Abstract
Background:
Multiple sclerosis (MS) has a remarkable geographic distribution inversely paralleling that of regional ultraviolet radiation, supporting the hypothesis that vitamin D plays a central role in the disease etiology. The major histocompatibility complex exerts the largest genetic contribution to MS susceptibility, but much risk remains unexplained and direct gene-environment interaction is a strong candidate for this additional risk. Such interactions may hold the key for disease prevention.
Recent developments:
Several recent studies strengthen the candidacy of vitamin D as a key player in the causal cascade to MS. This includes a newly identified gene-environment interaction between vitamin D and the main MS-linked HLA-DRB1*1501 allele and evidence showing that vitamin D levels are significantly lower in patients with MS as compared to controls. Also, a recent study in twins with MS supports the notion that vitamin D levels are under regulation by genetic variation in the 1α-hydroxylase and vitamin D receptor genes, perhaps pointing to their importance in the disease pathogenesis.
Conclusions:
These findings have important practical implications for studies of disease mechanisms and prevention. Missing genetic risk may partly be explained by gene-environment interactions. More practically important is that these observations highlight a pressing need to determine if vitamin D supplementation can reduce the risk of multiple sclerosis (MS). However, the timing of action and the tissues in which this interaction takes place are not clear and future studies in prospective cohorts and animal models will be essential for deciphering the role of vitamin D in MS.
GLOSSARY
- MHC
= major histocompatibility complex;
- MS
= multiple sclerosis;
- VDR
= vitamin D receptor;
- VDRE
= vitamin D response element.
In 1902, Sir Archibald Garrod1 noted that “the influences of diet can mask some inborn errors of metabolism,” giving birth to the concept of gene-environment interactions in human disease. The impact of background genes in the phenotype have finally been given more play2 but now, over a century later, gene-environment interactions are being hailed as the future of public health. Their potential in disease prevention strategies seems vast.
A unifying theme emerging from recent work is that gene-environment interactions are likely to underpin many complex neurologic traits. Such interactions can be observed at statistical and biological levels. An example of statistical interaction is seen between smoking and the CFH gene. Both the presence of the CFH Y402H risk variant and a history of smoking synergistically confer an increased risk to age-related macular degeneration, as compared with either factor alone.3 Although the mechanism behind the interaction is not fully understood, this has led to lifestyle recommendations that may prevent age-related macular degeneration in high-risk individuals.
A biological interaction is defined as the observation of a direct physical or chemical reaction and coparticipation between environmental and genetic factors in the causal mechanism of disease development. Examples of fully understood interactions are rare. Here we consider a recent study that puts the theory of biological gene-environment interactions into practice by demonstrating direct links between a leading candidate environmental factor, vitamin D, and the main susceptibility region which contains the HLA-DRB1 locus. Accordingly, we review the existing evidence for a possible role of vitamin D in MS and discuss the relevance of recent findings.
MS: GENES AND ENVIRONMENT
Epidemiologic studies in MS provide overwhelming evidence that both genes and environment are important in the etiology of the disease. If genetic factors are held constant, the environment and stochastic events presumably set the disease threshold. Although the effect of the environment in MS is not necessarily mediated by a single factor, latitude demonstrates the strongest association.4 In the Northern hemisphere, MS prevalence shows a north-south gradient, mirrored by a south-north gradient in the Southern hemisphere. In accordance with the disease geography, sunlight, specifically through its role in generating active vitamin D, has been implicated as a key environmental factor in MS.5
Recent support for this theory comes from the finding that patients with MS studied prospectively had significantly low levels of circulating vitamin D [25(OH)D] during their adolescence prior to disease onset compared to age-, sex-, and ethnicity-matched controls.6 In the Caucasian population, there was a 41% decrease in MS risk for every 50 nmol/L increase in 25(OH)D. Other key observations that had lent credence to this hypothesis include age-dependent low risk of MS in UK migrants to sunny South Africa,7 differential twin concordance rates by latitude of birthplace,8 and month of birth effects highlighting a risk factor that varies seasonally in MS.9 A detailed list of reports supporting this concept is summarized in table 1 and expanded in table e-1 on the Neurology® Web site at www.neurology.org.
Table 1 Circumstantial evidence for a role of vitamin D in multiple sclerosis
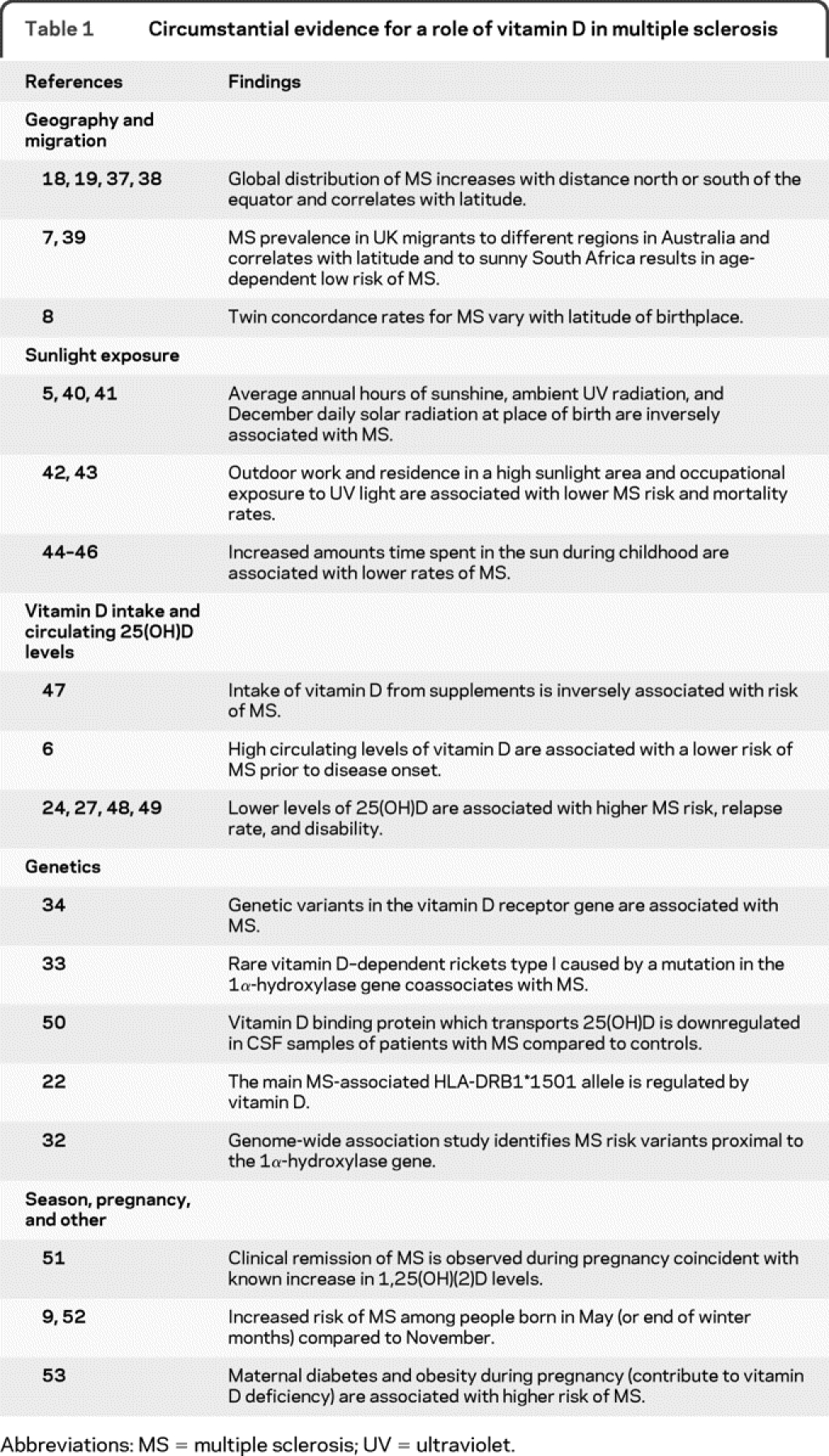
However, the environment cannot explain why African American, Asian, and North American Aboriginal individuals are inherently more resistant to MS compared to white individuals, despite residing in temperate regions with high disease prevalence.10 This emphasizes the important role of genetics. The largest MS genetic association in Northern Europeans is with the extended major histocompatibility complex (MHC) haplotype HLA-DQB1*0602-DQA1*0102-DRB1*1501-DRB5*0101(DR2).11 Other haplotypes in this region also demonstrate epistatic interactions to modify risk, highlighting the MHC as the key susceptibility locus in MS.
Several investigators have suggested that the frequency of MS tracks the distribution of northern European genes,12–14 and that susceptibility genes show much of the same geographical patterns as the distribution of MS.15 For example, increased prevalence of MS in Scotland compared to England has been credited to the higher frequency of the DR2 haplotype in the general Scottish population.16 This is especially true in the Orkneys, where the frequency of DR2 and the disease incidence are both the highest recorded in the world.17
However, the geography of the disease cannot be solely linked to genetic clines, as individuals from the same ethnic derivation can also have very different risks for MS in areas separated by latitude.18 US data reveal a north-south as well as an east-west gradient attributed to the latitude differences and the pattern of Scandinavian immigration.19 Collectively, these observations point to the conclusion that the distribution of MS cannot be explained by any single known environmental or genetic factor in isolation. A heterogeneous distribution of genes, environmental factors, and particularly their interplay appears to be required.
INTERACTION BETWEEN VITAMIN D AND HLA-DRB1
Ultraviolet radiation–dependent metabolism provides the major source of vitamin D in humans but at high latitudes solar radiation is too low to produce adequate levels of vitamin D, particularly in the winter and adjacent months.20 Biological effects of the active form of vitamin D (1,25-dihydroxyvitamin D3) are mediated by the vitamin D receptor (VDR). This receptor is a member of the steroid receptor superfamily and influences the rate of transcription of vitamin D–responsive genes by binding to vitamin D response elements (VDREs) in the genome. The effects of vitamin D on MHC class II gene expression have long been appreciated and early studies demonstrate that vitamin D can alter HLA-DR antigen expression and presentation.21
In an attempt to couple genetics with the environment, Ramagopalan et al.22 recently searched known MS susceptibility loci for VDREs, to determine if they could be regulated by vitamin D. A single VDRE in the HLA-DRB1 promoter region was identified. Strikingly, this VDRE showed haplotype-specific differences, being highly conserved (no mutations on over 600 chromosomes) in the major MS-associated DR2 haplotype bearing the HLA-DRB1*15 allele but not conserved generally among non–MS-associated haplotypes. Functional assays were then used to demonstrate that this VDRE influenced gene expression, thereby conferring 1,25-dihydroxyvitamin D3 sensitivity to HLA-DRB1*15. The variant VDRE present on other, non–MS-associated HLA-DRB1 haplotypes was not responsive to 1,25-dihydroxyvitamin D3. It should be noted that several MS and autoimmune disease–associated haplotypes not addressed in this study could perhaps harbor conserved VDREs and call for a more detailed examination of this region, but the non–MS-associated HLA-DRB1*04, 07, and 09 haplotypes contain polymorphisms, resulting in a nonfunctional VDRE.
These experiments provide evidence for a direct biological interaction between HLA-DRB1, the main MS susceptibility locus, and vitamin D, a key candidate for mediating the environmental effect. The role of this interaction in disease etiology remains to be discovered but it is plausible that a lack of vitamin D in early childhood can affect the expression of HLA-DRB1 in the thymus. It can be speculated that a general reduction in the expression of disease-associated class II alleles including HLA-DRB1*15 in the thymus during early life might result in loss of central tolerance, perhaps increasing the risk of autoimmunity in later life.
PUTTING THE INTERACTION IN CONTEXT
Understanding this HLA–vitamin D interaction will undoubtedly be critical for dissecting disease mechanisms and future prevention strategies. The frequency of the MS-associated HLA-DRB1*1501 allele varies substantially between ethnic backgrounds, being high in Caucasian individuals and low in African and Asian individuals. Therefore, this finding partially illuminates the geographical distribution of MS and offers some explanation as to why low prevalence of MS is observed in races with dark skins living in temperate clines regardless of heightened vitamin D deficiency due to the high ultraviolet-filtering capability of cutaneous melanin. Moreover, given that the frequency of HLA-DRB1*15 is significantly higher in female patients with MS as compared to male patients with MS,23 this interaction, at least in part, may explain why higher levels of vitamin D are associated with a lower incidence of MS, specifically in women.24
In addition, a recent report on multigenerational MS pedigrees provides evidence for an increased penetrance of HLA-DRB1*15 over time in a female-specific manner, paralleling the increasing risk of MS in females.25 The intriguing possibility that decreasing levels of vitamin D in the general population26 due to changes in lifestyle (lack of outdoor activities, increased use of sun protection, and high obesity levels) have amplified the frequency of HLA-DRB1*15 in MS over time warrants further consideration. Female bias may arise from gender differences in lifestyle choices and vitamin D metabolism. Indeed, lower vitamin D intakes and circulating concentrations of 25(OH)D have been observed in females compared to males and estrogen has been shown to control vitamin D3–mediated resistance to experimental autoimmune encephalomyelitis.
The timing of action and tissues in which this interaction might occur can only be speculated upon. It is tempting to hypothesize that the increased risk of MS among people born in May reflects maternal end-of-winter deficiencies in vitamin D. The recent observation that the month of birth effect is mediated by patients with MS bearing the HLA-DRB1*1501 allele as compared to those with non-HLA-DRB1*1501 alleles supports this notion. Epigenetics may underpin gene-environment interactions and the resulting expression changes due to fetal reprogramming could later predispose adult disease. Such an argument can be further extrapolated to include the grandmaternal environment as she houses cells of 3 generations during her pregnancy.
However, the role of vitamin D in MS may extend beyond its interaction with HLA-DRB1*15 and many MS relevant loci may be regulated by vitamin D. For example, active vitamin D has been shown to upregulate the biological activity of indoleamine 2,3-dioxygenase, resulting in a significant increase in the number of CD4+ CD25+ T-regulatory cells.27 Accordingly, a recent study shows that serum levels of 25(OH)D correlate with the ability of T-regulatory cells to suppress T-cell proliferation in patients with MS.28 This observation perhaps accounts for the potential beneficial effects of vitamin D via a peripheral mechanism in adulthood and following disease onset.
VITAMIN D LEVELS AND SUPPLEMENTATION
Can vitamin D supplementation reduce the risk of MS? To answer this question, let us consider a classic example of gene-environment interaction. Tryon's29 artificial selection experiment produced a remarkable difference in maze-running ability in 2 selected lines of rats after 7 generations of selecting the “best” and “worst” maze-running rats and breeding within their respective categories. Amazingly, the difference in ability disappeared in a single generation, if those rats were raised in an enriched environment with more objects to explore. This elegantly demonstrates that a genetic effect is only seen under some environmental conditions. Similarly, it is reasonable to hypothesize that the effect of HLA-DRB1*15 is only significant in the absence of sufficient vitamin D levels.
Given that a high frequency of vitamin D insufficiency has been observed in the general population, a strong argument can and has been made for supplementation in the general population. We discuss critical time periods relevant to MS in table 2. There is an emerging consensus that public health recommendations for vitamin D intake (200 IU/day) fall below the level required for protection against MS and other vitamin D–related diseases (1,000–4,000 IU/day).30 Tailoring vitamin D supplementation to genetic profile (i.e., selecting for the presence of HLA-DRB1*15) may be the most efficient method of identifying benefits.
Table 2 Possible time periods for a vitamin D effect
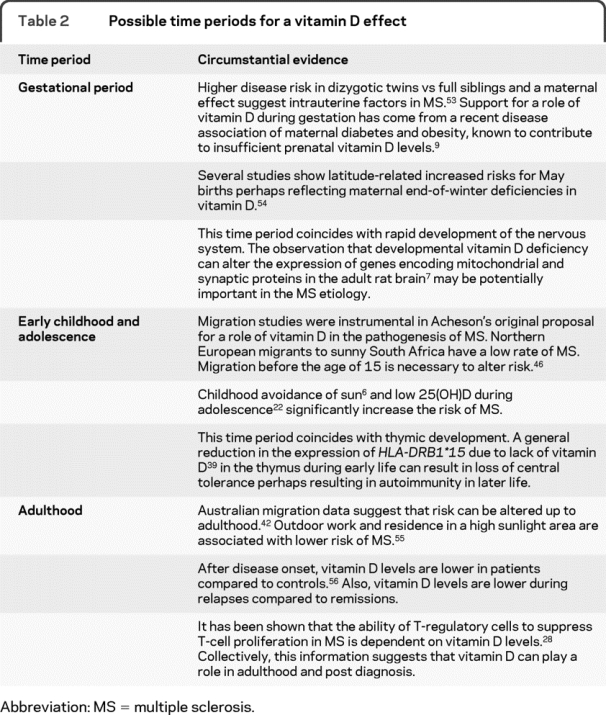
Evidence also suggests that levels of active vitamin D are genetically regulated and are influenced by variation in the 1α-hydroxylase gene,31 which controls the rate-limiting step in making vitamin D active. Therefore, it is not surprising to find that a recent large Australian study reports an association of polymorphisms proximal to the 1α-hydroxylase gene with MS,32 although the causal variant remains to be fine mapped. Further support for a role of 1α-hydroxylase in MS comes from previous coassociation of rare vitamin D–dependent rickets type 1 (caused by a mutation in the 1α-hydroxylase gene) with MS.33 Whether these mutations interact with the vitamin D–regulated HLA-DRB1*15 allele needs to be tested.
Similarly, the vitamin D receptor gene has tentatively been disease-associated in some populations34 but not replicated in others.35 It is intriguing that studies reporting positive associations with both of these genes were performed in populations with relatively high exposure to vitamin D. Therefore, it can be hypothesized that such polymorphisms are perhaps more penetrant in MS cohorts from countries with sufficient exposure to vitamin D levels. Indeed, this has been shown to be true in type 1 diabetes, where ultraviolet radiation levels in the winter could inversely determine the association of vitamin D receptor gene polymorphisms in several individual studies.36
CONCLUSION
Several lines of evidence discussed in this review are circumstantial and each individual study is open to an alternative interpretation. However, there is no single alternative interpretation that could easily explain all lines of evidence supporting a role of vitamin D in MS pathogenesis. Recent findings substantially strengthen the case for a role of vitamin D in MS and implications are now too great for prevention to remain an unanswered question. The interaction between HLA-DRB1*15 and vitamin D provides new insight into how vitamin D status may contribute to MS pathogenesis and pinpoints the MHC as the likely site of environment-gene interaction in this disease.
Future studies in prospective cohorts and animal models will be essential for understanding where and when vitamin D plays a role in MS. This will forge new avenues of research ultimately resulting in appropriate intervention strategies for high-risk individuals. As a result, vitamin D represents a key player that will be at the heart of future MS research and clinical practice.
ACKNOWLEDGMENT
The authors thank Dr. Sarah Orton and Dr. Julia Morahan for discussions and review of the manuscript.
DISCLOSURE
L. Handunnetthi receives research support from the Medical Research Council of the United Kingdom. Dr. Ramagopalan receives research support from the Multiple Sclerosis Society of the United Kingdom and the Multiple Sclerosis Society of Canada Scientific Research Foundation. Dr. Ebers serves on the editorial boards of the International Multiple Sclerosis Journal and Multiple Sclerosis; has received a speaker honorarium from Roche; served as a consultant to UCB; and receives research support from Bayer Schering Pharma, the Multiple Sclerosis Society of the United Kingdom, and the Multiple Sclerosis Society of Canada Scientific Research Foundation.
Supplementary Material
Address correspondence and reprint requests to Professor George C. Ebers, University Department of Clinical Neurology, Level 3, West Wing, John Radcliffe Hospital, Oxford OX3 9DU, UK george.ebers@clneuro.ox.ac.uk
Supplemental data at www.neurology.org
Disclosure: Author disclosures are provided at the end of the article.
Received October 2, 2009. Accepted in final form March 11, 2010.
REFERENCES
- 1.Garrod AE. The incidence of alkaptonuria: a study in chemical individuality: 1902 [classical article]. Yale J Biol Med 2002;75:221–231. [PMC free article] [PubMed] [Google Scholar]
- 2.Scriver CR, Waters PJ. Monogenic traits are not simple: lessons from phenylketonuria. Trends Genet 1999;15:267–272. [DOI] [PubMed] [Google Scholar]
- 3.Despriet DD, Klaver CC, Witteman JC, et al. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA 2006;296:301–309. [DOI] [PubMed] [Google Scholar]
- 4.Lauer K. Ecologic studies of multiple sclerosis. Neurology 1997;49:S18–S26. [DOI] [PubMed] [Google Scholar]
- 5.Acheson ED, Bachrach CA, Wright FM. Some comments on the relationship of the distribution of multiple sclerosis to latitude, solar radiation, and other variables. Acta Psychiatr Scand Suppl 1960;35:132–147. [DOI] [PubMed] [Google Scholar]
- 6.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296:2832–2838. [DOI] [PubMed] [Google Scholar]
- 7.Dean G. Multiple sclerosis in migrants to South Africa. Isr J Med Sci 1971;7:1568. [PubMed] [Google Scholar]
- 8.Islam T, Gauderman WJ, Cozen W, Hamilton AS, Burnett ME, Mack TM. Differential twin concordance for multiple sclerosis by latitude of birthplace. Ann Neurol 2006;60:56–64. [DOI] [PubMed] [Google Scholar]
- 9.Willer CJ, Dyment DA, Sadovnick AD, Rothwell PM, Murray TJ, Ebers GC. Timing of birth and risk of multiple sclerosis: population based study. BMJ 2005;330:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtzke JF, Beebe GW, Norman JE Jr. Epidemiology of multiple sclerosis in U.S. veterans: 1: race, sex, and geographic distribution. Neurology 1979;29:1228–1235. [DOI] [PubMed] [Google Scholar]
- 11.Ramagopalan SV, Ebers GC. Genes for multiple sclerosis. Lancet 2008;371:283–285. [DOI] [PubMed] [Google Scholar]
- 12.Davenport C. Multiple sclerosis from the standpoint geographic distribution and race. Arch Neurol Psychiatry 1922;8:51–58. [Google Scholar]
- 13.Sutherland JM. Observations on the prevalence of multiple sclerosis in Northern Scotland. Brain 1956;79:635–654. [DOI] [PubMed] [Google Scholar]
- 14.Poser CM. Viking voyages: the origin of multiple sclerosis? An essay in medical history. Acta Neurol Scand Suppl 1995;161:11–22. [DOI] [PubMed] [Google Scholar]
- 15.Dunne C, McGuigan C, Crowley J, et al. Human leucocyte antigen class II polymorphism in Irish patients with multiple sclerosis. Tissue Antigens 2006;68:257–262. [DOI] [PubMed] [Google Scholar]
- 16.Robertson N, Compston A. Surveying multiple sclerosis in the United Kingdom. J Neurol Neurosurg Psychiatry 1995;58:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swingler RJ, Compston D. The distribution of multiple sclerosis in the United Kingdom. J Neurol Neurosurg Psychiatry 1986;49:1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vukusic S, Van Bockstael V, Gosselin S, Confavreux C. Regional variations in the prevalence of multiple sclerosis in French farmers. J Neurol Neurosurg Psychiatry 2007;78:707–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulman D, Ebers GC. The geography of MS reflects genetic susceptibility. J Trop Geogr Neurol 1992;2: 66–72. [Google Scholar]
- 20.Kimlin MG. Geographic location and vitamin D synthesis. Mol Aspects Med 2008;29:453–461. [DOI] [PubMed] [Google Scholar]
- 21.Rigby WF, Waugh M, Graziano RF. Regulation of human monocyte HLA-DR and CD4 antigen expression, and antigen presentation by 1,25-dihydroxyvitamin D3. Blood 1990;76:189–197. [PubMed] [Google Scholar]
- 22.Ramagopalan SV, Maugeri NJ, Handunnetthi L, et al. Expression of the multiple sclerosis-associated MHC class II Allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet 2009;5:e1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hensiek AE, Sawcer SJ, Feakes R, et al. HLA-DR 15 is associated with female sex and younger age at diagnosis in multiple sclerosis. J Neurol Neurosurg Psychiatry 2002;72:184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kragt J, van Amerongen B, Killestein J, et al. Higher levels of 25-hydroxyvitamin D are associated with a lower incidence of multiple sclerosis only in women. Mult Scler 2009;15:9–15. [DOI] [PubMed] [Google Scholar]
- 25.Chao MJ, Ramagopalan SV, Herrera BM, et al. Epigenetics in multiple sclerosis susceptibility: difference in transgenerational risk localizes to the major histocompatibility complex. Hum Mol Genet 2009;18:261–266. [DOI] [PubMed] [Google Scholar]
- 26.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr 2008;88:558S–564S. [DOI] [PubMed] [Google Scholar]
- 27.Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of vitamin D in multiple sclerosis. Brain 2009;132:1146–1160. [DOI] [PubMed] [Google Scholar]
- 28.Smolders J, Thewissen M, Peelen E, et al. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One 2009;4:e6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tryon RC. Genetic differences in maze-learning ability in rats. Yearbook of the National Society for the Study of Education 1940;39:111–119. [Google Scholar]
- 30.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007;85:649–650. [DOI] [PubMed] [Google Scholar]
- 31.Orton SM, Herrera BM, Yee IM, et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol 2006;5:932–936. [DOI] [PubMed] [Google Scholar]
- 32.Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet 2009;41:824–828. [DOI] [PubMed] [Google Scholar]
- 33.Torkildsen O, Knappskog PM, Nyland HI, Myhr KM. Vitamin D-dependent rickets as a possible risk factor for multiple sclerosis. Arch Neurol 2008;65:809–811. [DOI] [PubMed] [Google Scholar]
- 34.Tajouri L, Ovcaric M, Curtain R, et al. Variation in the vitamin D receptor gene is associated with multiple sclerosis in an Australian population. J Neurogenet 2005;19:25–38. [DOI] [PubMed] [Google Scholar]
- 35.Yeo TW, Maranian M, Singlehurst S, Gray J, Compston A, Sawcer S. Four single nucleotide polymorphisms from the vitamin D receptor gene in UK multiple sclerosis. J Neurol 2004;251:753–754. [DOI] [PubMed] [Google Scholar]
- 36.Ponsonby AL, Pezic A, Ellis J, et al. Variation in associations between allelic variants of the vitamin D receptor gene and onset of type 1 diabetes mellitus by ambient winter ultraviolet radiation levels: a meta-regression analysis. Am J Epidemiol 2008;168:358–365. [DOI] [PubMed] [Google Scholar]
- 37.Kurtzke JF. A reassessment of the distribution of multiple sclerosis: part one. Acta Neurol Scand 1975;51:110–136. [DOI] [PubMed] [Google Scholar]
- 38.Miller DH, Hammond SR, McLeod JG, Purdie G, Skegg DC. Multiple sclerosis in Australia and New Zealand: are the determinants genetic or environmental? J Neurol Neurosurg Psychiatry 1990;53:903–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammond SR, English DR, McLeod JG. The age-range of risk of developing multiple sclerosis: evidence from a migrant population in Australia. Brain 2000;123:968–974. [DOI] [PubMed] [Google Scholar]
- 40.Sutherland JM, Tyrer JH, Eadie MJ. The prevalence of multiple sclerosis in Australia. Brain 1962;85:149–164. [DOI] [PubMed] [Google Scholar]
- 41.van der Mei IA, Ponsonby AL, Blizzard L, Dwyer T. Regional variation in multiple sclerosis prevalence in Australia and its association with ambient ultraviolet radiation. Neuroepidemiology 2001;20:168–174. [DOI] [PubMed] [Google Scholar]
- 42.Freedman DM, Dosemeci M, Alavanja MC. Mortality from multiple sclerosis and exposure to residential and occupational solar radiation: a case-control study based on death certificates. Occup Environ Med 2000;57:418–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westberg M, Feychting M, Jonsson F, Nise G, Gustavsson P. Occupational exposure to UV light and mortality from multiple sclerosis. Am J Ind Med 2009;52:353–357. [DOI] [PubMed] [Google Scholar]
- 44.van der Mei IA, Ponsonby AL, Dwyer T, et al. Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case-control study. BMJ 2003;327:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kampman MT, Wilsgaard T, Mellgren SI. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol 2007;254:471–477. [DOI] [PubMed] [Google Scholar]
- 46.Islam T, Gauderman WJ, Cozen W, Mack TM. Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology 2007;69:381–388. [DOI] [PubMed] [Google Scholar]
- 47.Munger KL, Zhang SM, O'Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004;62:60–65. [DOI] [PubMed] [Google Scholar]
- 48.van der Mei IA, Ponsonby AL, Dwyer T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol 2007;254:581–590. [DOI] [PubMed] [Google Scholar]
- 49.Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler 2008;14:1220–1224. [DOI] [PubMed] [Google Scholar]
- 50.Qin Z, Qin Y, Liu S. Alteration of DBP levels in CSF of patients with MS by proteomics analysis. Cell Mol Neurobiol 2009;29:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korn-Lubetzki I, Kahana E, Cooper G, Abramsky O. Activity of multiple sclerosis during pregnancy and puerperium. Ann Neurol 1984;16:229–231. [DOI] [PubMed] [Google Scholar]
- 52.Sotgiu S, Pugliatti M, Sotgiu MA, et al. Seasonal fluctuation of multiple sclerosis births in Sardinia. J Neurol 2006;253:38–44. [DOI] [PubMed] [Google Scholar]
- 53.Gardener H, Munger KL, Chitnis T, Michels KB, Spiegelman D, Ascherio A. Prenatal and perinatal factors and risk of multiple sclerosis. Epidemiology 2009;20:611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eyles D, Almeras L, Benech P, et al. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J Steroid Biochem Mol Biol 2007;103:538–545. [DOI] [PubMed] [Google Scholar]
- 55.Bahlo M, Booth DR, Broadley SA, et al. Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet 2009;41:824–828. [DOI] [PubMed] [Google Scholar]
- 56.Cooper RM, Zubek JP. Effects of enriched and restricted early environments on the learning ability of bright and dull rats. Can J Psychol 1958;12:159–164. [DOI] [PubMed] [Google Scholar]
- 57.Swank RL, Lerstad O, Strom A, Backer J. Multiple sclerosis in rural Norway its geographic and occupational incidence in relation to nutrition. N Engl J Med 1952;246:722–728. [DOI] [PubMed] [Google Scholar]
- 58.Alonso A, Hernan MA. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology 2008;71:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldacre MJ, Seagroatt V, Yeates D, Acheson ED. Skin cancer in people with multiple sclerosis: a record linkage study. J Epidemiol Community Health 2004;58:142–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tremlett H, van der Mei IA, Pittas F, et al. Monthly ambient sunlight, infections and relapse rates in multiple sclerosis. Neuroepidemiology 2008;31:271–279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


