Abstract
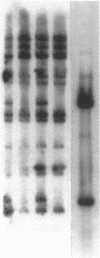
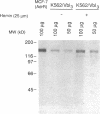
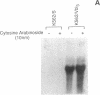
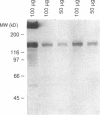
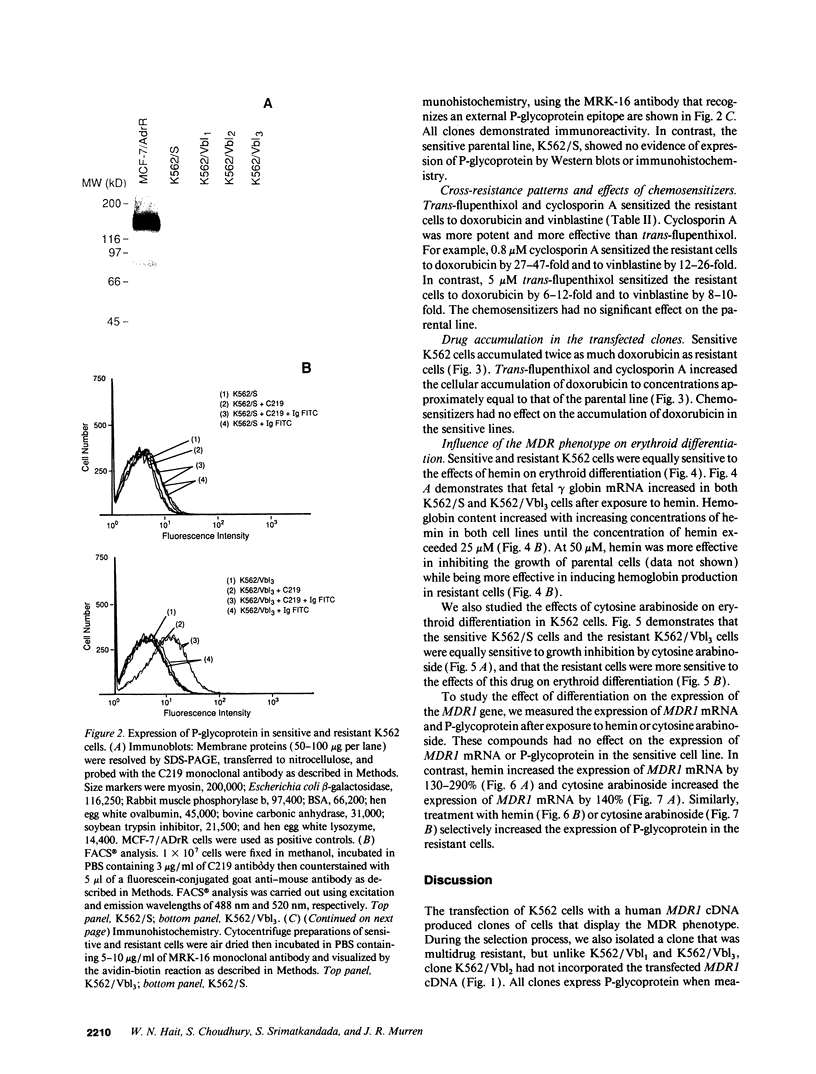
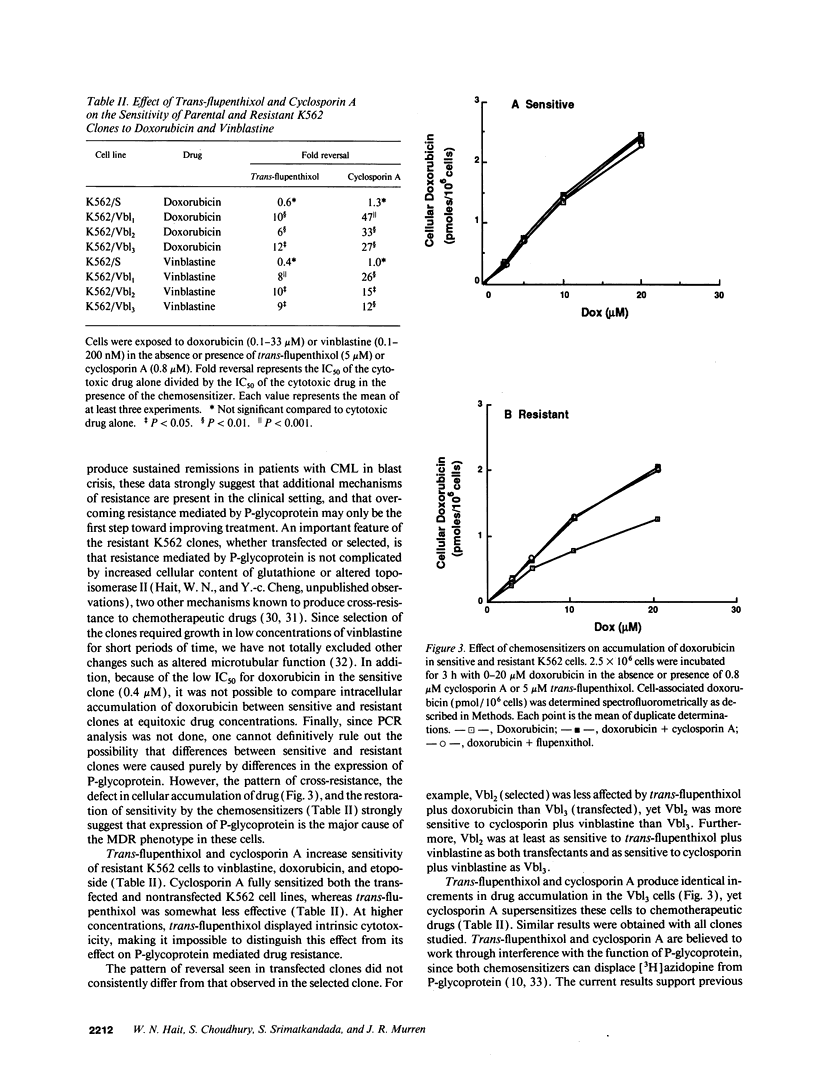
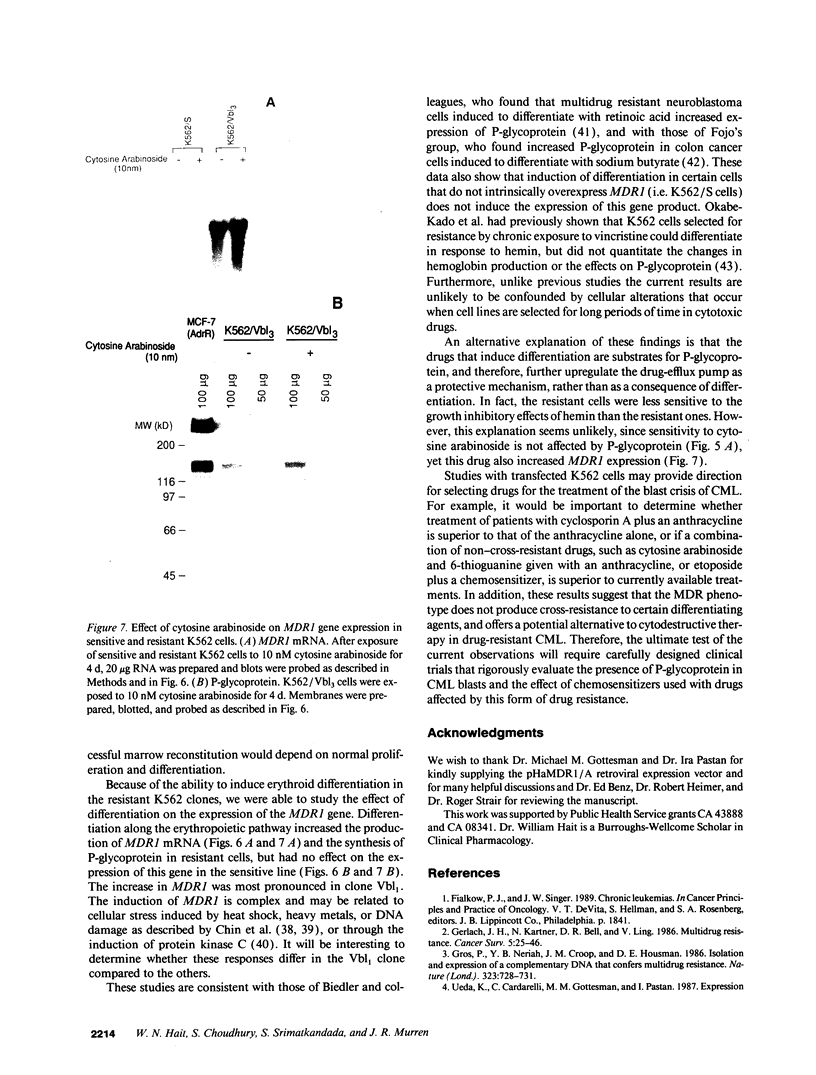
The blast crisis of chronic myelogenous leukemia (CML) is refractory to most forms of cancer chemotherapy, but may be amenable to drugs that differentiate rather than kill leukemic cells. One mechanism implicated in resistance to cytodestructive drugs is overexpression of P-glycoprotein, the MDR1 gene product. While several classes of drugs sensitize multidrug-resistant (MDR) cells by interfering with the function of P-glycoprotein in vitro, few sensitizers have been effective in vivo. We have developed a preclinical model of MDR/CML uncomplicated by other mechanisms of drug resistance to evaluate the effects of MDR1 overexpression on cytodestructive and differentiation therapy and the ability of sensitizers to restore chemosensitivity in this disease. The CML-derived cell line K562 was transfected with a human MDR1 cDNA from the pHaMDR1/A expression vector and selected with vinblastine. Resistant K562 clones were 20-30-fold resistant to vinblastine, were cross-resistant to doxorubicin and etoposide, and remained sensitive to cytosine arabinoside, 6-thioguanine, hydroxyurea, and mechlorethamine. Resistance was associated with decreased cellular accumulation of cytotoxic drug and was reversed by cyclosporin A and trans-flupenthixol. The MDR phenotype did not adversely affect the ability of K562 cells to produce fetal hemoglobin in response to hemin, and was associated with increased responsiveness of cells to differentiate with cytosine arabinoside. Upon differentiation, the resistant clones increased MDR1 mRNA and P-glycoprotein. These studies suggest that the overexpression of the MDR1 gene in CML may not adversely affect the ability to undergo erythroid differentiation and that these resistant K562 cell lines are good models for studying drug resistance mediated by P-glycoprotein in CML.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedler J. L., Casals D., Chang T. D., Meyers M. B., Spengler B. A., Ross R. A. Multidrug-resistant human neuroblastoma cells are more differentiated than controls and retinoic acid further induces lineage-specific differentiation. Prog Clin Biol Res. 1991;366:181–191. [PubMed] [Google Scholar]
- Chambers S. K., Hait W. N., Kacinski B. M., Keyes S. R., Handschumacher R. E. Enhancement of anthracycline growth inhibition in parent and multidrug-resistant Chinese hamster ovary cells by cyclosporin A and its analogues. Cancer Res. 1989 Nov 15;49(22):6275–6279. [PubMed] [Google Scholar]
- Chaudhary P. M., Roninson I. B. Activation of MDR1 (P-glycoprotein) gene expression in human cells by protein kinase C agonists. Oncol Res. 1992;4(7):281–290. [PubMed] [Google Scholar]
- Chin K. V., Chauhan S. S., Pastan I., Gottesman M. M. Regulation of mdr RNA levels in response to cytotoxic drugs in rodent cells. Cell Growth Differ. 1990 Aug;1(8):361–365. [PubMed] [Google Scholar]
- Chin K. V., Tanaka S., Darlington G., Pastan I., Gottesman M. M. Heat shock and arsenite increase expression of the multidrug resistance (MDR1) gene in human renal carcinoma cells. J Biol Chem. 1990 Jan 5;265(1):221–226. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Conscience J. F., Miller R. A., Henry J., Ruddle F. H. Acetylcholinesterase, carbonic anhydrase and catalase activity in Friend erythroleukemic cells, non-erythroid mouse cell lines and their somatic hybrids. Exp Cell Res. 1977 Mar 15;105(2):401–412. doi: 10.1016/0014-4827(77)90137-9. [DOI] [PubMed] [Google Scholar]
- Dusre L., Mimnaugh E. G., Myers C. E., Sinha B. K. Potentiation of doxorubicin cytotoxicity by buthionine sulfoximine in multidrug-resistant human breast tumor cells. Cancer Res. 1989 Feb 1;49(3):511–515. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Ford J. M., Bruggemann E. P., Pastan I., Gottesman M. M., Hait W. N. Cellular and biochemical characterization of thioxanthenes for reversal of multidrug resistance in human and murine cell lines. Cancer Res. 1990 Mar 15;50(6):1748–1756. [PubMed] [Google Scholar]
- Ford J. M., Hait W. N. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990 Sep;42(3):155–199. [PubMed] [Google Scholar]
- Ford J. M., Prozialeck W. C., Hait W. N. Structural features determining activity of phenothiazines and related drugs for inhibition of cell growth and reversal of multidrug resistance. Mol Pharmacol. 1989 Jan;35(1):105–115. [PubMed] [Google Scholar]
- Foxwell B. M., Mackie A., Ling V., Ryffel B. Identification of the multidrug resistance-related P-glycoprotein as a cyclosporine binding protein. Mol Pharmacol. 1989 Oct;36(4):543–546. [PubMed] [Google Scholar]
- Ganapathi R., Grabowski D. Enhancement of sensitivity to adriamycin in resistant P388 leukemia by the calmodulin inhibitor trifluoperazine. Cancer Res. 1983 Aug;43(8):3696–3699. [PubMed] [Google Scholar]
- Ganapathi R., Grabowski D., Ford J., Heiss C., Kerrigan D., Pommier Y. Progressive resistance to doxorubicin in mouse leukemia L1210 cells with multidrug resistance phenotype: reductions in drug-induced topoisomerase II-mediated DNA cleavage. Cancer Commun. 1989;1(4):217–224. [PubMed] [Google Scholar]
- Gerlach J. H., Bell D. R., Karakousis C., Slocum H. K., Kartner N., Rustum Y. M., Ling V., Baker R. M. P-glycoprotein in human sarcoma: evidence for multidrug resistance. J Clin Oncol. 1987 Sep;5(9):1452–1460. doi: 10.1200/JCO.1987.5.9.1452. [DOI] [PubMed] [Google Scholar]
- Goldstein L. J., Galski H., Fojo A., Willingham M., Lai S. L., Gazdar A., Pirker R., Green A., Crist W., Brodeur G. M. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989 Jan 18;81(2):116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Hait W. N., Stein J. M., Koletsky A. J., Harding M. W., Handschumacher R. E. Activity of cyclosporin A and a non-immunosuppressive cyclosporin against multidrug resistant leukemic cell lines. Cancer Commun. 1989;1(1):35–43. doi: 10.3727/095535489820875462. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Meador J., Sweet P., Stupecky M., Wetzel M., Murray S., Gupta S., Slater L. Enhancement by cyclosporin A of daunorubicin efficacy in Ehrlich ascites carcinoma and murine hepatoma 129. Cancer Res. 1987 Dec 1;47(23):6216–6219. [PubMed] [Google Scholar]
- Mickley L. A., Bates S. E., Richert N. D., Currier S., Tanaka S., Foss F., Rosen N., Fojo A. T. Modulation of the expression of a multidrug resistance gene (mdr-1/P-glycoprotein) by differentiating agents. J Biol Chem. 1989 Oct 25;264(30):18031–18040. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murren J. R., Hait W. N. Why haven't we cured multidrug resistant tumors? Oncol Res. 1992;4(1):1–6. [PubMed] [Google Scholar]
- Okabe-Kado J., Hayashi M., Honma Y., Hozumi M., Tsuruo T. Effects of inducers of erythroid differentiation of human leukemia K562 cells on vincristine-resistant K562/VCR cells. Leuk Res. 1983;7(4):481–485. doi: 10.1016/0145-2126(83)90043-7. [DOI] [PubMed] [Google Scholar]
- Pain J., Sirotnak F. M., Barrueco J. R., Yang C. H., Biedler J. L. Altered molecular properties of tubulin in a multidrug-resistant variant of Chinese hamster cells selected for resistance to vinca alkaloids. J Cell Physiol. 1988 Aug;136(2):341–347. doi: 10.1002/jcp.1041360218. [DOI] [PubMed] [Google Scholar]
- Pastan I., Gottesman M. M., Ueda K., Lovelace E., Rutherford A. V., Willingham M. C. A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P-glycoprotein in MDCK cells. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4486–4490. doi: 10.1073/pnas.85.12.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramu A., Glaubiger D., Fuks Z. Reversal of acquired resistance to doxorubicin in P388 murine leukemia cells by tamoxifen and other triparanol analogues. Cancer Res. 1984 Oct;44(10):4392–4395. [PubMed] [Google Scholar]
- Rutherford T. R., Clegg J. B., Weatherall D. J. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979 Jul 12;280(5718):164–165. doi: 10.1038/280164a0. [DOI] [PubMed] [Google Scholar]
- Slater L. M., Sweet P., Stupecky M., Wetzel M. W., Gupta S. Cyclosporin A corrects daunorubicin resistance in Ehrlich ascites carcinoma. Br J Cancer. 1986 Aug;54(2):235–238. doi: 10.1038/bjc.1986.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet P., Chan P. K., Slater L. M. Cyclosporin A and verapamil enhancement of daunorubicin-produced nucleolar protein B23 translocation in daunorubicin-resistant and -sensitive human and murine tumor cells. Cancer Res. 1989 Feb 1;49(3):677–680. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Tsuruo T., Sugimoto Y., Hamada H., Roninson I., Okumura M., Adachi K., Morishima Y., Ohno R. Detection of multidrug resistance markers, P-glycoprotein and mdr1 mRNA, in human leukemia cells. Jpn J Cancer Res. 1987 Dec;78(12):1415–1419. [PubMed] [Google Scholar]
- Ueda K., Cardarelli C., Gottesman M. M., Pastan I. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987 May;84(9):3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayuvegula B., Slater L., Meador J., Gupta S. Correction of altered plasma membrane potentials. A possible mechanism of cyclosporin A and verapamil reversal of pleiotropic drug resistance in neoplasia. Cancer Chemother Pharmacol. 1988;22(2):163–168. doi: 10.1007/BF00257315. [DOI] [PubMed] [Google Scholar]
- Warnke R., Levy R. Detection of T and B cell antigens hybridoma monoclonal antibodies: a biotin-avidin-horseradish peroxidase method. J Histochem Cytochem. 1980 Aug;28(8):771–776. doi: 10.1177/28.8.7003003. [DOI] [PubMed] [Google Scholar]