Summary
Dyskeratosis congenita (DC) is a rare inherited syndrome exhibiting marked clinical and genetic heterogeneity. It is characterised by mucocutaneous abnormalities, bone marrow failure and a predisposition to cancer. Bone marrow failure is the principal cause of premature mortality. Studies over the last 10 years have demonstrated that DC is principally a disease of defective telomere maintenance. All DC patients have very short telomeres and the genetically characterised cases of DC have mutations in six genes which either encode components of the telomerase complex (DKC1, TERC, TERT, NOP10, NHP2) or shelterin (TINF2); these are important in the elongation and protection of the telomeric end, respectively. These advances have led to the recognition of cryptic forms of DC, such as presentations with aplastic anaemia and myelodysplasia. They have also increased our understanding of normal haematopoiesis and provided new insights to the aetiology of some cases of aplastic anaemia and related haematological disorders.
Keywords: dyskeratosis congenita, bone marrow failure/aplastic anaemia, telomere, telomerase, shelterin
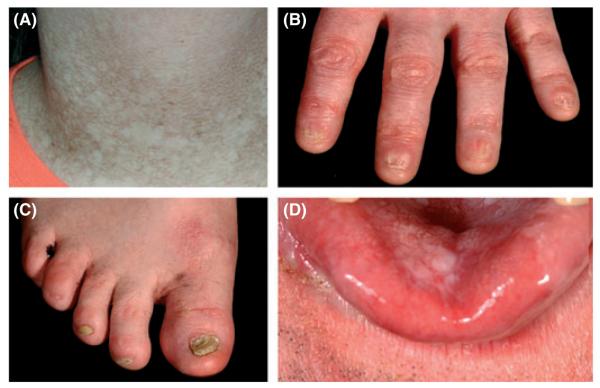
Our understanding of the spectrum of diseases encompassed by the banner Dyskeratosis congenita (DC) has increased at a tremendous rate over the last century. From its initial description in 1910 (Zinsser, 1910) through to the present day we are starting to understand its genetic basis, the underlying molecular mechanisms and the spectrum of related disorders all associated with DC, for a historical review see (Walne & Dokal, 2008). The classical triad of abnormalities associated with DC is one of abnormal skin pigmentation, nail dystrophy and oral leucoplakia (Fig 1). However, this triad is not always observed in a clinical setting. A wide spectrum of disorders affecting every system in the body, particularly the bone marrow has been associated with DC. In the early days our understanding of what was DC was largely derived from the clinic, as until 1998 nothing was known about the genetic mutations underlying the disease. Some basic genetics had been elucidated in as much that there were three modes of inheritance: X-linked recessive (OMIM #305000), autosomal dominant (OMIM #127550) and autosomal recessive (OMIM #2245230) and that the phenotype associated with each of these genetic forms varied widely (Dratchman & Alter, 1995).
Fig 1.
Photographs showing the classical mucocutaneous features of DC. (A) reticular skin pigmentation, (B) nail dystrophy of the fingers, (C) nail dystrophy of the toes, (D) tongue leucoplakia.
Classical DC and the evolution of its currently accepted diagnosis
Almost all papers describing work on DC describe the disease in terms of it being ‘multi system’; also there is ‘bone marrow failure’, ‘predisposition to cancer’ and ‘marked clinical and genetic heterogeneity’ (Dokal, 2000). All these additional definitions have come from the identification of more clinical cases. Initially, many of the individuals now diagnosed as having DC in terms of their genetics would have been classified as something else as they did not fit the classical definition of abnormal skin pigmentation, oral leucoplakia and nail dystrophy. It is not uncommon for bone marrow failure or an abnormality in another system to present before the more classic mucocutaneous features. Clinical features of DC often appear in childhood. The skin pigmentation and nail changes usually appear first, before the age of 10 years, and then bone marrow failure develops often before the age of 20 years with up to 90% of patients showing signs of bone marrow failure by the age of 30 years. The main causes of mortality in DC are bone marrow failure/immunodeficiency (60–70%), pulmonary complications (10–15%) and malignancy (10%), but there is considerable variation between patients with respect to age of onset and disease severity even within individual families. This causes considerable difficulty in making a diagnosis. Indeed, the evolution of the diagnostic criteria and the genetics has progressed hand in hand. Now the diagnosis of DC for inclusion into the Dyskeratosis Congenita Registry (DCR, currently based at Barts and The London, London, UK (Knight et al, 1998) has been relaxed from its original very rigid presentation with all three mucocutaneous features (nail dystrophy, abnormal skin pigmentation and leucoplakia) to inclusion if the index case presents with one or more of the mucocutaneous features in combination with hypoplastic bone marrow and two or more of the other somatic features known to occur in DC (Vulliamy et al, 2006). How this change came about will be discussed more (see below) in terms of the specific mutations involved.
Advances in the understanding of the genetic basis of DC
Until 1998 nothing was known about the genes involved in the pathology of DC; in the subsequent 10 years a total of six genes were identified as causing DC (Table I). However these six genes still do not account for all cases of DC, as approximately 50% of patients on the DCR still remain genetically uncharacterised.
Table I.
Mutations in the complexes associated with DC and related diseases.
| Gene (protein) | Number of mutations seen |
Mutation type | Disease | Additional references |
|---|---|---|---|---|
| Telomerase | ||||
| TERC | 32 | Heterozygous | AA, DC, ET, MDS PNH, PF | Du et al (2009) |
| TERT (TERT) | 32 | Heterozygous Biallelic |
AA, AD-DC, HH, PF AR-DC, HH |
Du et al (2009) |
| DKC1 (Dyskerin) | 54 | Hemizygous | X-linked DC, HH | |
| NHP2 (NHP2) | 3 | Biallelic | AR-DC | |
| NOP10 (NOP10) | 1 | Homozygous | AR-DC | |
| Shelterin | ||||
| TINF2 (TIN2) | 18 | Heterozygous | AA, AD-DC, HH, RS, S-DC | Walne et al (2008) |
From the telomerase database http://telomerase.asu.edu/diseases.html (Podlevsky et al, 2008) unless otherwise stated.
In the DCR (London) the approximate proportion of cases with each gene are DKC1 30%, TINF2 10-15%, TERC 5–10%, TERT 5%, NHP2 < 2%, NOP10 < 1% and uncharacterised 40–50%.
AA, aplastic anaemia; AD-DC, autosomal dominant dyskeratosis congenita; AR-DC, autosomal recessive dyskeratosis congenita; ET, essential thrombocythaemia; HH, Hoyeraal Hreidarsson syndrome; PF, pulmonary fibrosis; MDS, myelodysplastic syndrome; PNH, paroxysmal nocturnal haemoglobinurea; RS, Revesz syndrome; S-DC, sporadic dyskeratosis congenita.
X-linked DC
In 1986 the first DC gene was mapped to the X-chromosome (Connor et al, 1986). It was not until 1998 however that the gene DKC1, encoding dyskerin, was identified as the cause of X-lined DC and the first mutations were described (Heiss et al, 1998; Knight et al, 1999a). With the identification of mutations in DKC1, the first diagnostic tests became available either in terms of screening the patient for mutations in this gene or, in the case of skewed X-chromosome inactivation patterns, identification of disease carriers amongst unaffected female relatives (Vulliamy et al, 1997). Further screening for mutations in DKC1 provided the first evidence that DC was not a homogenous disorder and other syndromes with overlapping presentation can share the same genetic mutations. Hoyeraal-Hreidarsson (HH) syndrome is a severe multi-system disorder mainly affecting males that is characterised by growth retardation of prenatal onset, microcephaly, cerebellar hypoplasia and aplastic anaemia (Hoyeraal et al, 1970; Hreidarsson et al, 1988; Aalfs et al, 1995). Due to the overlap in features it was suggested, and subsequently proved, that HH is a severe variant of DC due to the presence of DKC1 mutations in males with the classical presentation of HH (Knight et al, 1999b). Without the genetics this association may not have been made as, in many cases, patients with HH do not live long enough to develop the diagnostic mucocutaneous features associated with DC nor possess all the features of HH. An accepted diagnosis of HH has been made based on patient information registered in the DCR: a diagnosis of HH can be made if a patient presents with four of the six most commonly recognised features of HH: intrauterine growth retardation, developmental delay, microcephaly, cerebellar hypoplasia, immunodeficiency or AA (Vulliamy et al, 2006). However DKC1 mutations are not the only cause of HH due to the presence of the disease in females implying that additional mutations (see below) can and do cause HH (Dokal, 2000).
Autosomal dominant DC
The next advance in our understanding of DC was the identification of mutations in TERC (telomerase RNA component) (Vulliamy et al, 2001a). Telomerase is a specialised polymerase that adds the telomeric repeat (TTAGGG) to the end of the 3′ lagging strand of DNA after replication. Due to the semi conservative nature of DNA replication, telomerase is essential to maintain telomere length in rapidly dividing cells, such as cells of the haematopoietic system. Without telomerase the telomeres would shorten with each successive round of replication until the telomeres reach a critical length where the cells would enter senescence. Telomerase is a ribonucleoprotein composed of two core components: a catalytic component which adds the repeats, telomerase reverse transcriptase (TERT) and TERC, which acts as the template. Mutations in cases of autosomal dominant DC were identified in TERC initially and the identification of mutations within this molecule led to the expansion of the DC phenotype (Fig 2) to include other haematological and non-haematological disorders. Firstly, heterozygous mutations in TERC were identified in patients with aplastic anaemia (Vulliamy et al, 2002) and soon after in patients with myelodysplasia (Yamaguchi et al, 2003). This started to push the original clinical diagnosis away from the classical triad to manifestations of bone marrow failure being the initial presenting feature. In some cases the more classical features develop after the underlying TERC mutation has been identified. This serves as a guide that patients with unexplained aplastic anaemia should be screened for TERC mutations to rule out a potential diagnosis of DC.
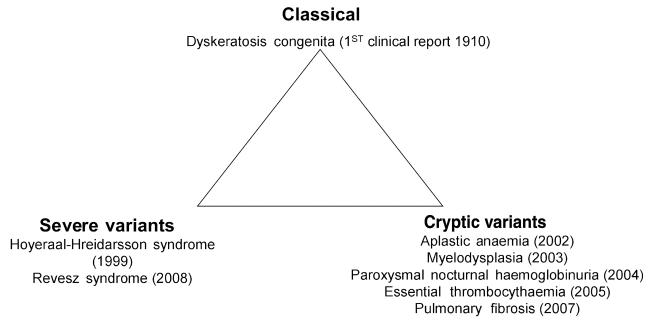
Fig 2.
Schematic representation highlighting the varying presentations of DC: Classical, severe variant and ‘cryptic’ forms. The year (in brackets) indicates when mutations in these variants were identified linking them to the DC phenotype.
The identification of mutations in DKC1 and TERC firmly established the pathology of defective telomere maintenance via the action of telomerase as being the principal underlying cause of DC. This led to further study of the telomerase complex to determine the genetic basis of the remaining uncharacterised patients, including those with autosomal recessive inheritance. The next gene to have mutations identified was TERT, the enzymatic component of the telomerase complex. However the clinical presentation was not necessarily pure DC. Many patients presented with aplastic anaemia or the inheritance was non-Mendelian, suggesting that perhaps heterozygous mutations within TERT were acting more as risk factors rather than disease causing (Vulliamy et al, 2005; Yamaguchi et al, 2005). In vitro evidence for the effect of the mutations was also unclear in that in some cases specific mutations could cause a reduction in telomerase activity as measured by the telomerase repeat amplification protocol (TRAP) assay, whereas others had no effect. However, in some cases TERT mutations were shown to give a pure DC phenotype. Homozygous mutations were identified in patients with both autosomal recessive DC and the more severe variant HH. In both of these situations the level of telomerase activity was severely reduced but the levels of TERC expression was not affected (Marrone et al, 2007; Du et al, 2009). Again, mutations in TERT have been identified in other diseases not necessarily associated with DC. Some patients with idiopathic pulmonary fibrosis have been shown to have mutations in either TERT or TERC (Armanios et al, 2007; Tsakiri et al, 2007). This is not too surprising considering that pulmonary complications, particularly after haematopoietic stem cell transplantation, are a major cause of mortality in DC. However in the pulmonary fibrosis patients, there are few clinical signs resembling a DC phenotype, at best there may be some mild to moderate anaemia but usually no other recognised features. These findings suggest that pulmonary fibrosis could also be a telomere maintenance disorder (Cronkhite et al, 2008).
Autosomal recessive DC
Some progress has also been made in understanding the genetic basis of autosomal recessive DC. A large linkage study of 16 consanguineous families comprising 25 affected individuals did not identify a single common locus (Walne et al, 2007). Although three genes have been identified as causing autosomal recessive DC, in each case there are only a small number of families associated with each mutation. The first causative gene to be identified was NOP10. The mutation identified in a large family from Saudi Arabia affected a highly conserved residue. As a result of this mutation all the affected individuals had reduced telomere length and reduced TERC levels. Screening of the DCR found no other family with a pathogenic mutation in NOP10. As mentioned above, homozygous and also biallelic mutations have been identified in TERT. These mutations give very different profiles regarding TRAP activity and telomere length with both being greatly reduced compared with heterozygous TERT mutations (Marrone et al, 2007). More recently three mutations have been identified in autosomal recessive DC patients in NHP2. One of these occurs in the homozygous state and the other is as a compound heterozygote. Again telomere lengths and TERC levels are reduced in patients compared with normal controls (Vulliamy et al, 2008). Both NOP10 and NHP2 are components of H/ACA ribonucleoprotein complex (H/ACA RNP). This complex is comprised of a RNA molecule and four proteins, dyskerin, GAR1 as well as NOP10 and NHP2. These four proteins are highly conserved and have been shown to be associated with ribosome biogenesis, pre mRNA splicing and telomere maintenance (Meier, 2005). Mutations have been identified in all components of this H/ACA RNP complex in patients with DC except for GAR1. The reason for this is unclear, but it may be due to its location relative to the RNA component. Modelling of the telomerase complex has placed GAR1 making no contact with the RNA molecule and GAR1 is not required for the structural integrity of the H/ACA RNP (Meier, 2006; Vulliamy et al, 2008).
A link to shelterin
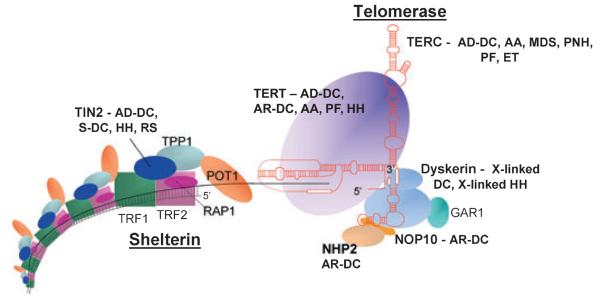
More recently a different protein complex, shelterin, has been implicated in the pathology of DC. Shelterin has at least three effects on telomeres. It determines the structure of the telomeric terminus, it has been implicated in the generation of t-loops and it controls the synthesis of telomeric DNA by telomerase. Without the protective activity of shelterin, telomeres are no longer hidden from DNA damage repair mechanisms and so chromosome ends are incorrectly processed by the DNA repair pathways. The shelterin complex is comprised of six proteins: telomeric-repeat binding protein 1 (TERF1, TRF1), telomeric-repeat binding protein 2 (TERF2, TRF2), TRF1-interacting nuclear factor 2 (TINF2, TIN2), TERF2-interacting protein (TERF2IP, RAP1), TIN2-interacting protein 1 (ACD, protein names include TPP1, TINT1, PIP1 and PTOP) and protection of telomeres (POT1, POT1) (gene name, protein name abbreviation respectively) (de Lange, 2005). TRF1, TRF2 and POT1 of the shelterin complex (Fig 3) bind directly to the telomeric DNA: TRF1 and TRF2 bind to double stranded DNA and POT1 to the single stranded DNA overhang. The composition and protein interaction of the components of shelterin complex appears to be highly ordered with TIN2 playing a pivotal role (Kim et al, 1999; Ye et al, 2004; O'Connor et al, 2006; Chen et al, 2007; Gilson & Geli, 2007). Mutations have been identified in the TINF2 component of shelterin in patients with DC, HH, aplastic anaemia and Revesz syndrome (Savage et al, 2008; Walne et al, 2008). This discovery extends the range of the DC spectrum of diseases even further. Revesz syndrome is characterised by bilateral exudative retinopathy, bone marrow hypoplasia, nail dystrophy, fine hair, cerebellar hypoplasia and growth retardation and, from this brief description of the key clinical features, the overlap with DC is apparent (Revesz et al, 1992; Kajtar & Mehes, 1994). Patients with TINF2 mutations tend to have similar characteristics in terms of disease severity: all have severe disease and this is associated with very short telomere lengths. Interestingly, nearly all the patients had de novo mutations which gives rise to a different mechanism that causes the disease. In patients with heterozygous TERC mutations, studies have shown that the phenomenon of genetic anticipation is involved; a parent of an affected child has a particular mutation but no overt signs of disease. However in the child with the same heterozygous TERC mutation the disease manifests itself at a much younger age (Vulliamy et al, 2004). This adds another level of complexity to DC. Mutations in one gene (TERC) that take a generation to cause an obvious effect can cause the same disease as mutations in another gene (TINF2) that arise instantly.
Fig 3.
Schematic representation of the telomerase and shelterin complexes involved in telomere maintenance. Protein names in bold are mutated in DC and associated disorders as listed. No human mutations have been described in the other components. AA, aplastic anaemia; AD-DC, autosomal dominant dyskeratosis congenita; AR-DC, autosomal recessive dyskeratosis congenita; ET, essential thrombocythaemia; HH, Hoyeraal Hreidarsson syndrome; PF, pulmonary fibrosis; MDS, myelodysplastic syndrome; PNH, paroxysmal nocturnal haemoglobinurea; RS, Revesz syndrome; S-DC, sporadic dyskeratosis congenita.
Haematological involvement in DC
Over the years it has become increasingly obvious that not all patients with DC present with the mucocutaneous features as the initial presentation. The usual progression of abnormalities is ectodermal dystrophy in the first decade followed by approximately 90% of patients developing haematopoietic abnormalities (bone marrow hypoplasia and dysplasia) by the third decade. The link between haematological abnormalities and mucocutaneous abnormalities is developmental, with defects in the epidermal tissues in DC suggesting a defect in the primitive mesoderm and ectoderm. As haemopoietic cells, marrow stromal cells, endothelial cells and dermis all originate from the primitive mesoderm it is easy to see how mutations affecting these primitive cells can lead to the two dissimilar aspects of the disease. Early studies have shown that there is a reduction or absence of the multi-lineage colony-forming cells in patients with DC (Colvin et al, 1984; Marsh et al, 1992; Yamaguchi et al, 2005), and more recently these have been shown to differ according to the mutation involved i.e. patients with DKC1 mutations have a reduction in colony number whereas patients with mutations in TERC tend have no detectable colonies in the peripheral blood (Kirwan et al, 2008a). This is the first evidence of a haematological correlation with DC subtype as patients with TERC mutations often present with aplastic anaemia before mucocutaneous features whereas the presentation usually tends to be more classical in those with DKC1 mutations. A very recent study has suggested it may be possible to partially correct some of the haematological defects associated with DC. By using only exogenously expressed TERC in primary T cells and B cell derived lines from patients with mutations in DKC1 or TERC, Kirwan et al (2008b) showed that telomerase activity was increased in both cell types. Cell survival and telomere length were also improved in the B cell derived lines. The results from this study could potentially lead to a therapeutic development in this difficult to treat disease (Kirwan et al, 2008b).
Telomere lengths
Telomeres consist of long TTAGGG repeats and the associated proteins of the shelterin complex at the ends of chromosomes that are essential for maintaining chromosome integrity by preventing chromosome ends from being recognised as DNA breaks (Chan & Blackburn, 2002). Telomeres progressively shorten with successive rounds of cell division due to the semi conservative replication that takes place at the 3′ end of the chromosome. Telomerase can help to maintain telomere lengths in some cell types, but telomerase is mainly restricted to cells, such as germ cells, stem cells and their immediate progeny, activated T cells and monocytes, but, in cells where telomerase is not present, telomere shortening is part of the normal process of cellular aging (Blasco, 2007).
Various premature aging syndromes have been characterised by increased rates of telomere shortening and this can be used to help understand the pathology of the disease. The association between telomere length and DC was first observed by Mitchell et al (1999) in cells from patients with dyskerin mutations. Regardless of the genetic basis, the universal feature of DC is that patients have short telomeres compared with healthy age-matched individuals (Vulliamy et al, 2001b). But short telomeres are not unique to DC patients, as individuals with heart failure, Ataxia telangiectasia, Bloom syndrome and Werner syndrome, for example, can also present with shortened telomeres (Blasco, 2007; Savage & Alter, 2008). The main difference is the relative telomere length. A study by Alter et al (2007) measured telomere length in various blood cell types in patients with DC, their relatives and other patients with different inherited bone marrow failure syndromes. They found that DC patients had very short telomeres in the majority of the leucocyte subset studied (less than 1st centile compared with normal controls). However there was no correlation between telomere length and the severity of bone marrow failure, presence of the diagnostic triad or other common symptoms. Telomere lengths in the DC patient group also tended to be shorter than in patients with other bone marrow failure syndromes, and because of this, telomere length can, with caution, be treated as a surrogate marker for DC but only in the context of bone marrow failure (Du et al, 2009). It remains to be validated whether up-front telomere length measurements in new patients presenting with aplastic anaemia in general can be used to identify the cryptic forms of DC.
Modelling dyskeratosis congenita in mice
Over the years many groups have tried to model DC in the mouse with varying success (Autexier, 2008). The main drawback to all the models is that none are a faithful replication of the disease seen in humans due to the highly variable presentation seen. One of the earliest models was by Ruggero et al (2003) who developed the hypomorphic Dkc1 mutant. This model reproduced many features seen in DC, such as reduced telomere length in later generations, severe anaemia and reduced cellularity in the bone marrow, reduction in telomerase activity, limited dyskeratosis of the skin and an increased predisposition to tumours particularly in the lung and mammary glands. One major down side to this model is that impaired pseudouridylation is seen in very early generations whereas this has not been reported in DC patients (Ruggero et al, 2003).
Many models involving Terc knockout or Tert knockout mice have been generated but these introduce defects that are not seen in the patients as a null in either of these two genes would probably be non-viable in humans (Herrera et al, 1999; Chiang et al, 2004). One example of a model that tried to reproduce what happens in humans has been developed by Gu et al (2008). This group introduced a 2 kb deletion that removed exon 15 of dyskerin (Dkc1). They showed for the first time that telomere length and telomerase can be separated in that dyskerin interacts with telomerase and affects telomere maintenance in a telomere length-independent manner (Gu et al, 2008).
Recently another model of the phenotype of DC has been produced (Hockemeyer et al, 2008); these mice demonstrate short telomeres, hyper pigmentation of the skin, nail abnormalities, bone marrow failure and reduced life span. The main problem with this model, which knocks out Pot1b in the mouse, is that this form of POT is not present in humans and also no mutations have been described in the POT gene to date. It can therefore be argued that although this model can be used to study some of the effects of defective telomere maintenance, again it is done in a fashion that is not comparable to the disease state seen in humans. This mouse model has clarified the notion that DC is caused by telomere dysfunction and there is a close relationship between the interactions of the shelterin complex, which is involved in telomere protection, and telomerase, which is involved in telomere elongation. Knockout mice for both Pot1b and Terc had vastly reduced viability whereas Terc+/− mice have increased survival but they do display some of the characteristic phenotypes seen in DC. There is no doubt that these models are of scientific value in studying the myriad effects of telomere biology but their accuracy of reproducing DC in an easily manipulated system is not so clear.
Management of DC
Currently there is no curative treatment for DC. The variation in presentation makes it difficult to treat, with bone marrow failure/immunodeficiency being the main cause of premature mortality. Use of the anabolic steroid oxymetholone and haematopoietic growth factors (such as erythropoietin, granulocyte macrophage colony-stimulating factor and granulocyte colony-stimulating factor (Erduran et al, 2003) can produce improvement in the haematopoietic function. Although the mechanism of action of oxymetholone is not well understood, it is thought to function by promoting the growth of haematopoietic progenitors indirectly through the effect of cytokine production and by supporting haemopoietic production in times of stress (Beran et al, 1982; Kim et al, 2005; Hosseinimehr et al, 2006). It has been found that approximately two thirds of patients with DC will respond to oxymetholone, in some cases the responses can last several years and involve all lineages. Patients with DC can respond to a dose of 0·5 mg oxymetholone/kg/d and this can be increased, if necessary to 2–5 mg/kg/d (I. Dokal, unpublished observation). It is important to monitor for side effects (e.g. liver toxicity). It is possible to maintain reasonable blood counts by this approach in many patients.
The only long term cure for the haemopoietic abnormalities is allogeneic haematopoietic stem cell transplantation, but this is not without risk. There is still significant mortality associated with bone marrow transplants for DC patients when compared with other bone marrow failure syndromes. One of the main reasons for this is the high level of pulmonary/vascular complications that present in these patients probably as a result of the underlying telomere defect. The conditioning regimen appears to have an impact on patient survival. The standard myeloablative conditioning regimes are associated with frequent and severe adverse effects, such as lung fibrosis, interstitial pneumonia and veno-occlusive disease. Recently, the adoption of non-myeloablative fludarabine based protocols (also known as reduced intensity conditioning: RIC) has allowed for successful engraftment in some patients with fewer complications and lower toxicity (de la Fuente & Dokal, 2007). The long-term survival however is unknown at present but the initial response is encouraging as a more effective treatment for DC. Of the 30 patients with DC who have undergone stem cell transplantation, 9 patients received RIC and the remaining 21 underwent conventional transplantation. Only 2/9 RIC patients have died whereas 15 of 21 patients who received conventional therapy died (Ostronoff et al, 2007). A recent case of an unrelated cord transplant has been described, again using the fludarabine-based reduced-intensity conditioning regime in a girl with HH (Coman et al, 2008). When this study was published the patient was still alive 37 months post-transplant and is believed to be only the third HH patient to receive a bone marrow transplant. There are very few reports in the literature of stem cell transplantation using unrelated donors; of the four cases reported; three received a myeloablative transplant and all three died. The remaining case described by Coman et al survived using the less severe RIC regime (Ostronoff et al, 2007; Coman et al, 2008). Further ongoing studies will be able to give a more accurate indication of disease-free survival rates and transplant-related mortality in both patients with DC and related syndromes such as HH.
DC has been accepted as being a haematological disease due to the high prevalence of bone marrow failure seen in patient groups. What has become more obvious over the years is how wide the spectrum of disorders associated with DC extends into other disease types. DC can be seen as an aging disorder as patients have increasingly short telomeres, often present with premature grey hair and have increased risk of osteoporosis and dental loss. At the other end of the age spectrum it is a developmental disorder due to microcephaly, intrauterine growth retardation, learning difficulties and developmental/mental retardation. It is also a cancer syndrome because of the increased risk of malignancy (haematological, squamous cell carcinomas of the skin and gastrointestinal tract and carcinomas of the larynx bronchus and tongue) particularly after the third decade (Baykal et al, 2003). It is now becoming more apparent that DC can be identified in a dental cohort. The oral phenotype of DC is characterised by leucoplakia, decreased root/crown ratios and mild taurodontism. From a clinical view point a diagnosis of DC or other bone marrow failure syndromes should be considered if a young patient presents with leucoplakia, particularly in those with no history of tobacco use (Auluck, 2007; Atkinson et al, 2008). Treatment of these DC complications remains unsatisfactory and will be a challenge for the future. Equally it will be important to establish whether treatments aimed at the restoration of telomere length (as discussed above), have any therapeutic benefit in DC and related haematological disorders.
The expansion of diseases that are now associated with DC has changed our view of what exactly DC is. As stated earlier we need to move away from the very rigid initial diagnostic criteria of the presence of the mucocutaneous triad of skin pigmentation nail dystrophy and oral leucoplakia. In the absence of an internationally accepted diagnosis the best criteria still appears to be that of Vulliamy et al (2006), i.e. the presentation of one or more of the mucocutaneous features in combination with hypoplastic bone marrow and two or more of the other somatic features known to occur in DC (such as hair loss, abnormal dentition, malignancy, pulmonary disease, short stature, liver disease, developmental delay etc). As shown in Fig 2, there is a spectrum of diseases that are all related by their causative mutation and shared pathology of defective telomere maintenance. It is apparent that they are not all pure DC but it is important for clinicians to be aware of the overlap between the similar diseases, particularly when considering therapy options. Defining DC in terms of telomere length can also be misleading. Although in general most patients with classical DC do have significantly shortened telomeres compared with age-matched controls, there are some with telomere lengths within the normal range, so these patients would be incorrectly diagnosed. Equally, in family studies, asymptomatic individuals have been identified to have short telomeres and it remains unclear how they will develop overt disease features. One of the main outstanding issues is the number of patients even with classical DC in whom the genetic mutation is still unknown. Presentation cannot always give the answer as there are many examples where the index case has died at a very young age and the diagnosis of DC has only been made when a subsequent member of the family survived long enough for the more characteristic features to become apparent.
Conclusions
The advances in our understanding of DC have increased remarkably over the last 10 years but there are still huge advances to be made. Six genes have been identified that cause DC and this in turn has led to the broadening of the definition of DC. Many other diseases, such as aplastic anaemia, Revesz syndrome and idiopathic pulmonary fibrosis, can now be encompassed by mutations describing the DC phenotype – ‘spectrum of diseases due to defective telomere maintenance’. These advances have also increased our understanding of normal haematopoiesis and highlighted the critical role of telomerase and telomeres in human biology.
Acknowledgements
We would like to thanks all colleagues past (Stuart Knight, Anna Marrone, David Stevens and Philip Mason) and present (Richard Beswick, Mike Kirwan and Tom Vulliamy) and all the patients and clinicians upon whom the research in our laboratory depends. Our work is supported by funding from The Wellcome Trust and The Medical Research Council, UK.
References
- Aalfs CM, van den Berg H, Barth PG, Hennekam RC. The Hoyeraal-Hreidarsson syndrome: the fourth case of a separate entity with prenatal growth retardation, progressive pancytopenia and cerebellar hypoplasia. European Journal of Pediatrics. 1995;154:304–308. doi: 10.1007/BF01957367. [DOI] [PubMed] [Google Scholar]
- Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, Willner JP, Peters JA, Giri N, Lansdorp PM. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110:1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. The New England Journal of Medicine. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- Atkinson JC, Harvey KE, Domingo DL, Trujillo MI, Guadagnini JP, Gollins S, Giri N, Hart TC, Alter BP. Oral and dental phenotype of dyskeratosis congenita. Oral Diseases. 2008;14:419–427. doi: 10.1111/j.1601-0825.2007.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auluck A. Dyskeratosis congenita. Report of a case with literature review. Medicina Oral, Patologia Oral Y Cirugia Bucal. 2007;12:E369–E373. [PubMed] [Google Scholar]
- Autexier C. POT of gold: modeling dyskeratosis congenita in the mouse. Genes & Development. 2008;22:1731–1736. doi: 10.1101/gad.1695808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykal C, Kavak A, Gulcan P, Buyukbabani N. Dyskeratosis congenita associated with three malignancies. Journal of European Academy of Dermatology and Venereology. 2003;17:216–218. doi: 10.1046/j.1468-3083.2003.00585.x. [DOI] [PubMed] [Google Scholar]
- Beran M, Spitzer G, Verma DS. Testosterone and synthetic and androgens improve the in vitro survival of human marrow progenitor cells in serum-free suspension cultures. The Journal of Laboratory and Clinical Medicine. 1982;99:247–253. [PubMed] [Google Scholar]
- Blasco MA. Telomere length, stem cells and aging. Nature Chemical Biology. 2007;3:640–649. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- Chan SW, Blackburn EH. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene. 2002;21:553–563. doi: 10.1038/sj.onc.1205082. [DOI] [PubMed] [Google Scholar]
- Chen LY, Liu D, Songyang Z. Telomere maintenance through spatial control of telomeric proteins. Molecular and Cellular Biology. 2007;27:5898–5909. doi: 10.1128/MCB.00603-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YJ, Hemann MT, Hathcock KS, Tessarollo L, Feigenbaum L, Hahn WC, Hodes RJ. Expression of telomerase RNA template, but not telomerase reverse transcriptase, is limiting for telomere length maintenance in vivo. Molecular and Cellular Biology. 2004;24:7024–7031. doi: 10.1128/MCB.24.16.7024-7031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin BT, Baker H, Hibbin JA, Gordon-Smith EC, Gordon M. Haemopoietic progenitor cells in dyskeratosis congenita. British Journal of Haematology. 1984;56:513–515. doi: 10.1111/j.1365-2141.1984.tb03981.x. [DOI] [PubMed] [Google Scholar]
- Coman D, Herbert A, McGill J, Lockwood L, Hallahan A. Unrelated cord blood transplantation in a girl with Hoyeraal-Hreidarsson syndrome. Bone Marrow Transplantation. 2008;42:293–294. doi: 10.1038/bmt.2008.163. [DOI] [PubMed] [Google Scholar]
- Connor JM, Gatherer D, Gray FC, Pirrit LA, Affara NA. Assignment of the gene for dyskeratosis congenita to Xq28. Human Genetics. 1986;72:348–351. doi: 10.1007/BF00290963. [DOI] [PubMed] [Google Scholar]
- Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, Garcia CK. Telomere shortening in familial and sporadic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine. 2008;178:729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokal I. Dyskeratosis congenita in all its forms. British Journal of Haematology. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- Dratchman RA, Alter BP. Dyskeratosis congenita. Dermatologic Clinics. 1995;13:33–39. [PubMed] [Google Scholar]
- Du HY, Pumbo E, Ivanovich J, An P, Maziarz RT, Reiss UM, Chirnomas D, Shimamura A, Vlachos A, Lipton JM, Goyal RK, Goldman F, Wilson DB, Mason PJ, Bessler M. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood. 2009;113:309–316. doi: 10.1182/blood-2008-07-166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erduran E, Hacisalihoglu S, Ozoran Y. Treatment of dyskeratosis congenita with granulocyte-macrophage colony-stimulating factor and erythropoietin. Journal of Pediatric Hematology and Oncology. 2003;25:333–335. doi: 10.1097/00043426-200304000-00015. [DOI] [PubMed] [Google Scholar]
- de la Fuente J, Dokal I. Dyskeratosis congenita: advances in the understanding of the telomerase defect and the role of stem cell transplantation. Pediatric Transplantation. 2007;11:584–594. doi: 10.1111/j.1399-3046.2007.00721.x. [DOI] [PubMed] [Google Scholar]
- Gilson E, Geli V. How telomeres are replicated. Nature Reviews. 2007;8:825–838. doi: 10.1038/nrm2259. [DOI] [PubMed] [Google Scholar]
- Gu BW, Bessler M, Mason PJ. A pathogenic dyskerin mutation impairs proliferation and activates a DNA damage response independent of telomere length in mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10173–10178. doi: 10.1073/pnas.0803559105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nature Genetics. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- Herrera E, Samper E, Martin-Caballero J, Flores JM, Lee HW, Blasco MA. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. The EMBO Journal. 1999;18:2950–2960. doi: 10.1093/emboj/18.11.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Palm W, Wang RC, Couto SS, de Lange T. Engineered telomere degradation models dyskeratosis congenita. Genes & Development. 2008;22:1773–1785. doi: 10.1101/gad.1679208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinimehr SJ, Zakaryaee V, Froughizadeh M. Oral oxymetholone reduces mortality induced by gamma irradiation in mice through stimulation of hematopoietic cells. Molecular and Cellular Biochemistry. 2006;287:193–199. doi: 10.1007/s11010-005-9111-5. [DOI] [PubMed] [Google Scholar]
- Hoyeraal HM, Lamvik J, Moe PJ. Congenital hypoplastic thrombocytopenia and cerebral malformations in two brothers. Acta Paediatrica Scandinavica. 1970;59:185–191. doi: 10.1111/j.1651-2227.1970.tb08986.x. [DOI] [PubMed] [Google Scholar]
- Hreidarsson S, Kristjansson K, Johannesson G, Johannsson JH. A syndrome of progressive pancytopenia with microcephaly, cerebellar hypoplasia and growth failure. Acta Paediatrica Scandinavica. 1988;77:773–775. doi: 10.1111/j.1651-2227.1988.tb10751.x. [DOI] [PubMed] [Google Scholar]
- Kajtar P, Mehes K. Bilateral coats retinopathy associated with aplastic anaemia and mild dyskeratotic signs. American Journal of Medical Genetics. 1994;49:374–377. doi: 10.1002/ajmg.1320490404. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kaminker P, Campisi J. TIN2, a new regulator of telomere length in human cells. Nature Genetics. 1999;23:405–412. doi: 10.1038/70508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Hwang JH, Cheon JM, Park NS, Park SE, Park SJ, Yun HJ, Kim S, Jo DY. Direct and indirect effects of androgens on survival of hematopoietic progenitor cells in vitro. Journal of Korean Medical Science. 2005;20:409–416. doi: 10.3346/jkms.2005.20.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan M, Vulliamy T, Beswick R, Walne AJ, Casimir C, Dokal I. Circulating haematopoietic progenitors are differentially reduced amongst subtypes of dyskeratosis congenita. British Journal of Haematology. 2008a;140:719–722. doi: 10.1111/j.1365-2141.2008.06991.x. [DOI] [PubMed] [Google Scholar]
- Kirwan M, Beswick R, Vulliamy T, Nathwani AC, Walne AJ, Casimir C, Dokal I. Exogenous TERC alone can enhance proliferative potential, telomerase activity and telomere length in lymphocytes from dyskeratosis congenita patients. British Journal of Haematology. 2008b doi: 10.1111/j.1365-2141.2008.07516.x. doi:10.1111/j.1365-2141.2008.07516.x. [DOI] [PubMed] [Google Scholar]
- Knight S, Vulliamy T, Copplestone A, Gluckman E, Mason P, Dokal I. Dyskeratosis Congenita (DC) registry: identification of new features of DC. British Journal of Haematology. 1998;103:990–996. doi: 10.1046/j.1365-2141.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- Knight SW, Heiss NS, Vulliamy TJ, Greschner S, Stavrides G, Pai GS, Lestringant G, Varma N, Mason PJ, Dokal I, Poustka A. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. American Journal of Human Genetics. 1999a;65:50–58. doi: 10.1086/302446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SW, Heiss NS, Vulliamy TJ, Aalfs CM, McMahon C, Richmond P, Jones A, Hennekam RC, Poustka A, Mason PJ, Dokal I. Unexplained aplastic anaemia, immunodeficiency, and cerebellar hypoplasia (Hoyeraal-Hreidarsson syndrome) due to mutations in the dyskeratosis congenita gene, DKC1. British Journal of Haematology. 1999b;107:335–339. doi: 10.1046/j.1365-2141.1999.01690.x. [DOI] [PubMed] [Google Scholar]
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes & Development. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- Marrone A, Walne A, Tamary H, Masunari Y, Kirwan M, Beswick R, Vulliamy T, Dokal I. Telomerase reverse-transcriptase homozygous mutations in autosomal recessive dyskeratosis congenita and Hoyeraal-Hreidarsson syndrome. Blood. 2007;110:4198–4205. doi: 10.1182/blood-2006-12-062851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JC, Will AJ, Hows JM, Sartori P, Darbyshire PJ, Williamson PJ, Oscier DG, Dexter TM, Testa NG. “Stem cell” origin of the hematopoietic defect in dyskeratosis congenita. Blood. 1992;79:3138–3144. [PubMed] [Google Scholar]
- Meier UT. The many facets of H/ACA ribonucleoproteins. Chromosoma. 2005;114:1–14. doi: 10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier UT. How a single protein complex accommodates many different H/ACA RNAs. Trends in Biochemical Sciences. 2006;31:311–315. doi: 10.1016/j.tibs.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- O'Connor MS, Safari A, Xin H, Liu D, Songyang Z. A critical role for TPP1 and TIN2 interaction in high-order telomeric complex assembly. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11874–11879. doi: 10.1073/pnas.0605303103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostronoff F, Ostronoff M, Calixto R, Florencio R, Domingues MC, Souto Maior AP, Sucupira A, Tagliari C. Fludarabine, cyclophosphamide, and antithymocyte globulin for a patient with dyskeratosis congenita and severe bone marrow failure. Biology of Blood and Marrow Transplantation. 2007;13:366–368. doi: 10.1016/j.bbmt.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Podlevsky JD, Bley CJ, Omana RV, Qi X, Chen JJ. The telomerase database. Nucleic Acids Research. 2008;36:D339–D343. doi: 10.1093/nar/gkm700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revesz T, Fletcher S, al-Gazali LI, DeBuse P. Bilateral retinopathy, aplastic anaemia, and central nervous system abnormalities: a new syndrome? Journal of Medical Genetics. 1992;29:673–675. doi: 10.1136/jmg.29.9.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero D, Grisendi S, Piazza F, Rego E, Mari F, Rao PH, Cordon-Cardo C, Pandolfi PP. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science. 2003;299:259–262. doi: 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- Savage SA, Alter BP. The role of telomere biology in bone marrow failure and other disorders. Mechanisms of Ageing and Development. 2008;129:35–47. doi: 10.1016/j.mad.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. American Journal of Human Genetics. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliamy TJ, Knight SW, Dokal I, Mason PJ. Skewed X-inactivation in carriers of X-linked dyskeratosis congenita. Blood. 1997;90:2213–2216. [PubMed] [Google Scholar]
- Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001a;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cells Molecules and Diseases. 2001b;27:353–357. doi: 10.1006/bcmd.2001.0389. [DOI] [PubMed] [Google Scholar]
- Vulliamy T, Marrone A, Dokal I, Mason PJ. Association between aplastic anaemia and mutations in telomerase RNA. Lancet. 2002;359:2168–2170. doi: 10.1016/S0140-6736(02)09087-6. [DOI] [PubMed] [Google Scholar]
- Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nature Genetics. 2004;36:447–449. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Walne A, Baskaradas A, Mason PJ, Marrone A, Dokal I. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cells Molecules and Diseases. 2005;34:257–263. doi: 10.1016/j.bcmd.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- Vulliamy T, Beswick R, Kirwan M, Marrone A, Digweed M, Walne A, Dokal I. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8073–8078. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walne AJ, Dokal I. Dyskeratosis Congenita: a historical perspective. Mechanisms of Ageing and Development. 2008;129:48–59. doi: 10.1016/j.mad.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Walne AJ, Vulliamy T, Marrone A, Beswick R, Kirwan M, Masunari Y, Al-Qurashi FH, Aljurf M, Dokal I. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Human Molecular Genetics. 2007;16:1619–1629. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112:3594–3600. doi: 10.1182/blood-2008-05-153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, Young NS. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodys-plastic syndrome. Blood. 2003;102:916–918. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, Young NS. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. The New England Journal of Medicine. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- Ye JZ, Donigian JR, van Overbeek M, Loayza D, Luo Y, Krutchinsky AN, Chait BT, de Lange T. TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. The Journal of Biological Chemistry. 2004;279:47264–47271. doi: 10.1074/jbc.M409047200. [DOI] [PubMed] [Google Scholar]
- Zinsser F. Atrophia cutis reticularis cum pigmentations, dystrophia unguium et leukoplakis oris (poikioodermia atrophicans vascularis jacobi) Ikonographia Dermatologica. 1910;5:219–223. [Google Scholar]