Abstract
Background:
Idiopathic intracranial hypertension (IIH) typically affects young, obese women. We examined 2 groups of atypical patients with IIH: those with a normal body mass index (BMI) and those at least 50 years of age.
Methods:
A retrospective cohort study of 407 consecutive adult patients with IIH with known BMI from 3 centers was undertaken. Demographics, associated factors, visual acuity, and visual fields were collected at presentation and follow-up.
Results:
We identified 18 IIH patients (4%) with normal BMI and 19 (5%) aged 50 years or older at the time of diagnosis who were compared with the remainder of the cohort. Medication-induced IIH was more frequent in patients with IIH with normal BMI (28 vs 7%, p = 0.008). No patient with IIH with a normal BMI had severe visual loss in either eye (0 vs 17%, p = 0.09). Older patients with IIH had a lower BMI, but were still generally obese (33 vs 38, p = 0.04). Older patients were less likely to report headache as initial symptom (37 vs 76%, p < 0.001) and more likely to complain of visual changes (42 vs 21%, p = 0.03). Treatment of any type was less likely in older patients (significant for medications: 74 vs 91%, p = 0.004), and they were more likely to have persistent disc edema at last follow-up (median Frisén grade: 1 vs 0, p = 0.002), but had similar, if not better, visual outcomes compared with younger patients. A case-control study did not identify any new medication or risk factor associations.
Conclusions:
Patients with normal body mass index and those 50 years or older make up a small proportion of patients with idiopathic intracranial hypertension (IIH), but appear to have better visual outcomes than more typical patients with IIH.
GLOSSARY
- BMI
= body mass index;
- IIH
= idiopathic intracranial hypertension;
- MR
= magnetic resonance.
Although idiopathic intracranial hypertension (IIH) typically occurs in young, obese women, it may occur outside of this characteristic demographic. Two previous reports have specifically evaluated the characteristics of older patients with IIH and suggested differences in presentation and outcomes. To our knowledge, no previous studies have studied the characteristics of normal-weight patients with IIH. The purpose of this study was to compare the characteristics of patients with IIH with a body mass index (BMI) less than 25 to overweight and obese patients with IIH and the characteristics of patients with IIH 50 years or older to younger, adult patients with IIH.
METHODS
Standard protocol approvals, registrations, and patient consents.
Each participating university's Institutional Review Board approved the study with a waiver of consent.
Study conduct.
All consecutive charts for patients given the diagnosis code of IIH or disc edema seen by the neuro-ophthalmology services at Emory University (1989–2007), University of Mississippi (1989–2007), and Wayne State University (2001–2007) were identified and reviewed. Only patients with definite IIH diagnosed according to the modified Dandy criteria were included: 1) signs and symptoms of increased intracranial pressure, 2) no localizing signs except abducens nerve palsy, 3) CSF opening pressure ≥25 cm with normal CSF composition, and 4) normal neuroimaging (ruling out venous sinus thrombosis).1
Although the patient database was created using a retrospective chart review, all patients had been evaluated in a standardized fashion by experienced neuro-ophthalmologists, including documentation of body habitus, blood pressure, and complete neuro-ophthalmic examination with formal visual fields, fundus photography, review of neuroimaging tests, and recording of factors associated with IIH. Demographic information regarding age, gender, and race were collected. Patients younger than 18 years of age at diagnosis were excluded. Race was assessed by the judgment of the examiner based on patient appearance. Medication use (current and recent), the presence or absence of several associated factors (recent weight gain, known sleep apnea, anemia [hemoglobin <12 g/dL], systemic hypertension, endocrine disorders, and pregnancy), symptoms (headache, tinnitus, diplopia, and transient visual obscuration), Snellen visual acuity, formal visual fields (static perimetry using a Humphrey automated perimeter and kinetic perimetry using a Goldmann perimeter), and dilated ophthalmoscopic appearance were recorded. Medications considered possibly contributing included vitamin A preparations, minocycline, cyclosporine, doxycycline, tetracycline, depot progestins, and recent discontinuation of steroids. The contributing medications were grouped by their presence or absence in each patient for analysis. Patients without known height or weight were excluded from analysis. BMI was calculated for use in statistical analyses according to the World Health Organization BMI guidelines.2 Prediagnosis duration of symptoms, CSF opening pressure, height, weight, medical treatments, surgical treatments, follow-up duration, and visual outcome were also recorded.
Snellen visual acuity was converted to logMAR visual acuity for analysis. Formal visual fields were systematically reviewed for all patients. All visual field defects, whether obtained with static or kinetic perimetry, were graded on a 1 to 4 scale as 1) normal; 2) enlargement of the blind spot; 3) nasal or temporal defect; or 4) diffusely constricted, as previously published.3,4 In addition, mean deviations were recorded for those patients who underwent static automated perimetry. Papilledema was graded with the Frisén staging scheme5 by systematic review of fundus photography: stage 0 defines a normal optic nerve head and stage 5, severe papilledema. Severe visual loss in an eye was defined by the United States criteria for legal blindness (best-corrected visual acuity less than or equal to 20/200 or total central visual field less than 20 degrees) and assessed at the last available visit.
All patients had definite IIH by the modified Dandy criteria, but 2 aspects of our population merit further mention. First, although all patients underwent a lumbar puncture that documented elevation of CSF opening pressure, the specific value was sometimes unavailable: 3 (16%) of the patients with BMI <25, 2 (11%) of the patients aged 50 or older, and 56 (15%) of the remainder of the cohort. Second, clinically appropriate neuroimaging was performed on all patients to rule out cerebral venous thrombosis. However, as the patient population is representative of an actual clinical practice, there were occasionally practical limitations to obtaining ideal imaging studies, such as body habitus preventing entry into imaging gantries and changes in the clinical usage of MRI and magnetic resonance (MR) venography over the study period.6 MRIs were all reviewed at the time of diagnosis and MR venography or CT venography was obtained when there was a question regarding possible cerebral venous thrombosis. Those patients who could not have MRI had head CT with contrast, often accompanied by CT venography.
Data analysis.
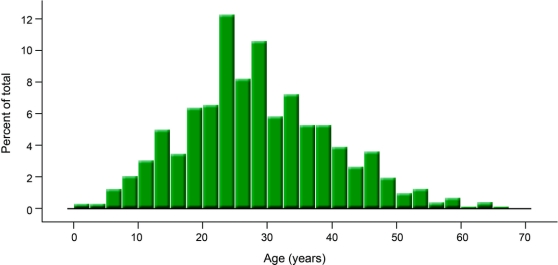
Two groups of patients were identified and analyzed separately: 1) patients considered to be of normal weight by World Health Organization BMI guidelines (BMI <25), and 2) patients aged 50 years or older at the time of diagnosis. This age cutoff was identified using a data-driven approach as the age approximately 2 standard deviations above the mean age of all 721 patients with IIH in our database, including pediatric cases (mean: 29 years, SD: 11 years, figure 1). For each of these atypical patients, a control patient with IIH matched by race, presence or absence of hypertension, and year of visit was randomly selected from the remaining cohort.
Figure 1 Age distribution of 721 patients with idiopathic intracranial hypertension
Statistical analysis was performed with R: A language and environment for statistical computing (R Foundation for Statistical Computing, http://www.R-project.org). Continuous and ordinal variables were compared between groups using the Mann-Whitney U test. Pearson χ2 with Yates' continuity correction or Fisher exact test, as appropriate, were used to compare the frequency distribution of categorical variables between groups. These tests were 2-tailed, and significance was set at 5%. Univariate analyses for normal weight and older age vs other factors were undertaken on the entire population. In the case of missing data, the number of patients available for each analysis was reported. McNemar χ2 was used to test for medication and past medical history associations between cases and controls.
RESULTS
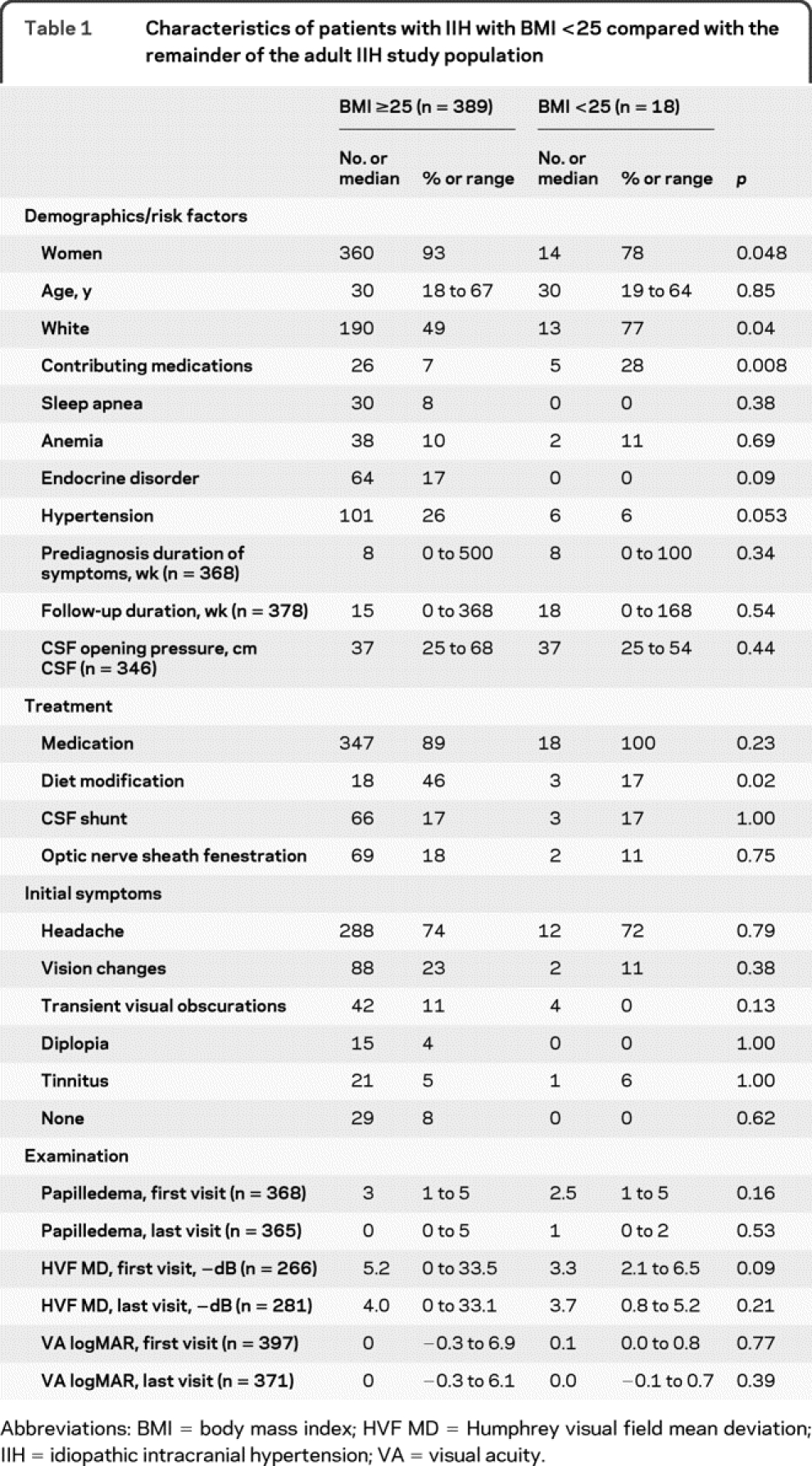
We identified 407 consecutive, adult patients with IIH with known BMI. Of these, 18 (4%) had a normal BMI (<25). There were 341 patients (84%) who had a BMI ≥30. Among patients with normal BMI (table 1), medication-induced IIH was more frequent (28 vs 7%, p = 0.008). The implicated medications in the patients with normal BMI were vitamin A preparations (2 patients), depot progestins (2 patients), and recent steroid discontinuation (1 patient). The patients with normal BMI were more likely to be men (22 vs 7%, p = 0.048) and less likely to receive diet modification therapy (17 vs 46%, p = 0.02). No patient with IIH with a normal BMI had severe visual loss in either eye (0 vs 17%, p = 0.09).
Table 1 Characteristics of patients with IIH with BMI <25 compared with the remainder of the adult IIH study population
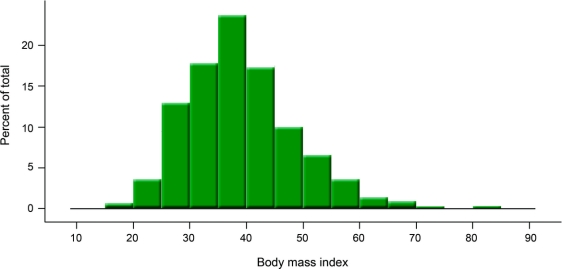
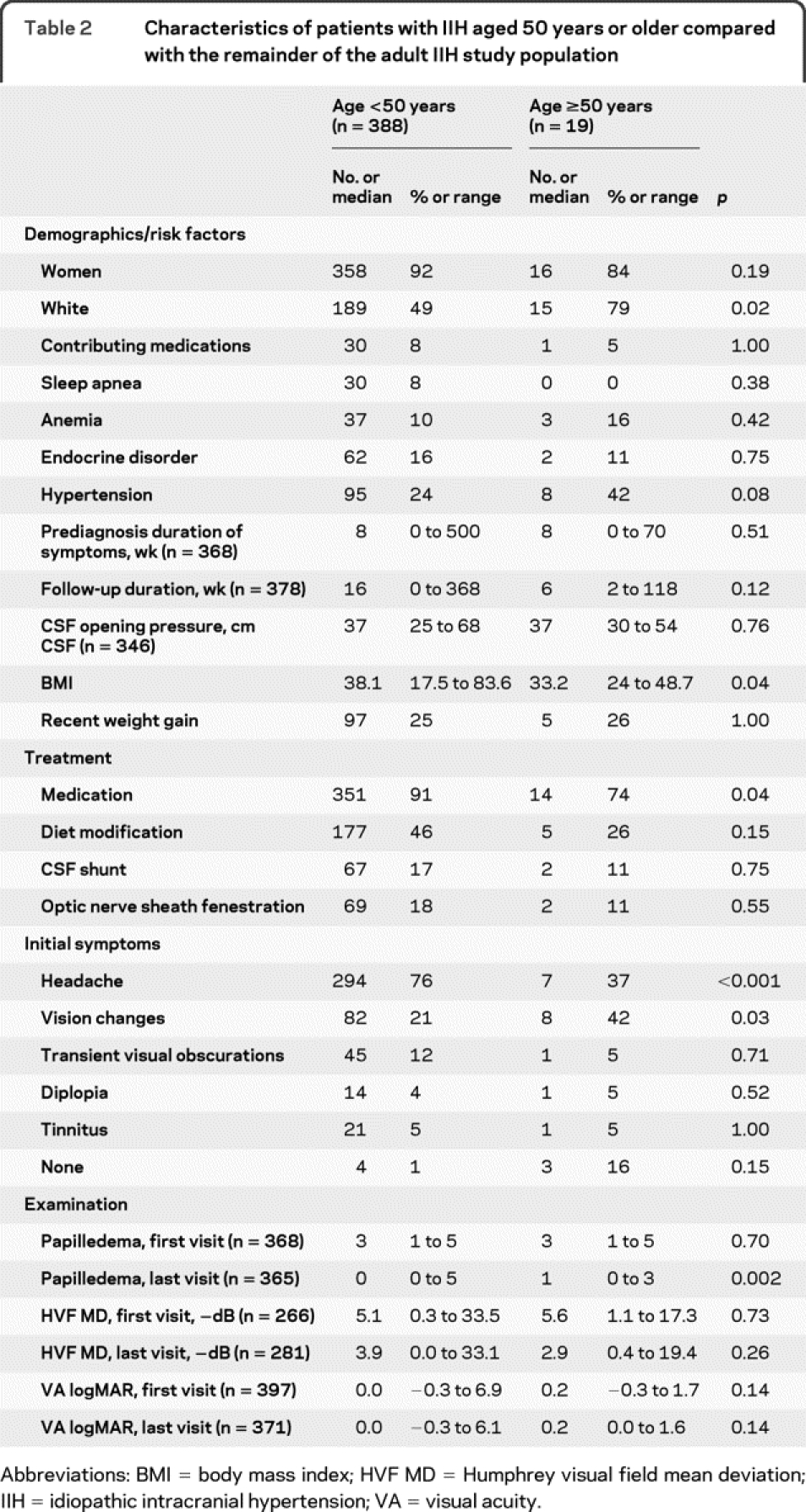
There were 19 patients with IIH (5%) who were at least 50 years old at the time of diagnosis (range 50–67 years, figure 2). Five patients (1%) were older than 60. Older patients with IIH (table 2) had a lower BMI, but were still generally obese (33 vs 38, p = 0.04). They were less likely to report headache as an initial sign of their illness (36 vs 76%, p < 0.001) and more likely to complain of visual changes (42 vs 21%, p = 0.03). Treatment of any type was less likely (significant for medications: 74 vs 90%, p = 0.04). These older patients were more likely to have mild, persistent disc edema at last follow-up (median Frisén grade: 1 vs 0, p = 0.002), but appeared to have similar, if not better, visual outcomes compared with younger patients. Only one older IIH patient had severe visual loss in either eye (5% vs 15%, p = 0.33).
Figure 2 Body mass index distribution of 407 patients with idiopathic intracranial hypertension
Table 2 Characteristics of patients with IIH aged 50 years or older compared with the remainder of the adult IIH study population
Both patients with normal BMI and older patients were more likely to be white (77%–79% vs 49%, p = 0.04) than the rest of the cohort. The case-control part of the study did not identify any new medication or risk factor associations.
DISCUSSION
Our study's proportion of obese patients was similar to other studies, which report rates between 74% and 94%, but direct comparisons are difficult because these studies use various definitions of obesity.7–15 Although other studies have evaluated the proportion and characteristics of obese patients with IIH, we are unaware of other studies that have specifically examined the characteristics of nonobese patients with IIH. We found that 4% of our patients with IIH were of normal weight, and of these, over a quarter were exposed to a medication that might be associated with IIH. An additional quarter of normal weight patients were atypical in some other fashion: they were men or were at least 50 years of age. Thus, as expected from clinical experience, otherwise typical IIH occurs very rarely in patients of normal weight and careful evaluation for alternate causes should be undertaken when the diagnosis is considered in persons with a normal BMI.
Given the high proportion of medication-related IIH among our patients with normal weight, we attempted to identify new medication associations with IIH through a case-control study between our atypical patients with IIH and other patients with IIH, but we did not find any such association. However, by only using other patients with IIH rather than normal persons as controls, our power to find such associations would have been reduced, because other patients with IIH, despite their more typical features, may also have had an unknown medication or other risk factor that contributed to their development of IIH.
Patients 50 years or older also represented only a small percentage of patients with IIH, but both older and normal weight patients shared several characteristics. First, and most importantly, both groups appeared to have similar, if not, better visual outcomes compared with the typical patient with IIH. A previous descriptive series of 14 patients with IIH aged 44–88 years of age suggested that the visual prognosis was generally good in older patients.16 This same series also suggested, as our study confirms, that older patients are less likely to have headache and are not as obese as the typical IIH patient. Another descriptive series of 23 patients with IIH older than 40 years found, as we did, that older patients with IIH had fewer complaints of headache and were more likely to complain of visual disturbances.17 Older patients appeared more likely to have better visual outcomes based on a reduced rate of severe visual loss and better visual field parameters. Our older patients also received less surgical and less medical therapy (table 2), further suggesting that they had less progressive visual loss and milder symptoms. These improved visual outcomes occurred despite an increased frequency of vision-related symptoms as their initial complaint. This is in direct contrast to men with IIH, who more frequently had poorer visual outcomes in the setting of more frequent vision-related presentations.3 The better visual outcomes among older patients with IIH may be related to their relatively mild, although persistent, papilledema seen at last follow-up. Indeed, we also observed among patients with normal BMI what might represent a similar trend toward milder, but persistent papilledema with equal or better visual outcomes at last follow-up. It is worth noting that previous studies have not clearly supported a direct relationship between the degree of papilledema and visual outcome, except for cases of severe or atrophic papilledema.18
Patients with normal BMI and older patients were about 1.5 times more likely to be white. In the case of the normal weight patients, this may represent confounding related to differences in the baseline prevalence of obesity between whites (24%) and blacks (36%). Among women, those at highest risk for IIH, the difference is even greater: 39% of black women are obese, compared with only 22% of white women.19 Similarly, confounding may be responsible for the higher proportion of white subjects seen among our older patients with IIH because 16% of blacks are 55 or older in the US population, compared with 24% of whites.20 These characteristics of the underlying population would tend to lead to an overrepresentation of white subjects among both nonobese and older patients. We also found trends toward more frequent hypertension in both more obese and older patients with IIH, but this is again likely due to confounding, because these groups of patients would be expected to be at higher risk of hypertension compared with their less obese, younger counterparts.
The main limitations of our study are its retrospective nature and our investigation of the rarest aspects of IIH, inherently limiting the number of patients under direct study. However, all of our patients were systematically evaluated by experienced neuro-ophthalmologists and our agreement with the findings of other previous small series of older patients suggests the external validity of our results. In addition, patients with normal weight were less likely to be treated with diet modification, which, while unsurprising, lends support for the internal validity of our dataset. Finally, it is likely that we have seen a higher proportion of atypical patients with IIH because all sites were tertiary referral centers that might be expected to see a higher proportion of such patients. If this is the case, it only strengthens our conclusions regarding the rarity of IIH among patients who are either normal weight or who are at least 50 years of age, and the importance of careful evaluation for alternate etiologies in these patients.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Beau B. Bruce.
COINVESTIGATORS
David Monaghan, BS (University of Mississippi, Site Subinvestigator); Marie D. Acierno, MD (Louisiana State University, Site Subinvestigator); Ronald A. Braswell, MD (University of Alabama, Birmingham, Site Subinvestigator); Pisit Preechawat, MD (Ramathibodi Hospital, Mahidol University, Thailand, Site Subinvestigator).
DISCLOSURE
Dr. Bruce receives research support from the NIH/PHS (UL1-RR025008 and KL2-RR025009 [Scholar]); and received the American Academy of Neurology Practice Research Fellowship. Dr. Kedar reports no disclosures. Dr. Van Stavern has received travel expenses and speaker honoraria from Pfizer Inc. and Teva Pharmaceutical Industries Ltd. Dr. Corbett serves as an Associate Editor for the Journal of Neuro-ophthalmology and The Neurologist, as a section editor for Current Neurology & Neuroscience Reviews, and on the editorial board of the Turkish Journal of Neurology; serves as a consultant for Eisai Inc.; receives royalties from the publication of Practical Viewing of the Optic Disc (Elsevier Health Sciences, 2002); receives research support from Eisai Inc. and the NIH/NEI (U10-EY017281 [Co-Director, IIHTT]); and has provided expert testimony and served as a witness on the topic of idiopathic intracranial hypertension. Dr. Newman serves as an Associate Editor of the American Journal of Ophthalmology and the Journal of Neuro-ophthalmology and serves on the editorial board of the Journal of the Neurological Sciences; receives royalties from the publication of Walsh And Hoyt's Clinical Neuro-ophthalmology, 5th and 6th eds. (Lippincott Williams & Wilkins, 1998, 2005), Walsh & Hoyt's Clinical Neuro-Ophthalmology: The Essentials, 1st and 2nd eds. (Lippincott Williams & Wilkins, 1999, 2008), Blue Books of Neurology: Neuro-Ophthalmology (Butterworth Heinemann Elsevier, 2008), and Neuro-ophthalmology Illustrated (Thieme, 2009); and is a recipient of the Research to Prevent Blindness and a Lew R. Wasserman Merit Award. Dr. Biousse serves on the editorial boards of the American Journal of Ophthalmology, Reviews in Neurological Diseases, and the Journal of Neuro-ophthalmology; receives royalties from the publication of Walsh And Hoyt's Clinical Neuro-ophthalmology, 5th and 6th eds. (Lippincott Williams & Wilkins, 1998, 2005), Walsh & Hoyt's Clinical Neuro-Ophthalmology: The Essentials, 1st and 2nd eds. (Lippincott Williams & Wilkins, 1999, 2008), Blue Books of Neurology: Neuro-Ophthalmology (Butterworth Heinemann Elsevier, 2008), and Neuro-ophthalmology Illustrated (Thieme, 2009); and receives research support from the NIH (PHS Grant UL1-RR025008).
Address correspondence and reprint requests to Dr. Beau Bruce, Neuro-ophthalmology Unit, Emory Eye Center, 1365-B Clifton Road, NE, Atlanta, GA 30322 bbbruce@emory.edu
Study funding: Supported by Research to Prevent Blindness and the NIH (RR025009, RR025008, and EY06360).
Disclosure: Author disclosures are provided at the end of the article.
Received December 29, 2009. Accepted in final form March 1, 2010.
REFERENCES
- 1.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002;59:1492–1495. [DOI] [PubMed] [Google Scholar]
- 2.Physical status: the use and interpretation of anthropometry: report of a WHO expert committee. WHO Tech Rep Ser 1995;854:1–452. [PubMed] [Google Scholar]
- 3.Bruce BB, Kedar S, Van Stavern GP, et al. Idiopathic intracranial hypertension in men. Neurology 2009;72:304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruce BB, Preechawat P, Newman NJ, Lynn MJ, Biousse V. Racial differences in idiopathic intracranial hypertension. Neurology 2008;70:861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frisén L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry 1982;45:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology 1999;53:1537–1542. [DOI] [PubMed] [Google Scholar]
- 7.Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri: population studies in Iowa and Louisiana. Arch Neurol 1988;45:875–877. [DOI] [PubMed] [Google Scholar]
- 8.Galvin JA, Van Stavern GP. Clinical characterization of idiopathic intracranial hypertension at the Detroit Medical Center. J Neurol Sci 2004;223:157–160. [DOI] [PubMed] [Google Scholar]
- 9.Kesler A, Gadoth N. Epidemiology of idiopathic intracranial hypertension in Israel. J Neuroophthalmol 2001;21:12–14. [DOI] [PubMed] [Google Scholar]
- 10.Radhakrishnan K, Ahlskog JE, Cross SA, Kurland LT, O'Fallon WM. Idiopathic intracranial hypertension (pseudotumor cerebri): descriptive epidemiology in Rochester, Minn, 1976 to 1990. Arch Neurol 1993;50:78–80. [DOI] [PubMed] [Google Scholar]
- 11.Radhakrishnan K, Ahlskog JE, Garrity JA, Kurland LT. Idiopathic intracranial hypertension. Mayo Clin Proc 1994;69:169–180. [DOI] [PubMed] [Google Scholar]
- 12.Radhakrishnan K, Sridharan R, Ashok PP, Mousa ME. Pseudotumour cerebri: incidence and pattern in North-Eastern Libya. Eur Neurol 1986;25:117–124. [DOI] [PubMed] [Google Scholar]
- 13.Radhakrishnan K, Thacker AK, Bohlaga NH, Maloo JC, Gerryo SE. Epidemiology of idiopathic intracranial hypertension: a prospective and case-control study. J Neurol Sci 1993;116:18–28. [DOI] [PubMed] [Google Scholar]
- 14.Wall M, George D. Idiopathic intracranial hypertension: a prospective study of 50 patients. Brain 1991;114:155–180. [PubMed] [Google Scholar]
- 15.Kesler A, Hadayer A, Goldhammer Y, Almog Y, Korczyn AD. Idiopathic intracranial hypertension: risk of recurrences. Neurology 2004;63:1737–1739. [DOI] [PubMed] [Google Scholar]
- 16.Bandyopadhyay S, Jacobson DM. Clinical features of late-onset pseudotumor cerebri fulfilling the modified dandy criteria. J Neuroophthalmol 2002;22:9–11. [DOI] [PubMed] [Google Scholar]
- 17.Zayit-Soudry S, Leibovitch I, Kesler A. Idiopathic intracranial hypertension after 40 years of age: clinical features in 23 patients. Eur J Ophthalmol 2008;18:989–993. [DOI] [PubMed] [Google Scholar]
- 18.Orcutt JC, Page NG, Sanders MD. Factors affecting visual loss in benign intracranial hypertension. Ophthalmology 1984;91:1303–1312. [DOI] [PubMed] [Google Scholar]
- 19.Differences in prevalence of obesity among black, white, and Hispanic adults: United States, 2006–2008. MMWR Morb Mortal Wkly Rep 2009;58:740–744. [PubMed] [Google Scholar]
- 20.US Census Bureau. Annual population estimates by sex, race, and Hispanic origin, selected years from 1990 to 2000. Available at: http://www.census.gov/popest/archives/1990s/nat_sex_race_hispanic.html. Accessed April 27, 2010.