Abstract
Background
Determination of parathyroid hormone (PTH) is the most commonly used surrogate marker for bone turnover in stage 5 chronic kidney disease patients on dialysis (CKD-5D patients). The objective of the current study was to evaluate the predictive value of various PTH measurements for identifying low or high bone turnover rate.
Study design
Diagnostic test study.
Settings & Participants
141 CKD-5D patients from 15 US hemodialysis centers.
Index Tests
Intact PTH, PTH 1–84, and PTH ratio (ratio of level of PTH 1–84 to level of large carboxy-terminal PTH fragments).
Reference Test or Outcome
Bone turnover determined by bone histomorphometry
Other Measurements
Demographic and treatment related factors, serum calcium and phosphorus.
Results
Histologically, patients presented with a broad range of bone turnover abnormalities. In White patients (n=70), the iPTH cut-off of >420 pg/ml resulted in classifying 84% of the patients correctly as high turnover. A PTH ratio <1.0 when added to an intact PTH <420 pg/ml increased the positive predictive value for low bone turnover from 74% to 90% in these patients. In Black patients (n=71), adding a PTH ratio <1.2 to an intact PTH <340pg/ml increased the positive predictive value for low bone turnover from 48% to 90%. Adding a PTH ratio >1.6 to an intact PTH between 340 and 790 pg/ml increased the positive predictive value for high bone turnover from 56% to 71%.
Limitations
Since the research protocol called for carefully controlled blood specimen handling, drawing of blood and routine specimen handling might be less stringent in clinical practice. By limiting study participation to Black and White CKD-5D patients, we cannot comment on the roles of intact PTH, PTH 1–84 and the PTH ratio in other racial/ethnic groups.
Conclusion
In Black CKD-5D patients, addition of the PTH ratio to intact PTH measurements is helpful for diagnosing low and high bone turnover. In White CKD-5D patients, it aids in the diagnosis of low bone turnover.
INTRODUCTION
Abnormalities of bone turnover are major components of renal osteodystrophy,1–2 and bone biopsy is considered the gold standard for diagnosis of the turnover component of renal osteodystrophy.3–4 Since this procedure is invasive, and requires specialized processing of bone samples,4–6 it is not suitable for routine monitoring of renal osteodystrophy. Accordingly, clinicians often rely on measurements of serum parathyroid hormone (PTH) levels for the noninvasive assessment of bone turnover abnormalities in chronic kidney disease stage 5 patients on maintenance dialysis therapy (CKD-5D patients); this practice, however, carries several limitations.
The first generation of PTH assays was introduced with the development of a competitive protein binding radioimmunoassay for human PTH and used polyclonal antisera raised against PTH purified from animal parathyroid glands.7 Although subsequent assays employed antibodies against human PTH, initial experiments with these single-antibody “first generation” assays showed considerable variance in sensitivity and specificity.8–9 Secondary to the short half-life and the complex metabolism of PTH, there is a large variety of lower molecular weight PTH fragments present in serum.10–11 The half-lives of these fragments vary greatly, especially in the setting of decreased glomerular filtration rate.12
Immunometric “sandwich” assays utilizing 2 antibodies were developed in the 1980’s as “second generation” PTH assays.13–14 The first antibody is usually directed toward the carboxy-terminal region and the second antibody toward the amino-terminal 34 amino acids; accordingly, it was suggested that these assays are more accurate because they measure the full-length, or “intact” PTH molecule (iPTH).14 Studies involving bone biopsies in CKD-5D patients showed, however, that the predictability of bone turnover is limited with the exclusive use of these PTH assays.15–16 This finding could be explained by more recent studies showing that “second generation” iPTH assays recognize not only the full-length (84 amino acid) molecule, which is designated PTH 1–84, but also PTH fragments with large truncations at the amino terminus.17 These large carboxy-terminal PTH fragments have been shown to biologically antagonize the effect of the full-length protein on bone 18 and to accumulate in CKD.19 In spite of this new information on the lack of specificity of second-generation PTH assays, the misleading term “intact” continues to be used.
In an effort to overcome these limitations, a new type of PTH assay was introduced in the late 1990s with an amino-terminal antibody that was altered to more specifically detect PTH 1–84.20–21 This assay – considered a “third generation” assay – is, however, currently not the most widely used PTH assay.
In today’s clinical practice, second-generation iPTH measurements are used to non-invasively assess bone turnover and guide therapeutic decisions. The Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines recommend 150–300 pg/ml as the desirable iPTH concentration in CKD-5D patients, based on measurements using the iPTH Nichols® Allegro IRMA assay.22 This assay is, however, no longer commercially available, and recent studies report significant variability between the currently available “second generation” iPTH kits and the Nichols® Allegro assay, as well as between the iPTH assays themselves.23–25 Most importantly, comparison of bone histologic changes at similar levels of PTH showed over- or underestimation of bone turnover with the use of the currently available iPTH assays.26
Considering the serious clinical implications of potential misjudgments of bone turnover based on information from second generation iPTH assays, it appears highly desirable to look for additional means to increase the predictive value of PTH measurements for bone turnover. This study was conducted to evaluate if diagnostic accuracy for histologically determined bone turnover in Black and White CKD-5D patients could be increased by using results from the third-generation PTH 1–84 assay or by considering the PTH ratio (the ratio of the level of PTH 1–84 to level of large carboxy-terminal PTH fragments) in addition to iPTH or to PTH 1–84 measurements.
METHODS
Study design
Patients agreeing to participate in the Renal Bone Disease Registry of the University of Kentucky were enrolled prospectively in this study. Participation in the registry consisted of a blood draw after overnight fast at time of bone biopsy and agreement to histomorphometric evaluation of the bone biopsy sample. The protocol was approved by the Institutional Review Board of the University of Kentucky. The study has been conducted in adherence to the Declaration of Helsinki, and all patients provided informed consent.
Patients
The study was conducted between 2000 and 2006. During this time, 470 patients were enrolled in the registry. Out of these patients, 172 were biopsied by the investigators. All of these 172 patients agreed to determination of iPTH and PTH 1–84 in blood. These patients were from 15 dialysis centers in Kentucky and surrounding states. All patients were receiving stable dialysis treatment with standard dialysate calcium (2.5 mEq/L).
Inclusion criteria were age ≥18 years; Dialysis vintage ≥3 months; Mental competence; Willingness to participate in the study; Naïve to vitamin D or on a steady dose of vitamin D analogs for ≥6 months.
Exclusion criteria were Hematocrit ≤30%; Parathyroidectomy or failed kidney transplant during the past 6 months; Pregnancy; Uncontrolled systemic illnesses or organ diseases that may affect bone (except type 1 or 2 diabetes mellitus); Treatment during the last 6 months with drugs known to affect bone metabolism such as calcimimetics, bisphosphonates, or steroids (except treatment with vitamin D analogs as outlined above and phosphate binders); Chronic alcoholism and/or drug addiction; bone biopsy sample of inadequate specimen quality for histomorphometry.
Laboratory analysis
Blood was drawn at time of bone biopsy after an overnight fast. Samples were immediately processed and stored at −80°C. All samples were analyzed at the end of the study at the Registry laboratory. iPTH levels were determined in plasma by a second-generation iPTH assay (Total Intact PTH kit, Scantibodies Laboratory Inc, www.scantibodies.com; normal range 14–66 pg/mL; intra- and interassay CV <5% and <7%). PTH 1–84 levels were measured by a third-generation PTH assay (Whole PTH immunoradiometric kit, Scantibodies Laboratory Inc; normal range 5–39 pg/mL; intra- and interassay CV <5% and <7%). Levels of large carboxy-terminal PTH fragments were calculated by subtracting the measured value of PTH 1–84 from iPTH level. Ratios of measured levels of PTH 1–84 to large carboxy-terminal PTH fragments were computed.
Bone biopsy and mineralized bone histology
Anterior iliac crest bone biopsies were done after tetracycline labeling under local anesthesia and conscious sedation. The labeling schedule consisted of a 2-day oral administration of tetracycline hydrochloride (250mg bid) followed by a drug-free interval of 10 days and subsequent oral administration of demeclocycline hydrochloride (300mg bid) for 4 days. Bone biopsies were performed 3–4 days after the second label. Bone samples were obtained with the one-step electrical drill technique (Straumann Medical, www.straumann.com). In the Registry bone laboratory, bone samples were processed undecalcified and 4 and 7μm sections were stained with the modified Masson-Goldner trichrome stain, the aurin tricarboxylic acid stain, and solochrome azurin for assessment of stainable aluminum in bone.5 Unstained sections were prepared for phase contrast and fluorescent light microscopy. Histomorphometric analysis of bone was done at standardized sites in cancellous bone using the semi-automatic method (Osteoplan II, Kontron, www.kontron.com) at 200× magnification. The ratio of bone formation rate to bone surface was measured. Activation frequency was calculated by measuring mineralizing surface per bone surface including all double tetracycline labels and ½ of single tetracycline labels.
Statistical analysis
Data are presented as mean±SD (standard deviation) or frequency counts and percentages unless otherwise indicated. The classification of “low”, “normal”, and “high” bone turnover was based on our normative database.5, 27 Low bone turnover was defined as activation frequency <0.49 year−1, normal bone turnover as activation frequency of 0.49–0.72 year−1, and high bone turnover as activation frequency >0.72 year−1. Differences in patient characteristics between the bone turnover groups were evaluated using ANOVA tests for continuous variables and chi-square or Fisher’s exact test for frequency counts. Pearson correlation coefficients were used to investigate bivariate relationships. Multiple linear regression analysis was used to determine independent associations of predictor variables with log(activation frequency). Variables for inclusion were selected based on statistical significance from bivariate associations (P≤0.2). Backward elimination using inclusion P≤0.05 determined the final model in each case.
In the next analytical step, classification trees for allocating patients into a bone turnover group using iPTH and the PTH ratio, or using PTH 1–84 and the PTH ratio, were generated from a Classification and Regression Tree (CART) analysis. CART provides a flexible, translational approach for determining multi-parameter clinical decision rules.28 In general, classification trees are built by sequentially identifying, in order of importance, parameters and cutoffs for them that can be used for allocating subjects into two or more groups (e.g., low, normal, or high bone turnover in the present study). The CART procedure automatically evaluates interactions among parameters which may have linear or nonlinear associations with the outcome group thus including information from traditional regression analysis techniques. In addition, CART also provides the optimal parameter cutoff value(s) for the classification(s) that can be used in clinical practice. Because of its data-driven flexibility, CART can be viewed as a “nonparametric” classification procedure that eliminates the inherent loss of information associated with the common practice of dichotomizing continuous diagnostic data and/or with the use of decision rules that are based on a single input parameter. All calculations were carried out by the R 2.7.1 statistical package (R Foundation for Statistical Computing, www.r-project.org). The R function used for cart was “rpart”.
RESULTS
Selection criteria were fulfilled by 141 patients: 22 patients were excluded because of drugs known to affect bone metabolism (calcimimetic [n=17], dilantin [n=2]; steroid [n=3]), failed kidney transplant within the previous 6 months [n=5], and bone sample inadequate for bone histomorphometry [n=4]. The study included approximately the same number of Black and White patients (71 vs. 70), and none of the patients had stainable aluminum in bone. Characteristics of the study population are given in Table 1.
Table 1.
Characteristics of the study population.
| Total | Low turnover | Normal turnover | High turnover | P | |
|---|---|---|---|---|---|
| N | 141 | 52 | 21 | 68 | |
| Age (years) | 49.9 ± 15.1 | 53.3 ± 17.0 | 47.6 ± 12.7 | 48.0 ± 13.9 | 0.1 |
| Dialysis Duration (months) | 36.5 ± 35.3 | 34.0 ± 31.0 | 22.4 ± 17.3 | 39.3 ± 40.9 | 0.2 |
| Gender | 0.8 | ||||
| Female (%) | 69 (48.9) | 24 (34.8) | 10 (14.5) | 35 (50.7) | |
| Male (%) | 72 (51.1) | 28 (38.9) | 11 (15.3) | 33 (45.8) | |
| Race | 0.01 | ||||
| White (%) | 70 (49.6) | 34 (50.0) | 6 (8.6) | 29 (41.4) | |
| Black (%) | 71 (50.4) | 17 (24.0) | 15 (21.1) | 39 (54.9) | |
| Diabetes | 0.5 | ||||
| Yes (%) | 38 (27.0) | 17 (44.7) | 5 (13.2) | 16 (42.1) | |
| No (%) | 103 (73.0) | 35 (34.0) | 16 (15.5) | 52 (50.5) | |
| Vitamin D | 0.03 | ||||
| Yes (%) | 52 (36.9) | 13 (25.0) | 12 (23.1) | 27 (51.9) | |
| No (%) | 89 (63.1) | 39 (43.8) | 9 (10.1) | 41 (46.1) | |
| Phosphate Binder | 0.02 | ||||
| None (%) | 15 (10.6) | 9 (60.0) | 2 (13.3) | 4 (26.7) | |
| Calcium based (%) | 73 (51.8) | 32 (43.8) | 10 (13.7) | 31 (42.5) | |
| Non calcium based (%) | 53 (37.6) | 11 (20.8) | 9 (17.0) | 33 (62.2) | |
| Total PTH (pg/ml) | 525.3 ± 500.0 | 260.2 ± 342.6 | 494.1 ± 452.2 | 737.6 ± 520.9 | <0.001 |
| PTH 1–84 (pg/ml) | 286.0 ± 318.4 | 101.0 ± 104.9 | 287.7 ± 260.2 | 426.9 ± 369.5 | <0.001 |
| PTH ratio | 1.5 ± 1.8 | 0.8 ± 0.5 | 2.2 ± 3.1 | 1.7 ± 1.8 | 0.01 |
| Calcium level (mg/dl) | 9.1 ± 0.9 | 9.2 ± 1.0 | 8.7 ± 0.8 | 9.2 ± 0.9 | 0.06 |
| Phosphorus (mg/dl) | 6.1 ± 1.9 | 5.7 ± 1.9 | 6.4 ± 2.2 | 6.3 ± 1.8 | 0.1 |
| Activation frequency (year−1) | 0.81 ± 0.66 | 0.18 ± 0.12 | 0.60 ± 0.07 | 1.32 ± 0.56 | <0.001 |
| BFR/BS (mm3/cm2/yr) | 4.2 ± 3.2 | 1.0 ± 0.8 | 3.4 ± 0.5 | 6.7 ± 2.7 | <0.001 |
Continuous variables are summarized as mean ± standard deviation; categorical variables are summarized as count (percent). Differences in patient characteristics between the bone turnover groups were evaluated using ANOVA tests for continuous variables and chi-square or Fisher’s exact test for frequency counts.
Abbreviations and definitions: PTH = parathyroid hormone; PTH 1–84 = full-length PTH measured by a third-generation assay; PTH ratio = ratio of level of PTH 1–84 to level of large carboxy-terminal PTH fragments, BFR/BS = ratio of bone formation rate to bone surface.
Note: Conversion factors for units: serum calcium in mg/dl to mmol/L, × 0.2495; serum phosphorus in mg/dl to mmol/L, × 0.3229; no conversion necessary for PTH in pg/ml and ng/L.
Since the two measures of bone turnover (activation frequency and ratio of bone formation rate to bone surface) were highly correlated (r=0.97, P<0.001), patients were divided into the bone turnover categories “low”, “normal”, and “high” based on the outcome measure activation frequency (see Methods). There were no statistically significant differences between the groups with respect to age, gender, duration of dialysis, presence of diabetes mellitus, serum calcium and serum phosphorus levels (all P>0.05). Patients with high bone turnover were more likely to be black (P=0.01, to be on vitamin D analog (P=0.03) and/or phosphate binder (P=0.02) therapy. Serum levels of iPTH and of PTH 1–84 correlated well (r=0.91, P<0.001), and they were higher with higher bone turnover (P<0.001). The PTH ratio was lower in patients with low bone turnover when compared to normal or high bone turnover (P=0.01).
Since the distribution of PTH measurements and the PTH ratio were skewed to the right, these variables were log transformed for further statistical analysis. The PTH ratio and measurements of iPTH and PTH 1–84 covered a wide range and correlated well with activation frequency (r=0.42; r=0.57, and r=0.66 respectively; all P<0.001). Similar correlations were found with ratio of bone formation rate to bone surface (r=0.25; r=0.57, and r=0.67 respectively; all P<0.001).
In White patients, multiple linear regression revealed significant associations between bone turnover and iPTH (estimated β=0.535, 95%CI: 0.288–0.781, P<0.001), and the PTH ratio (estimated β=0.507, 95%CI: 0.194–0.821, P=0.002) on the one hand and PTH 1–84 (estimated β=0.596, 95%CI: 0.377–0.815, P<0.001), and the PTH ratio (estimated β=0.334, 95%CI: 0.013–0.654, P=0.04) on the other hand when adjusted for vitamin D usage, serum calcium, serum phosphorus and phosphate binder use. For Black patients, significant associations were found between bone turnover and iPTH (estimated β=0.471, 95%CI: 0.198–0.745, P=0.001), and the PTH ratio (estimated β=0.129, 95%CI: 0.007–0.251, P=0.04) when adjusted for the same variables. In this patient population, only PTH 1–84 (estimated β=0.482, 95%CI: 0.228–0.737, P<0.001) was associated with bone turnover but not the PTH ratio (estimated β=0.090, 95%CI: -0.024–0.206, P<0.1).
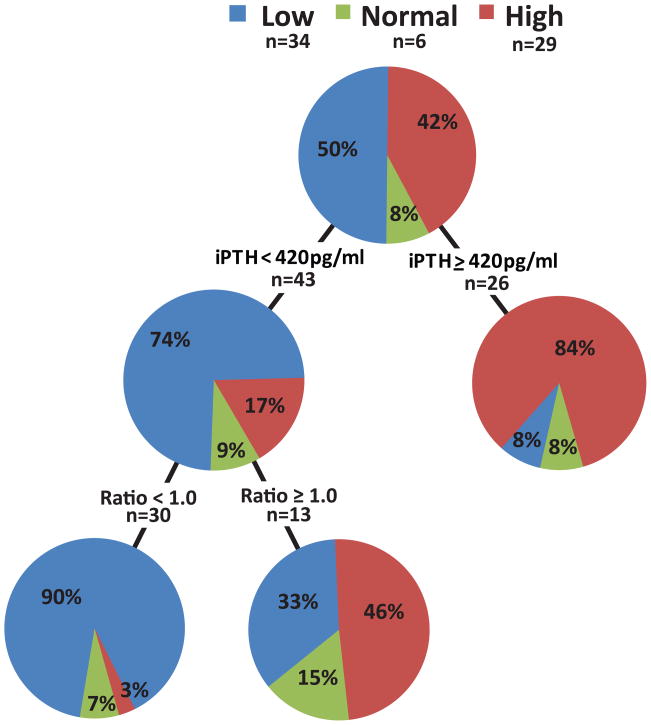
Classification and Regression Tree (CART) analysis (see Methods) was carried out by using iPTH or PTH 1–84 singly or in combination with the PTH ratio as predictor variables for low, normal and high bone turnover to identify optimal cut-off values. In White patients, the iPTH cut-off of ≥420pg/ml (identified by CART analysis as the optimal cut-off point) resulted in classifying 84% of the patients correctly as high turnover (Figure 1a). Levels of iPTH <420pg/ml showed, however, only moderate sensitivity for classification of patients with low turnover (74%). Adding the PTH ratio with a cut-off of <1.0 (identified by CART analysis as the optimal cut-off point) to iPTH measurements increased the proportion of correctly classified White patients with low bone turnover to 90%. CART analysis did not yield a cut-off value for the PTH ratio that would have increased in a statistically significant way the positive predictive value (PPV) for high turnover.
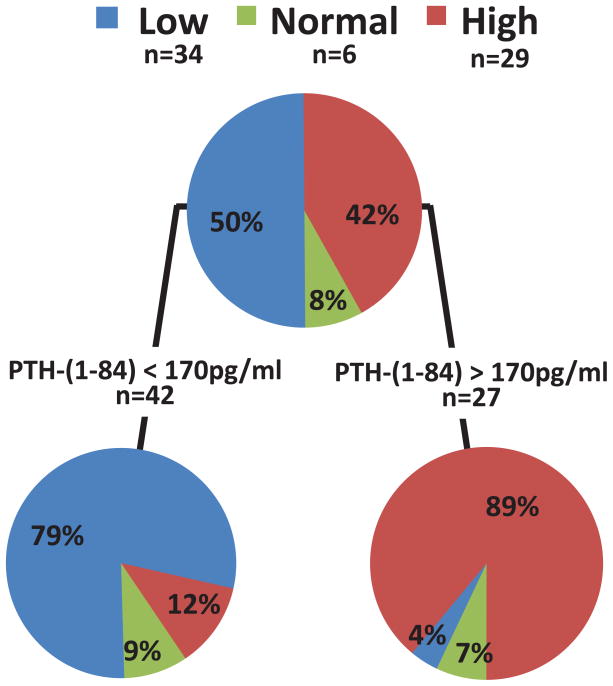
Figure 1.
Figure 1 a and b: Classification and Regression Tree (CART) analysis using (a) intact PTH and (b) PTH 1–84 in combination with the PTH ratio for bone turnover assessment in White chronic kidney disease stage 5 patients.
Abbreviations and definitions: l=low bone turnover; n=normal bone turnover; h=high bone turnover; PPV low = positive predictive value for low bone turnover; PPV high = positive predictive value for high bone turnover; iPTH = intact PTH; PTH ratio = ratio of level of PTH 1–84 to level of large carboxy-terminal PTH fragments.
When using PTH 1–84 measurements, CART analysis reported a PPV of 89% for high turnover for values ≥170pg/ml, and a PPV of 79% for low turnover for values <170pg/ml (Figure 1b). Addition of the PTH ratio did not result in a statistically significant improvement of the model.
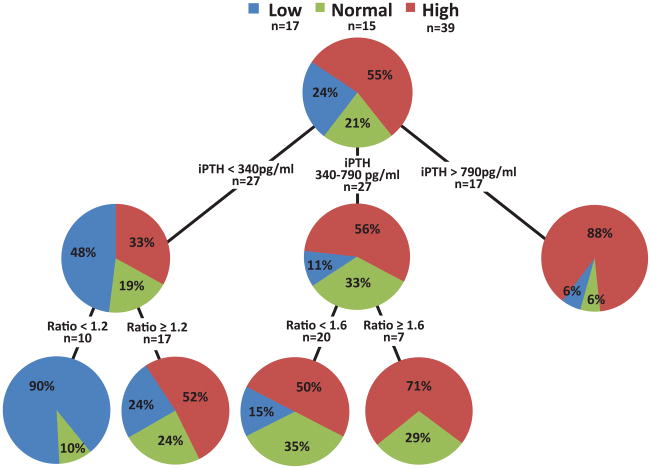
When considering Black patients, the iPTH cut-off <340pg/ml correctly identified only 48% of patients with low bone turnover (Figure 2a). Adding the PTH ratio with a cut-off <1.2 increased this proportion to 90%. For high bone turnover, an iPTH cut-off ≥790pg/ml correctly identified 88% of patients. In the range between 340 and 790pg/ml, 56% of patients presented with high bone turnover and 33% with normal bone turnover. Adding the PTH ratio with a cut-off ≥1.6 to values in this iPTH range increased the yield for patients with high turnover to 71%. Adding the PTH ratio to this iPTH range did not increase in a statistically significant way the PPV for normal or low bone turnover.
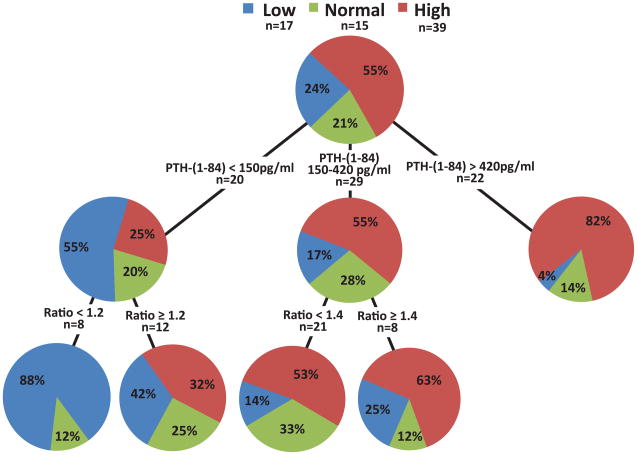
Figure 2.
Figure 2 a and b: Classification and Regression Tree (CART) analysis using (a) intact PTH and (b) PTH 1–84 in combination with the PTH ratio for bone turnover assessment in Black chronic kidney disease stage 5 patients.
Abbreviations and definitions: l=low bone turnover; n=normal bone turnover; h=high bone turnover; PPV low = positive predictive value for low bone turnover; PPV high = positive predictive value for high bone turnover; PPV non-low = positive predictive value for normal and high bone turnover; iPTH = intact PTH; PTH ratio = ratio of level of PTH 1–84 to level of large carboxy-terminal PTH fragments.
While PTH 1–84 values <150pg/ml had a poor PPV of 55% for low bone turnover in Black patients, adding a PTH ratio <1.2 increased this PPV to 88% (Figure 2b). A PTH ratio ≥1.2 yielded a PPV of 67% for normal or high bone turnover. PTH 1–84 values between 150 and 420pg/ml had a PPV of 55% for high bone turnover; adding the PTH ratio ≥1.4 increased the PPV for high bone turnover to 63%. Values of PTH 1–84 >420pg/ml had a PPV for high turnover of 82%; accordingly, adding the PTH ratio did not increase the predictive value of the model in a statistically significant way.
DISCUSSION
Diagnosis of bone turnover in CKD-5D patients is of great clinical importance because of the recently shown associations between low bone turnover and vascular calcifications.29–30 The gold standard for diagnosis of bone turnover is bone biopsy.22 Histomorphometric determination of activation frequency gives the probability that a new cycle of remodeling will be initiated at any point on the bone surface by the event of activation.31–32 and is a highly representative parameter of bone turnover.33 We found activation frequency to correlate closely with ratio of bone formation rate to bone surface in CKD-5D patients with renal osteodystrophy.34
During the past decade, the primary focus of renal osteodystrophy therapy was directed toward avoiding high bone turnover states in CKD-5D patients. More recently, however, the prevalence of low bone turnover was reported to be as high as the prevalence of high bone turnover.35 The increasing prevalence of low bone turnover can be explained by the more frequent initiation of dialysis therapy in CKD-5D patients who are known to be predisposed to lower turnover such as diabetics and the elderly, and the more excessive use of medications for lowering bone turnover such as calcitriol, calcitriol analogs, and calcimimetics.36–39
Based on these observations, there is a growing need for the precise noninvasive assessment of bone turnover abnormalities in CKD patients. Although determinations of serum PTH concentrations are frequently used for this purpose, recent studies showed significant differences between different iPTH assays regarding biochemical results. 19, 23 Importantly, it was recently demonstrated that the 5 most commonly used iPTH assays have limited sensitivity and specificity for assessing bone turnover in CKD-5D patients.26 These data limit the usefulness of iPTH measurements for diagnosing specific bone turnover states and restrict their applications to mere screening for bone turnover abnormalities. Adding results of total alkaline phosphatase measurements or bone specific alkaline phosphatase to iPTH results does not improve diagnostic accuracy26, 34 since alkaline phosphatase can reflect osteoblastic activity in bone,40 and osteoblast-like-cell activity in vascular smooth muscle.41–42 Our data show that using PTH 1–84 alone does not provide significantly improved PPVs for diagnosing bone turnover abnormalities over the use of iPTH in CKD-5D patients.
The specific focus of the current study was to address the addition of the PTH ratio to iPTH and PTH 1–84 for diagnosing the level of bone turnover in this patient population. Previously, it was shown that a PTH ratio cut-off of 1.0 is helpful in the assessment of bone turnover in this patient population; the majority of these patients were, however, white.34 Subsequent findings identified differences in bone response to PTH between Black and White CKD-5D patients.43 Divergent results reported by other investigators on the value of the PTH ratio for diagnosing bone turnover might have been related to differences in racial composition of the studied populations, to differences in specimen handling, and to an insufficient time between cessation of vitamin D therapy and PTH measurements.44–45 The current study was conducted strictly following specimen handling instructions, included only patients naïve to vitamin D or on a steady dose of vitamin D analogs for at least 6 months, and the data was analyzed separately for Black and White CKD-5D patients. It is of note that, independent of the use of vitamin D analogs, White patients presented more often with low bone turnover than Black patients.
A particular strength of the present study is the employment of Classification and Regression Tree (CART) analysis.28 While the conventional method of using regression analyses can be viewed as the default approach to evaluate the contribution of a diagnostic parameter (such as iPTH or the PTH ratio) for predicting an abnormality (such as bone turnover in CKD-5D patients), CART analysis includes regression modeling and improves upon this approach by searching and identifying the best cut-off values of statistically significant predictive parameters (taking into consideration test specificity and sensitivity) for different classes of the studied outcome. When addition of a predictive parameter does not yield statistically significant improvements in PPV for a specific class of an outcome, CART omits the parameter from the decision tree. This approach provides novel and clinically useful decision trees which aid in the interpretation of iPTH and PTH 1–84 results in Black and White CKD-5D patients. The decision trees also demonstrate the value of adding the PTH ratio for diagnosing specific bone turnover abnormalities and show that, irrespective of race, it is particularly helpful for diagnosing low bone turnover. Specifically, there are clear cut-off values for low (iPTH <420pg/ml in combination with a PTH ratio <1.0; or PTH 1–84 <170pg/ml) versus high bone turnover in White patients (iPTH ≥420pg/ml; or PTH 1–84 ≥170pg/ml). This distinction is not as easily accomplished in Black patients; while the combination of iPTH <340pg/ml in combination with a PTH ratio <1.2 clearly identifies patients with low turnover and iPTH >790pg/ml identifies high turnover, there is an iPTH range between 340 and 790pg/ml where low bone turnover can not be diagnosed accurately while a PTH ratio ≥1.6 aids in diagnosing high turnover. Similar limitations exist for the use of PTH 1–84 in this patient population. The apparent differences between Black and White CKD-5D patients regarding the precision and cut-off values for diagnosing bone turnover imply that there might be other factors in addition to PTH involved in the regulation of bone turnover in Black patients. This interesting finding justifies larger population based studies evaluating differences between Black and White CKD-5D patients. Although the above algorithms might appear somewhat complex for use in everyday clinical practice, the significant enhancements in PPVs for bone turnover and the avoidance of clinical misjudgments with their potentially serious consequences should justify their applications.
We would like to acknowledge the following limitations to our study: (1) Our study focuses specifically on the diagnostic values of iPTH, PTH 1–84 and the PTH ratio for bone turnover and was not designed to report prevalences of bone turnover abnormalities in unselected dialysis patients; (2) While our research protocol called for carefully controlled blood specimen handling, drawing of blood and routine specimen handling might be less stringent in clinical practice. (3) Since our study is limited to Black and White CKD-5D patients, we cannot comment on the roles of iPTH, PTH 1–84 and the PTH ratio in other racial/ethnic groups. (4) Although addition of a second biochemical test for the diagnosis of bone turnover add cost, recognition and appropriate management of bone turnover abnormalities should prevent clinical complications that would otherwise create the need for costly interventions. One study shows that using the PTH ratio together with iPTH versus iPTH alone to guide vitamin D dosage avoids low bone turnover in Black CKD-5D patients.46
In summary, these results provide an improved multiparameter algorithm for the noninvasive assessment of bone turnover abnormalities in Black and White CKD-5D patients. These algorithms address the importance of considering differences in race which shall help to choose the appropriate level of suppressive therapy for bone turnover. Future studies are needed to address the utility of this algorithm for improving patient outcomes and whether bone markers beyond PTH and alkaline phosphatase will provide further aid in diagnosing bone turnover in this fast growing patient population.
Acknowledgments
The authors would like to thank Guodong Wang, MD and Juliana Van Willigen for their technical assistance.
Support: The study was supported by the Dean’s Clinical Research Scholar Program, University of Kentucky, grant 1012112710 (Dr Herberth), National Institutes of Health grant RO1 DK51530 (Dr Malluche), and funding from the Kentucky Nephrology Research Trust (Dr Monier-Faugere).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Malluche HH, Monier-Faugere MC. Renal osteodystrophy: what’s in a name? Presentation of a clinically useful new model to interpret bone histologic findings. Clin Nephrol. 2006 Apr;65(4):235–242. doi: 10.5414/cnp65235. [DOI] [PubMed] [Google Scholar]
- 2.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006 Jun;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 3.Martin KJ, Olgaard K, Coburn JW, et al. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis. 2004 Mar;43(3):558–565. doi: 10.1053/j.ajkd.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Malluche HH, Monier-Faugere MC. The role of bone biopsy in the management of patients with renal osteodystrophy. J Am Soc Nephrol. 1994;4:1631–1642. doi: 10.1681/ASN.V491631. [DOI] [PubMed] [Google Scholar]
- 5.Malluche HH, Faugere MC. Atlas of Mineralized Bone Histology. New York: Karger; 1986. [Google Scholar]
- 6.Hruska KA, Teitelbaum SL. Renal Osteodystrophy. N Engl J Med. 1995;333:166–174. doi: 10.1056/NEJM199507203330307. [DOI] [PubMed] [Google Scholar]
- 7.Berson SA, Yalow RS. Immunochemical heterogeneity of parathyroid hormone in plasma. J Clin Endocrinol Metab. 1968;28(7):1037–1047. doi: 10.1210/jcem-28-7-1037. [DOI] [PubMed] [Google Scholar]
- 8.Fischer JA, Binswanger U, Dietrich FM. Human parathyroid hormone. Immunological characterization of antibodies against a glandular extract and the synthetic amino-terminal fragments 1–12 and 1–34 and their use in the determination of immunoreactive hormone in human sera. J Clin Invest. 1974 Dec;54(6):1382–1394. doi: 10.1172/JCI107885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manning RM, Hendy GN, Papapoulos SE, O’Riordan JL. Development of homologous immunological assays for human parathyroid hormone. J Endocrinol. 1980 Apr;85(1):161–170. doi: 10.1677/joe.0.0850161. [DOI] [PubMed] [Google Scholar]
- 10.Mayer GP, Keaton JA, Hurst JG, Habener JF. Effects of plasma calcium concentration on the relative proportion of hormone and carboxyl fragments in parathyroid venous blood. Endocrinology. 1979;104(6):1778–1784. doi: 10.1210/endo-104-6-1778. [DOI] [PubMed] [Google Scholar]
- 11.Segre GV, Niall HD, Sauer RT, Potts JT., Jr Edman degradation of radioiodinated parathyroid hormone: application to sequence analysis and hormone metabolism in vivo. Biochemistry. 1977;16(11):2417–2427. doi: 10.1021/bi00630a017. [DOI] [PubMed] [Google Scholar]
- 12.Martin KJ, Hruska KA, Freitag JJ, Klahr S, Slatopolsky E. The peripheral metabolism of parathyroid hormone. N Engl J Med. 1979;301(20):1092–1098. doi: 10.1056/NEJM197911153012005. [DOI] [PubMed] [Google Scholar]
- 13.Brown RC, Aston JP, Weeks I, Woodhead JS. Circulating intact parathyroid hormone measured by a two-site immunochemiluminometric assay. J Clin Endocrinol Metab. 1987 Sep;65(3):407–414. doi: 10.1210/jcem-65-3-407. [DOI] [PubMed] [Google Scholar]
- 14.Nussbaum SR, Zahradnik RJ, Lavigne JR, et al. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem. 1987 Aug;33(8):1364–1367. [PubMed] [Google Scholar]
- 15.Qi Q, Monier-Faugere MC, Geng Z, Malluche HH. Predictive value of serum parathyroid hormone levels for bone turnover in patients on chronic maintenance dialysis. Am J Kidney Dis. 1995;26(4):622–631. doi: 10.1016/0272-6386(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Hercz G, Sherrard DJ, Maloney NA, Segre GV, Pei Y. Relationship between intact 1–84 parathyroid hormone and bone histomorphometric parameters in dialysis patients without aluminum toxicity. Am J Kidney Dis. 1995;26(5):836–844. doi: 10.1016/0272-6386(95)90453-0. [DOI] [PubMed] [Google Scholar]
- 17.Brossard JH, Cloutier M, Roy L, Lepage R, Gascon-Barre M, D’Amour P. Accumulation of a non-(1–84) molecular form of parathyroid hormone (PTH) detected by intact PTH assay in renal failure: importance in the interpretation of PTH values. J Clin Endocrinol Metab. 1996;81(11):3923–3929. doi: 10.1210/jcem.81.11.8923839. [DOI] [PubMed] [Google Scholar]
- 18.Langub MC, Monier-Faugere MC, Wang G, Williams JP, Koszewski NJ, Malluche HH. Administration of PTH-(7–84) antagonizes the effects of PTH-(1–84) on bone in rats with moderate renal failure. Endocrinology. 2003 Apr;144(4):1135–1138. doi: 10.1210/en.2002-221026. [DOI] [PubMed] [Google Scholar]
- 19.Herberth J, Fahrleitner-Pammer A, Obermayer-Pietsch B, et al. Changes in total parathyroid hormone (PTH), PTH-(1–84) and large C-PTH fragments in different stages of chronic kidney disease. Clin Nephrol. 2006 May;65(5):328–334. doi: 10.5414/cnp65328. [DOI] [PubMed] [Google Scholar]
- 20.Gao P, Scheibel S, D’Amour P, et al. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1–84: Implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res. 2001;16:605–614. doi: 10.1359/jbmr.2001.16.4.605. [DOI] [PubMed] [Google Scholar]
- 21.John MR, Goodman WG, Gao P, Cantor TL, Salusky IB, Juppner H. A novel immunoradiometric assay detects full-length human PTH but not amino-terminally truncated fragments: implications for PTH measurements in renal failure. J Clin Endocrinol Metab. 1999;84(11):4287–4290. doi: 10.1210/jcem.84.11.6236. [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003 Oct;42(4 Suppl 3):S1–201. [PubMed] [Google Scholar]
- 23.Souberbielle JC, Boutten A, Carlier MC, et al. Inter-method variability in PTH measurement: implication for the care of CKD patients. Kidney Int. 2006 Jul;70(2):345–350. doi: 10.1038/sj.ki.5001606. [DOI] [PubMed] [Google Scholar]
- 24.Joly D, Drueke TB, Alberti C, et al. Variation in serum and plasma PTH levels in second-generation assays in hemodialysis patients: a cross-sectional study. Am J Kidney Dis. 2008 Jun;51(6):987–995. doi: 10.1053/j.ajkd.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Cantor T, Yang Z, Caraiani N, Ilamathi E. Lack of comparability of intact parathyroid hormone measurements among commercial assays for end-stage renal disease patients: implication for treatment decisions. Clin Chem. 2006 Sep;52(9):1771–1776. doi: 10.1373/clinchem.2006.071589. [DOI] [PubMed] [Google Scholar]
- 26.Herberth J, Monier-Faugere MC, Mawad H, et al. The five most commonly used intact parathyroid hormone assays are useful for screening but not for diagnosing bone turnover abnormalities in CKD-5 patients. Clin Nephrol. 2009;72:5–14. doi: 10.5414/cnp72005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malluche HH, Meyer W, Sherman D, Massry SG. Quantitative bone histology in 84 normal American subjects. Micromorphometric analysis and evaluation of variance in iliac bone. Calcif Tissue Int. 1982 Sep;34(5):449–455. doi: 10.1007/BF02411283. [DOI] [PubMed] [Google Scholar]
- 28.Breiman L, Friedman J, Stone CJ, Olshen RA. Classification and regression trees. Wadsworth International Group; Belmont, California: 1984. [Google Scholar]
- 29.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004 Jul;15(7):1943–1951. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- 30.Asci G, Ozkaya M, Duman S, Toz H, Savas R, Kayikcioglu M, Celik G, Ozbek S, Basci A, Monier-Faugere MC, Malluche HH, Ok E. The link between cardiovascular and bone disease in hemodialysis patients. Nephrol Dial Transplant. 2007;22(suppl vi):217. [Google Scholar]
- 31.Parfitt AM. The Physiologic and Clinical Significance of Bone Histomorphometric Data. In: Recker RR, editor. Bone Histomorphometry: Techniques and Interpretation. Boca Raton, FL: CRC Press, Inc; 1983. pp. 143–224. [Google Scholar]
- 32.Eriksen EF. Normal and pathological remodeling of human trabecular bone: three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone disease. Endocr Rev. 1986 Nov;7(4):379–408. doi: 10.1210/edrv-7-4-379. [DOI] [PubMed] [Google Scholar]
- 33.Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols and units. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 34.Monier-Faugere MC, Geng Z, Mawad H, et al. Improved assessment of bone turnover by the PTH-(1–84)/large C-PTH fragments ratio in ESRD patients. Kidney Int. 2001 Oct;60(4):1460–1468. doi: 10.1046/j.1523-1755.2001.00949.x. [DOI] [PubMed] [Google Scholar]
- 35.Malluche HH, Mawad H, Monier-Faugere MC. The importance of bone health in end-stage renal disease: out of the frying pan, into the fire? Nephrol Dial Transplant. 2004 Mar;19 (Suppl 1):i9–13. doi: 10.1093/ndt/gfh1002. [DOI] [PubMed] [Google Scholar]
- 36.Baker LR, Abrams L, Roe CJ, et al. 1,25(OH)2D3 administration in moderate renal failure: a prospective double-blind trial. Kidney Int. 1989;35(2):661–669. doi: 10.1038/ki.1989.36. [DOI] [PubMed] [Google Scholar]
- 37.Malluche HH, Monier-Faugere MC. Risk of adynamic bone disease in dialyzed patients. Kidney Int Suppl. 1992;38:S62–67. [PubMed] [Google Scholar]
- 38.Hernandez JD, Wesseling K, Salusky IB. Role of parathyroid hormone and therapy with active vitamin D sterols in renal osteodystrophy. Semin Dial. 2005 Jul–Aug;18(4):290–295. doi: 10.1111/j.1525-139X.2005.18404.x. [DOI] [PubMed] [Google Scholar]
- 39.Malluche HH, Monier-Faugere MC, Wang G, et al. An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin Nephrol. 2008 Apr;69(4):269–278. doi: 10.5414/cnp69269. [DOI] [PubMed] [Google Scholar]
- 40.Malluche HH, Faugere MC, Fanti P, Price PA. Plasma levels of bone Gla-protein reflect bone formation in patients on chronic maintenance dialysis. Kidney Int. 1984 Dec;26(6):869–874. doi: 10.1038/ki.1984.230. [DOI] [PubMed] [Google Scholar]
- 41.Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005 Jul 22;97(2):105–114. doi: 10.1161/01.RES.00000175571.53833.6c. [DOI] [PubMed] [Google Scholar]
- 42.Orita Y, Yamamoto H, Kohno N, et al. Role of Osteoprotegerin in Arterial Calcification. Development of New Animal Model. Arterioscler Thromb Vasc Biol. 2007 Jul 5; doi: 10.1161/ATVBAHA.107.147868. [DOI] [PubMed] [Google Scholar]
- 43.Sawaya BP, Butros R, Naqvi S, et al. Differences in bone turnover and intact PTH levels between African American and Caucasian patients with end-stage renal disease. Kidney Int. 2003 Aug;64(2):737–742. doi: 10.1046/j.1523-1755.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 44.Salusky IB, Goodman WG, Kuizon BD, et al. Similar predictive value of bone turnover using first- and second-generation immunometric PTH assays in pediatric patients treated with peritoneal dialysis. Kidney Int. 2003 May;63(5):1801–1808. doi: 10.1046/j.1523-1755.2003.00915.x. [DOI] [PubMed] [Google Scholar]
- 45.Coen G, Bonucci E, Ballanti P, et al. PTH 1–84 and PTH “7–84” in the noninvasive diagnosis of renal bone disease. Am J Kidney Dis. 2002;40(2):348–354. doi: 10.1053/ajkd.2002.34519. [DOI] [PubMed] [Google Scholar]
- 46.Fehmi H, Osman Y, Bhat S, et al. Absence of adynamic bone disease in African-Americans with CKD stage 5 after 3 years of vitamin D therapy guided by iPTH and the PTH-(1–84)/N-terminally truncated PTH fragments ratio. Clin Nephrol. 2009 Mar;71(3):267–275. doi: 10.5414/cnp71267. [DOI] [PubMed] [Google Scholar]