstract
Background:
Mitoxantrone is used for aggressive multiple sclerosis (MS), but concerns about safety, including cardiotoxicity and other laboratory measures, prevail.
Objective:
To evaluate the incidence and potential predictors of adverse events associated with mitoxantrone at the MS Clinic, University of British Columbia, Canada.
Methods:
Retrospective review of patients treated with mitoxantrone by standard protocol; maximum cumulative dose = 120 mg/m2. Left ventricular ejection fraction (LVEF) was measured with regular multiple-gated acquisition (MUGA) scans; blood cell counts and biochemical liver tests were performed before infusions. Generalized estimating equations were used to examine potential predictors of adverse events (graded according to the Common Toxicity Criteria, version 4) in patients with normal baseline and ≥1 follow-up MUGA or laboratory assessment.
Results:
All 163 patients (58% women) treated with mitoxantrone from 1999 to 2007 were reviewed. Mean baseline age was 41.9 (SD 10.8) years, cumulative dose was 59.7 (SD 26.0) mg/m2, and median follow-up duration was 14 months (maximum 6.5 years). By study end, 14% developed de novo cardiotoxicity (grade ≥2) as measured by decreased LVEF, 27% neutropenia (grade ≥1), 15% anemia (grade ≥1), and 15% liver toxicity (grade ≥1). Possible predictors of adverse events included sex, age, disease duration, and cumulative dose; only women exposed to a higher cumulative dose were at a greater risk of anemia (adjusted odds ratio 1.26, 95% confidence interval 1.08–1.48 per 10 mg/m2).
Conclusions:
Based on cardiac and laboratory assessments, mitoxantrone was reasonably well tolerated. However, cardiotoxicity was evident after doses well below current maximum recommended levels. A dose-response effect was not apparent. Findings emphasize the importance of monitoring; the long-term effects of mitoxantrone in multiple sclerosis require investigation.
GLOSSARY
- AST
= aspartate aminotransferase;
- BMI
= body mass index;
- CI
= confidence interval;
- GEE
= generalized estimating equation;
- LLN
= lower limit of normal;
- LVEF
= left ventricular ejection fraction;
- MS
= multiple sclerosis;
- MUGA
= multiple-gated acquisition;
- OR
= odds ratio;
- UBC
= University of British Columbia;
- ULN
= upper limit of normal.
Mitoxantrone is licensed in the United States and some European countries, and is used “off label” in other countries, including Canada, as a disease-modifying therapy for multiple sclerosis (MS).
Despite its promising therapeutic effects,1–4 widespread use of mitoxantrone for MS is hindered by concerns about potential adverse events. Cardiotoxicity is a major concern; mitoxantrone treatment can result in cardiomyopathy leading to reduced left ventricular ejection fraction (LVEF) and irreversible congestive heart failure.5 Risk increases with cumulative dose,6,7 limiting the recommended lifetime dose in MS to 140 mg/m2. Other potential adverse events include myelosuppression, leading to anemia, neutropenia or leukopenia, and liver toxicity.1,2,4,8–11 The mitoxantrone clinical trials for MS reported low rates of adverse events, whereas some postmarketing studies have revealed a higher incidence of adverse events, particularly subclinical cardiac events.12–15
We examined cardiotoxicity and other laboratory adverse events in a cohort of patients with MS treated with mitoxantrone during routine clinical practice. Sex, age, disease duration, and cumulative dose were investigated as possible predictors of adverse events.
METHODS
This retrospective review included all patients with MS treated with mitoxantrone at the University of British Columbia (UBC) MS Clinic, Vancouver, Canada, with first infusion between July 1999 and December 2007. Subsequent treatments and monitoring were followed up to January 2009. Mitoxantrone is used at the UBC MS clinic to treat patients with active and aggressive MS. Contraindications include use of a cardiotoxic or cytotoxic medication, history of heart disease, and pregnancy or breast-feeding.
The standard treatment protocol during the study period consisted of mitoxantrone (12 mg/m2 IV) infused over 30 minutes, preceded by methylprednisolone (1 g IV) and dolasetron (100 mg IV). Mitoxantrone was administered monthly for 3 months, and then 3-monthly to a maximum cumulative dose of 120 mg/m2. Cardiac function was monitored by a cardiologist and included multiple-gated acquisition (MUGA) scans before infusion (baseline), after the third and sixth doses, and then at the physician's discretion. Complete blood cell count and biochemical liver tests were performed before each infusion. Reduction or termination of infusions due to adverse events was at the physician's discretion.
Laboratory indication of mitoxantrone-induced cardiotoxicity (measured by LVEF) was the primary outcome of interest. Myelosuppression, anemia, and liver toxicity, as measured by neutrophil count, hemoglobin level, and increased aspartate aminotransferase (AST), were also investigated. Patients with normal baseline LVEF (≥50%), neutrophil count (≥ the lower limit of normal [LLN]), hemoglobin level (≥ LLN), or AST (≤ the upper limit of normal [ULN]) and with ≥1 follow-up test result were included in the respective analyses.
Adverse events were described according to the Common Toxicity Criteria for Adverse Events, version 4, as grade 1 (mild), 2 (moderate), 3 (severe), or 4 (life threatening).16
The influence of sex, age (≤35, 36–45, and >46 years), disease duration (≤5, 6–11, and ≥12 years) and cumulative dose of mitoxantrone on the odds of each subclinical adverse event occurring in repeated measures was explored using the generalized estimating equation (GEE). Cardiotoxicity was defined as a follow-up resting LVEF <50% or an absolute reduction in LVEF ≥10% points from baseline; equivalent to at least a grade 2 adverse event (the lowest grade available for this type of adverse event16). Grade 1 or higher events were used to define neutropenia (neutrophil count < LLN), anemia (hemoglobin < LLN), and liver toxicity (AST > UNL). ULN and LLN values were provided by each testing laboratory. All multivariable models were adjusted for ethnicity (white vs nonwhite) and body mass index (BMI).
Potential bias created by the exclusion of patients from the cardiotoxicity analysis was explored by comparing their characteristics to those of the included patients using the χ2 test and the t test. All analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago IL; 2006.).
Standard protocol approvals, registrations, and patient consents.
The UBC Clinical Ethics Board approved the study. Written informed consent was obtained from all patients participating in the study.
RESULTS
During the study period, 163 patients with MS received at least 1 infusion of mitoxantrone; their baseline characteristics and treatment details are shown in table 1. In total, 160 patients were included in at least 1 of the analyses; reasons for exclusion are listed in table 2.
Table 1 Characteristics of mitoxantrone-treated patients with multiple sclerosis (n = 163)
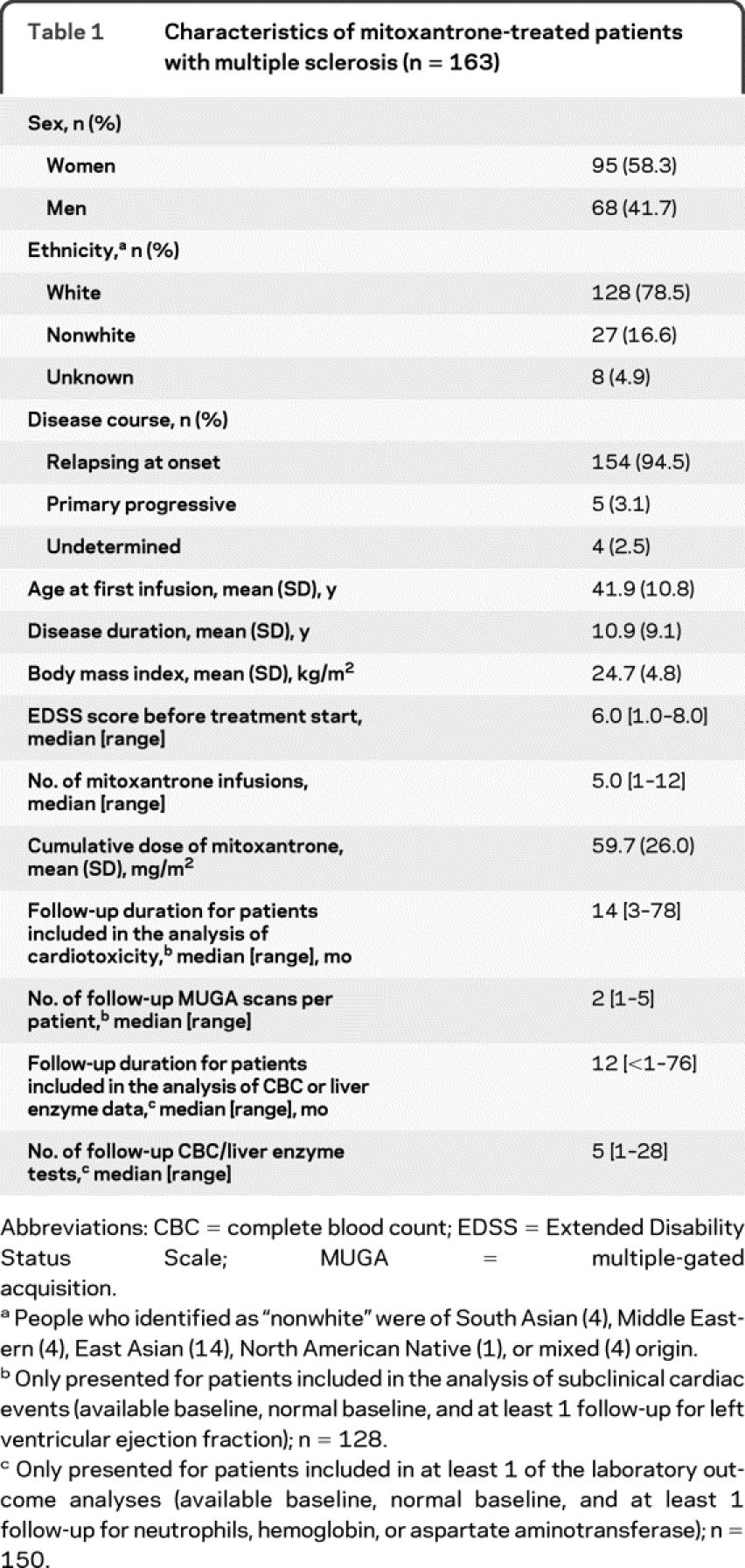
Table 2 Reason for exclusion from adverse event analyses
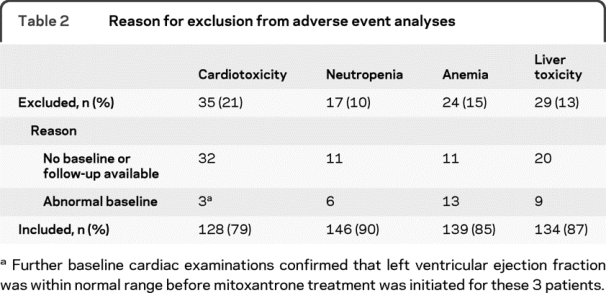
Patients excluded from the cardiotoxicity analysis (n = 35) were comparable to those included (n = 128) by sex, age, disease duration, ethnicity, clinical course, and BMI (p > 0.05). Excluded patients, however, received a lower cumulative dose of mitoxantrone (p < 0.001). All 163 patient files were reviewed for serious adverse events.
Cardiotoxicity.
Among 128 patients with normal baseline LVEF, 18 (14%) developed reduced LVEF (grade 2 toxicity) during follow-up. Of these, 3 (17%) recovered to normal at their next assessment, but 9 (50%) had 2 consecutive low MUGA scans. The remaining 6 had no further available follow-up. In GEE analyses, a subclinical reduction in LVEF was not associated with cumulative dose of mitoxantrone, sex, age, or disease duration (figure e-1A on the Neurology® Web site at www.neurology.org). One woman had nonfatal coronary heart failure 31 months after treatment end (cumulative dose 75.7 mg/m2), having demonstrated a significantly decreased LVEF during monitoring without full recovery.
Neutropenia.
Among 146 patients, 40 (27%) had 1 or more follow-up reduced neutrophil counts (grade ≥1); of these, neutropenia was evident in 26 (18% of all patients) outside of the nadir (i.e., excluding measurements taken 10–18 days after infusion). Seventeen cases had severely decreased counts (grade 3 or 4), but all recovered to at least 1.0 × 10e9/L (grade 2). Neutropenia was not associated with any of the considered variables in GEE analyses (figure e-1B).
Anemia.
Low hemoglobin levels developed in 21 of 139 patients (15%). The maximum toxicity reached (2 cases) was grade 2 (<100 g/L to 80 g/L) after low cumulative doses of mitoxantrone (<50 mg/m2) and being sustained well after treatment ended. Women had a greater odds of low hemoglobin than men (adjusted odds ratio [OR] 8.61, 95% confidence interval [CI] 2.25–32.85), and among women, anemia was associated with increasing dose of mitoxantrone (adjusted OR 1.26, 95% CI 1.08–1.48 per 10 mg/m2; figure e-1C).
Liver toxicity.
Increased AST developed in 21 of 134 patients (16%); the maximum toxicity reached was grade 2 (>3.0–5.0 × ULN). GEE analyses revealed no significant predictors of increased AST (figure e-1D).
DISCUSSION
The incidence of subclinical cardiotoxicity was higher than that reported in the phase II/III mitoxantrone clinical trials2,4 and by some,11,17,18 although not all,12–15 postmarketing studies.
This adverse event is considered moderate by recognized standard criteria,16 but the potential long-term consequences are unknown. Interestingly, no dose-response effect was observed, perhaps because of the relatively narrow dose range used in this cohort. This underlines the need for careful monitoring of all patients with MS during and after treatment as emphasized by the recent, more stringent US Food and Drug Administration guidelines.19 These recommendations include LVEF evaluation before every dose of mitoxantrone and annual evaluations after treatment cessation to detect late-occurring cardiotoxicity.
The observed differences between the results of clinical trials and postmarketing studies are likely due to variation in total follow-up, patient populations, timing of laboratory measurements, monitoring techniques (such as MUGA vs echocardiogram), and criteria for cardiotoxicity. Notably, the definition of decreased LVEF has been inconsistent between studies; greater homogeneity and clarity regarding the criteria used in reports of mitoxantrone-related cardiotoxicity would facilitate pooling and interpretation across studies.
As observed by others,7,17 we found no association of cardiac events with sex or age; we also found no association with MS disease duration at start of treatment. None of these indicators can be regarded as helpful prognostic factors for mitoxantrone associated cardiotoxic events.
The incidences of reversible anemia or neutropenia in patients with MS exposed to mitoxantrone were higher than those reported in the pivotal trial,4 but the incidences of liver toxicity were comparable. These adverse events were mostly transient and not severe. Anemia was far more common in women and was associated with a higher dose; women in particular should be carefully monitored for signs of anemia during mitoxantrone treatment for MS.
Patients with MS could potentially be more susceptible to cardiotoxic or other adverse events; there is evidence that both low ventricular ejection fraction20 and increased liver enzymes21 are more frequent in “untreated” patients with MS than expected. This emphasizes the need for careful monitoring when exposing patients with MS to potentially toxic treatments.
The lack of an untreated comparison group is a limitation of our study; however, serial LVEF measurements would be too invasive to justify enrollment of a control group outside of a clinical trial. Our data were collected retrospectively, and as a result, the documentation was unavoidably incomplete. Excluded patients were exposed to a lower cumulative dose of mitoxantrone and might have experienced more adverse events precipitating cessation of treatment, potentially resulting in an underestimate of the adverse event rate.
These results indicate that cardiotoxic events are evident in patients with MS after doses of mitoxantrone well below current maximum recommended levels. Thus, there is potential for cardiac injury even at the low doses given to patients with MS. Although mitoxantrone was reasonably well tolerated in our cohort over the short term, the long-term cardiac effects are unknown, and extended prospective follow-up is required to examine these and other serious potential side effects of treatment.
AUTHOR CONTRIBUTIONS
All statistical analyses were performed by Elaine Kingwell.
ACKNOWLEDGMENT
The UBC MS Clinic Neurologists contributed to this study through patient examination and data collection. Their contribution and access to their patient data are gratefully acknowledged; contributing neurologists were (in alphabetical order) V. Devonshire, MD; S. Hashimoto, MD; J. Hooge, MD; L. Kastrukoff, MD; J. Oger, MD; D. Paty, MD; P. Smyth, MD; and T. Traboulsee, MD.
DISCLOSURE
Dr. Kingwell receives research support from the Multiple Sclerosis Society of Canada (Postdoctoral Fellowship). Dr. Koch receives research support from the Canadian Institutes of Health Research (CIHR); and has received travel funding from the MS International Federation (Du Pré Grant; http://www.msif.org). Ms. Leung, Dr. Isserow, and Ms. Geddes report no disclosures. Dr. Rieckmann serves on scientific advisory boards for Novartis and Merck Serono; has received speaker honoraria from sanofi-aventis, Bayer Schering Pharma, Biogen Idec, Merck Serono, Novartis, and Teva Pharmaceutical Industries Ltd.; and holds the Multiple Sclerosis Society of Canada Research Chair. Dr. Tremlett has received speaker honoraria from the Swiss Multiple Sclerosis Society and the University of British Columbia Multiple Sclerosis Research Program; has received research support from the Canadian Institutes of Health Research (190898 [PI] and MOP-82738 [PI]), the US National Multiple Sclerosis Society, and the Multiple Sclerosis Society of Canada (Don Paty Career Development Award); and is a Michael Smith Foundation for Health Research Scholar.
Supplementary Material
Address correspondence and reprint requests to Dr. Elaine Kingwell, Faculty of Medicine (Neurology), UBC Hospital, 2211 Wesbrook Mall, University of British Columbia, Vancouver, BC, V6T 2B5 Canada elainejk@interchange.ubc.ca
Supplemental data at www.neurology.org
e-Pub ahead of print on April 28, 2010, at www.neurology.org.
Study funding: The BCMS database was funded by an unrestricted grant from Dr. Donald Paty and the MS/MRI Research Group.
Disclosure: Author disclosures are provided at the end of the article.
Received November 10, 2009. Accepted in final form March 9, 2010.
REFERENCES
- 1.Edan G, Miller D, Clanet M, et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry 1997;62:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millefiorini E, Gasperini C, Pozzilli C, et al. Randomized placebo-controlled trial of mitoxantrone in relapsing-remitting multiple sclerosis: 24-month clinical and MRI outcome. J Neurol 1997;244:153–159. [DOI] [PubMed] [Google Scholar]
- 3.van de Wyngaert FA, Beguin C, D'Hooghe MB, et al. A double-blind clinical trial of mitoxantrone versus methylprednisolone in relapsing, secondary progressive multiple sclerosis. Acta Neurol Belg 2001;101:210–216. [PubMed] [Google Scholar]
- 4.Hartung HP, Gonsette R, Konig N, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet 2002;360:2018–2025. [DOI] [PubMed] [Google Scholar]
- 5.Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf 2000;22:263–302. [DOI] [PubMed] [Google Scholar]
- 6.Mather FJ, Simon RM, Clark GM, Von Hoff DD. Cardiotoxicity in patients treated with mitoxantrone: Southwest Oncology Group phase II studies. Cancer Treat Rep 1987;71:609–613. [PubMed] [Google Scholar]
- 7.Ghalie RG, Edan G, Laurent M, et al. Cardiac adverse effects associated with mitoxantrone (Novantrone) therapy in patients with MS. Neurology 2002;59:909–913. [DOI] [PubMed] [Google Scholar]
- 8.Mauch E, Kornhuber HH, Krapf H, Fetzer U, Laufen H. Treatment of multiple sclerosis with mitoxantrone. Eur Arch Psychiatry Clin Neurosci 1992;242:96–102. [DOI] [PubMed] [Google Scholar]
- 9.Cohen BA, Mikol DD. Mitoxantrone treatment of multiple sclerosis: safety considerations. Neurology 2004;63:S28–S32. [DOI] [PubMed] [Google Scholar]
- 10.Jeffery DR. The argument against the use of cyclophosphamide and mitoxantrone in the treatment of multiple sclerosis. J Neurol Sci 2004;223:41–46. [DOI] [PubMed] [Google Scholar]
- 11.Hamzehloo A, Etemadifar M. Mitoxantrone-induced cardiotoxicity in patients with multiple sclerosis. Arch Iran Med 2006;9:111–114. [PubMed] [Google Scholar]
- 12.Avasarala JR, Cross AH, Clifford DB, Singer BA, Siegel BA, Abbey EE. Rapid onset mitoxantrone-induced cardiotoxicity in secondary progressive multiple sclerosis. Mult Scler 2003;9:59–62. [DOI] [PubMed] [Google Scholar]
- 13.Paul F, Dorr J, Wurfel J, Vogel HP, Zipp F. Early mitoxantrone-induced cardiotoxicity in secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 2007;78:198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hum S, Lapierre Y. A clinical retrospective report on mitoxantrone treatment in active multiple sclerosis patients. Neurology 2009;72:A237. Abstract.
- 15.Rivera V, Weinstock-Guttman B, Beagan J, Bennet R, Al-Sabbagh A, Dangond F. Final results from the registry to evaluate Novantrone. Mult Scler 2009;15:S254. Abstract.
- 16.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.02_2009-09-15_QuickReference_8.5x11.pdf. Accessed January 19, 2010.
- 17.Zingler VC, Nabauer M, Jahn K, et al. Assessment of potential cardiotoxic side effects of mitoxantrone in patients with multiple sclerosis. Eur Neurol 2005;54:28–33. [DOI] [PubMed] [Google Scholar]
- 18.Le Page E, Leray E, Taurin G, et al. Mitoxantrone as induction treatment in aggressive relapsing remitting multiple sclerosis: treatment response factors in a 5 year follow-up observational study of 100 consecutive patients. J Neurol Neurosurg Psychiatry 2008;79:52–56. [DOI] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration. Mitoxantrone Hydrochloride (marketed as Novantrone and generics)—Healthcare Professional Sheet. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126445.htm. Accessed August 20, 2009.
- 20.Olindo S, Guillon B, Helias J, Phillibert B, Magne C, Feve JR. Decrease in heart ventricular ejection fraction during multiple sclerosis. Eur J Neurol 2002;9:287– 291. [DOI] [PubMed] [Google Scholar]
- 21.Tremlett H, Seemuller S, Zhao Y, Yoshida EM, Oger JD, Petkau J. Liver test abnormalities in multiple sclerosis: findings from placebo-treated patients. Neurology 2006;67:1291–1293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



