Abstract
Background
Depressive symptoms are an established predictor of mortality and major adverse cardiac events (defined as nonfatal myocardial infarction or hospitalization for unstable angina or urgent/emergency revascularizations) in patients with acute coronary syndrome (ACS). This study was conducted to determine the acceptability and efficacy of enhanced depression treatment in patients with ACS.
Methods
A 3-month observation period to identify patients with ACS and persistent depressive symptoms was followed by a 6-month randomized controlled trial. From January 1, 2005, through February 29, 2008, 237 patients with ACS from 5 hospitals were enrolled, including 157 persistently depressed patients randomized to intervention (initial patient preference for problem-solving therapy and/or pharmacotherapy, then a stepped-care approach; 80 patients) or usual care (77 patients) and 80 nondepressed patients who underwent observational evaluation. The primary outcome was patient satisfaction with depression care. Secondary outcomes were depressive symptom changes (assessed with the Beck Depression Inventory), major adverse cardiac events, and death.
Results
At the end of the trial, the proportion of patients who were satisfied with their depression care was higher in the intervention group (54% of 80) than in the usual care group (19% of 77) (odds ratio, 5.4; 95% confidence interval [CI], 2.2–12.9 [P<.001]). The Beck Depression Inventory score decreased significantly more (t155=2.85 [P=.005]) for intervention patients (change, −5.7; 95% CI, −7.6 to −3.8; df=155) than for usual care patients (change, −1.9; 95% CI, −3.8 to −0.1; df=155); the depression effect size was 0.59 of the standard deviation. At the end of the trial, 3 intervention patients and 10 usual care patients had experienced major adverse cardiac events (4% and 13%, respectively; log-rank test, [P=.047]), as well as 5 nondepressed patients (6%) (for the intervention vs nondepressed cohort, [P=.49]).
Conclusion
Enhanced depression care for patients with ACS was associated with greater satisfaction, a greater reduction in depressive symptoms, and a promising improvement in prognosis.
PATIENTS WITH ACUTE COROnary syndrome (ACS) (myocardial infarction or unstable angina) who report even subsyndromal levels of depressive symptoms are at increased risk of ACS recurrence or mortality.1,2 This increased risk is observed over many years,3 is largely independent of other known risk factors for coronary heart disease (CHD),4 is strong,5 and has a dose-response association.6 The risk is particularly high for those whose depressive symptoms persist7 or are refractory to treatment.8,9 Although the association is not found in every study10 or with every ACS patient subgroup,11 systematic reviews,1,2,12 recent international data,13 and other accumulating research indicate that depression is a marker of increased risk of CHD events and mortality in this patient population. There have been calls for depression to be recognized as a risk marker14 and recommendations that patients with CHD be regularly screened for depression and be referred for treatment.15 However, we do not know whether patients with CHD and depressive symptoms, including many with subsyndromal symptoms, should be treated.
Screening for a reliable CHD risk marker without clear evidence of how to successfully treat the risk can be problematic.16 In the case of depression, the suffering associated with the disorder is arguably sufficient justification for treatment. Given the strength of the observational evidence, however, there have been surprisingly few trials to determine whether depression can be successfully treated in patients with ACS and the risk of ACS recurrence or mortality mitigated. The first sufficiently powered trial (Enhancing Recovery in Coronary Heart Disease [ENRICHD]; conducted in 2481 patients) to test this question found a significant but modest reduction in depressive symptoms but no mortality difference between cognitive behavioral depression therapy and usual care.17 A second trial (Myocardial Infarction and Depression–Intervention Trial [MIND-IT]; conducted in 331 patients) also found significant improvements in depression but no difference in the cardiac event rate between antidepressant treatment and usual care.18 These results were disappointing because the Sertraline Antidepressant Heart Attack Randomized Trial (conducted in 369 patients), although powered only for safety, had shown a promising trend for 6-month sertraline hydrochloride use to reduce the risk of severe cardiovascular events compared with placebo.19 Other small trials20 and a post hoc, post-randomization responder analysis of the ENRICHD trial21 showed similar results. Given these few trials, we do not yet know whether reducing depressive symptoms improves medical prognosis in patients with ACS.
The Coronary Psychosocial Evaluation Studies (COPES) intervention trial was designed to address several reasons why previous trials may not have led to greater reductions in depressive symptoms and improvements in medical prognosis. First, the COPES trial sought to better target at-risk patients by using a 3-month observation period after ACS to eliminate patients whose symptoms spontaneously remit or respond to usual care. This strategy identifies patients with persistently elevated depressive symptoms rather than those with a diagnosis of major depressive disorder only. Second, the COPES trial adopted an approach to depression care similar to that used for the Improving Mood–Promoting Access to Collaborative Treatment (IMPACT) trial,22 including stepped care and patient preference. This approach, tailored to patients with ACS, is designed to increase the acceptance of and satisfaction with depression treatment in this population because treatment acceptance has been low in previous trials.23 We hypothesized that the COPES intervention would result in greater satisfaction with depression care and improved depressive symptoms. We also compared the rates of major adverse cardiac events (MACEs) and mortality of the depressed patients in the intervention and usual care groups with those of an observational cohort of persistently nondepressed but otherwise medically eligible patients.
METHODS
RECRUITMENT, ENROLLMENT, AND INFORMED CONSENT
Participants were recruited at 5 hospital sites (Mount Sinai Hospital and New York Presbyterian Hospital, New York, New York; and New Haven Hospital, Hospital of St Raphael, and Veterans Affairs Connecticut Healthcare System−West Haven, New Haven, Connecticut) from January 1, 2005, through February 29, 2008. Full details of the design and methods are provided elsewhere.23
Study participants were identified prospectively by monitoring hospital admissions for ACS diagnoses.24 To ensure that only patients with persistent depressive symptoms were enrolled, trial eligibility required a score of 10 or higher on the Beck Depression Inventory (BDI)25 on assessments within 1 week of hospitalization for ACS and 3 months later. Patients with BDI scores below 5 at both assessment points who met all other eligibility criteria were included in a nondepressed observational cohort.
Exclusion criteria were assessed at the hospital visit and 3-month follow-up and included alcohol or other drug dependency, dementia, current or past psychosis or bipolar disorder, terminal illness, unavailability for follow-up, BDI score of 45 or higher, or suicidality by self-report or determined during a clinical interview.
The institutional review boards at all institutions approved the protocol, and all participants provided written informed consent. To ensure equipoise, the description of the study to patients and their physicians emphasized the possible benefits and limitations of both the intervention and usual care conditions.
RANDOMIZATION
At each site, eligible patients were randomized on a 1:1 basis within randomly ordered blocks of 4 or 6 patients according to a table of assignments prepared in advance by the trial statistician (J.E.S.). Using a Web-based program, project coordinators specified the strata, initials, and study identification number of the person to be randomized, and the program issued the group assignment.
INTERVENTION AND USUAL CARE PROTOCOLS
The intervention included the following 5 essential components adapted from the IMPACT study22: (1) an enhanced care approach, with treatment delivered by a clinical nurse specialist, psychologist, social worker, and/or psychiatrist; (2) patient choice of psychotherapy and/or pharmacotherapy; (3) a form of psychotherapy called problem-solving therapy (PST); (4) a stepped-care approach in which symptom severity was reviewed every 8 weeks and treatment was augmented according to predetermined decision rules23; and (5) a standardized instrument used to track depressive symptoms.
Problem-solving therapy, as developed for the IMPACT study, has been described in detail elsewhere.22,23,26 It is protocol driven, brief, problem focused, and designed to augment the patient's own skills. Patients are taught how to systematically evaluate and address individual psychosocial problems. The initiation of and regular engagement in pleasant activities chosen by the patient is encouraged. Visits initially occurred weekly, in person or by telephone, with each visit lasting approximately 30 to 45 minutes. Visit frequency was decreased or increased according to the progress of individual patients and their preference.
Pharmacotherapy treatment choices included sertraline, escitalopram oxalate, venlafaxine hydrochloride, bupropion hydrochloride, and mirtazapine. A study psychiatrist or nurse practitioner prescribed appropriate medication following standard clinical practice. Intervention patients choosing pharmacotherapy were initially seen at 1- to 2-week intervals for dose titration and thereafter every 3 to 5 weeks as needed for the remainder of the 6-month trial period. If a patient was already taking an antidepressant, treatment decisions were coordinated with the prescribing physician. At the end of the trial, patients were provided with 6 further months of medication if they could not afford it but were referred to their usual care provider for follow-up. Four patients took advantage of this offer.
Stepped-care decisions for patients randomized to the intervention group were guided by responses to the 9-item Patient Health Questionnaire,27 administered at each treatment visit and formally evaluated at 8-week intervals. Patients who did not show prespecified improvement were offered the choice of switching treatments (eg, from PST to medication), adding the other treatment, or intensifying the original treatment choice, based on the treatment team's recommendation (for details, see Burg et al23).
The control condition for the trial was usual care, as defined by the patient's treating physicians. Physicians of the intervention and usual care patients were informed that their patients were participating in a trial and that they had elevated depressive symptoms; physicians were also told whether the patient met the criteria for a major depressive episode.
DATA COLLECTION
At the time of the index ACS hospitalization, demographic, medical history, and prognostic variables were collected, including left ventricular ejection fraction and Global Registry of Acute Coronary Events (GRACE) risk score.28 At 3 months, just before randomization, a structured clinical interview (Depression Interview and Structured Hamilton questionnaire)29 was conducted by telephone to assess the presence of a current major depressive episode and psychiatric exclusion criteria. All other measures at hospitalization, 3 months after hospitalization, and at the end of the 6-month intervention (month 9) were assessed in person. Measures at months 5 and 7 were obtained by telephone. Interviewers and those collecting medical outcome data were blinded to intervention assignment.
OUTCOME MEASURES
The primary outcome was satisfaction with depression care because previous treatments may not have been acceptable to patients with CHD.17 Patients were asked, “Over the last 2 months, how would you rate the quality of professional care you have received for your symptoms of distress or depression?” Patients responded on a 5-point Likert scale (1, excellent; 5, poor) or indicated that they had received no care for these symptoms. Depression severity was assessed by the BDI,25 a well-validated depression measure that is predictive of medical outcomes in this population.30,31 A BDI score of 10 or higher is consistent with at least mild to moderate depression.
For each patient-reported hospitalization, supporting documentation was gathered from the hospital record. Hospital systems were also actively surveyed for events. An end-point committee of 2 board-certified cardiologists independently reviewed and classified each hospitalization; in case of disagreement, a third board-certified cardiologist adjudicated the final end point. Cardiologists were unaware of participants' depression or treatment status. For participants who could not be contacted or were reported deceased by a relative, the Social Security Death Index was searched to verify vital status, and death certificates were obtained. The first occurrence of a MACE (nonfatal myocardial infarction or hospitalization for unstable angina) or all-cause mortality was recorded.
ADVERSE EVENTS
Participants were asked about unanticipated problems or adverse events at each assessment (at 3, 5, 7, and 9 months) with the use of a standardized checklist covering major and minor cardiovascular symptoms and physical and psychiatric symptoms; these were presented regularly in a blinded fashion to the Data and Safety Monitoring Board.
STATISTICAL ANALYSIS
Differences between the intervention and usual care groups and between the trial participants and nondepressed cohort at baseline were evaluated using a t test for continuous variables and χ2 analysis for categorical variables. When baseline medical covariate data were incomplete for the GRACE and Charlson indexes, a regression-based approach was used to impute the best linear predicted score based on the available items.
Outcome Analyses
Descriptive statistics based on the raw data at baseline were used to characterize the sample. Linear and nonlinear (ie, logistic) multilevel repeated-measures modeling procedures were used to generate full-information maximum-likelihood estimates of all treatment effects (outcome at 9 months or change in outcome from months 3 to 9, after the 6-month intervention). By including all subjects and all available data, this approach yields intent-to-treat estimates that are valid under the assumption that the missing data are missing at random, conditional on the observed data.32,33 Wald χ2 statistics were used to test the statistical significance of group differences at 9 months and the differential change between groups (group×time interaction). The primary outcome was the percentage of patients who rated their depression care as excellent or very good at 9 months. Change in the BDI score was a secondary outcome. Effect size was calculated as the group difference in BDI change divided by the pooled SD at baseline. Kaplan-Meier survival curves for MACEs were estimated and compared using the log-rank test. All analyses were performed using SAS statistical software (version 9.2; SAS Institute Inc, Cary, North Carolina), including PROCs MIXED, NLMIXED, LIFETEST, and PHREG procedures.
Power Analysis
The 2-sided α was set at .05, and power was set at 0.90. The sample size was chosen to ensure this level of power to detect a 30% group difference (intervention vs usual care groups) in the proportion of patients who were satisfied with their depression care at the conclusion of the 6-month trial. This required enrolling 80 patients per group, allowing for 20% loss (eg, 64 per group with 9-month outcome data would provide a power of ≥0.93 to detect any 30% group difference in satisfaction, eg, 90% vs 60%, 65% vs 35%, or 35% vs 5%).
RESULTS
BASELINE CHARACTERISTICS
Patients randomized to the intervention and usual care groups were similar on all baseline variables (Table 1). In contrast, compared with those in the trial, patients in the nondepressed cohort differed on measures of depression (by definition), were less likely to be female, were more likely to be Hispanic, had more years of education, and were more likely to be married. Their index ACS was also more likely to be an ST-segment elevation or a non–ST-segment elevation myocardial infarction than unstable angina. Finally, the nondepressed cohort had significantly higher GRACE28 scores than the persistently depressed groups.
Table 1.
Characteristics of 237 Patients With ACSa
| Variable | Usual Care Group (n=77) | Intervention Group (n=80) | P Valueb,c | Nondepressed Cohort (n=80) | P Valueb,d |
|---|---|---|---|---|---|
| Female | 4 (53) | 43 (54) | .95 | 24 (30) | <.001 |
| Age, mean (SD), ye | 61.1 (10.6) | 59.3 (10.6) | .29 | 63.3 (10.3) | .03 |
| Hispanic | 32 (42) | 36 (45) | .66 | 50 (63) | .005 |
| African American | 17 (22) | 12 (15) | .25 | 23 (29) | .07 |
| Educatione | |||||
| Mean (SD), y | 13.0 (3.8) | 12.0 (4.0) | .35 | 14.3 (3.7) | .002 |
| ≥High school graduate | 53 (73) | 52 (67) | .43 | 61 (80) | .09 |
| ≥College graduate | 19 (26) | 20 (26) | .96 | 35 (46) | .002 |
| Marital statusf | |||||
| Single | 12 (16) | 18 (23) | .54 | 16 (21) | .01 |
| Married/partner | 36 (48) | 34 (44) | 49 (63) | ||
| Separated/divorced/widowed | 27 (36) | 26 (33) | 13 (17) | ||
| BDI depressive symptom score ≥16 at 3 mo25 | 52 (68) | 52 (65) | .74 | … | … |
| Diagnosis of major depressive episode at 3 mog | 21 (32) | 23 (33) | .90 | … | … |
| Hamilton Depression Rating at 3 mo, mean (SD)b,g | 13.3 (8.0) | 13.6 (6.3) | .83 | … | … |
| Type of ACS | |||||
| Unstable angina | 60 (78) | 58 (73) | .69 | 47 (59) | .02 |
| Non–ST-segment elevation myocardial infarction | 9 (12) | 13 (16) | 17 (21) | ||
| ST-segment elevation myocardial infarction | 8 (10) | 8 (10) | 16 (20) | ||
| GRACE score, mean (SD)28,e | 94 (23) | 91 (24) | .48 | 99 (23) | .03 |
| Left ventricular ejection fraction <40%h | 7 (11) | 7 (11) | .99 | 14 (21) | .06 |
Abbreviations: ACS, acute coronary syndrome; BDI, Beck Depression Inventory; ellipses, not applicable; GRACE, Global Registry of Acute Coronary Events.
Unless otherwise indicated, data are expressed as number (percentage) of patients.
Based on the χ2 test for categorical measures and independent-sample t test for continuous measures.
Indicates usual care group vs intervention group.
Indicates nondepressed cohort vs those in the randomized controlled trial (both groups combined).
The sample sizes were 73 in the usual care, 78 in the intervention, and 76 in the nondepressed groups.
The sample sizes were 75 in the usual care and 78 in the intervention and nondepressed groups.
The sample sizes were 65 in the usual care and 69 in the intervention groups.
The sample sizes were 63 in the usual care and intervention and 66 in the nondepressed groups.
TREATMENT PREFERENCES AND INTERVENTION IMPLEMENTATION
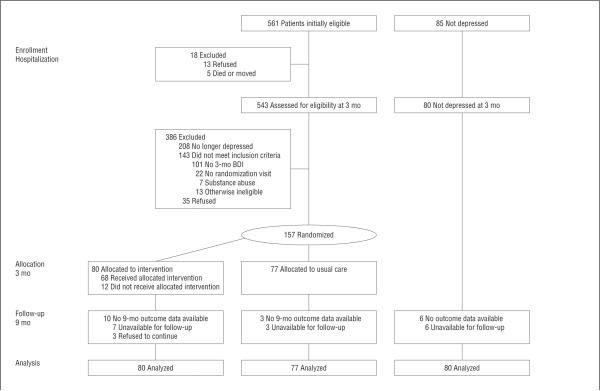
Of the 80 patients randomized to intervention, 60 (75%) initially chose PST, 16 (20%) chose antidepressant medication, and 2 (2.5%) chose both; two patients did not state a treatment preference (Figure 1). Thirteen patients (16%) did not receive any study treatment. The mean (SD) number of treatment sessions was 8.2 (5.2) for patients who initially chose PST, 6.0 (3.7) for patients who initially chose antidepressant medication, and 19.5 (6.4) for patients who initially chose both. The proportion of treatment sessions delivered by phone ranged between 0% and 94% (mean [SD], 38.8% [36.8%]).
Figure 1.
Patient flowchart. BDI indicates Beck Depression Inventory.
Of the patients who initially chose PST, 6 were additionally prescribed antidepressant medication during the course of their treatment. Of patients who initially chose antidepressant medication, 2 later additionally received PST and 1 switched to PST. Within the first 16 weeks of antidepressant treatment (during which 2 formal step reviews had been conducted by the entire depression care team), the dosage was increased once for 7 patients and twice for 2 patients. One patient's treatment was augmented with a second antidepressant; another patient's dosage was first decreased and later the medication was switched to another antidepressant type. Of the 2 patients who chose antidepressant treatment and PST at the beginning of the trial, 1 patient's dosage was changed multiple times. We did not capture antidepressant increases, switches, or therapy sessions for those randomized to usual care. Overall, 7 patients terminated treatment before their study provider advised it.
PREVALENCE OF ANTIDEPRESSANT AND PSYCHOTHERAPY USE BEFORE AND AFTER THE TRIAL
Approximately one-third of the trial participants (35%) reported taking antidepressants at the 3-month randomization; at the end of the trial this was 48% in the treatment group, but remained at 30% in the usual care group (odds ratio, 4.48; 95% confidence interval [CI], 1.05–19.2 [P=.04, intention-to-treat estimates]). Participation in psychotherapy was 11% and 20%, respectively, before randomization; at the end of the trial it had increased to 39% in the intervention group, with a decline to 12% in the usual care group (odds ratio, 10.1; 95% CI, 2.32–44.3 [P=.002, intention-to-treat estimates; therefore, patient numbers not presented]).
PRIMARY TRIAL OUTCOME
The percentage of patients reporting depression care as excellent or very good at month 3 (ie, randomization) was modestly and not significantly different between groups (P=.18) (Table 2). At 9 months, however, 54% of patients in the intervention group reported this level of satisfaction with depression care compared with 19% in the usual care group (odds ratio, 5.4; 95% CI, 2.2–12.9 [P<.001, intention-to-treat estimates]).
Table 2.
Satisfaction With Depression Care 3 and 9 Months After ACSa
| Variable | Usual Care Group (n=77) | Intervention Group (n=80) | OR (95% CI) | P Value |
|---|---|---|---|---|
| Rated depression care as excellent or very good at 3 mo, % (95% CI)b | 13.2 (6.5–19.6) | 21.6 (12.9–29.7) | 1.8 (0.8–4.5) | .18 |
| Rated depression care as excellent or very good at 9 mo, % (95% CI)b | 18.8 (10.4–26.7) | 54.2 (41.9–63.6) | 5.4 (2.2–12.9) | <.001 |
| Patients receiving no care at 3 mo, No. (%) | 56/76 (74) | 53/74 (72) | … | … |
| Patients receiving no care at 9 mo, No. (%) | 43/69 (62) | 19/70 (27) | … | … |
Abbreviations: ACS, acute coronary syndrome; CI, confidence interval; ellipses, not applicable; OR, odds ratio.
Data on depression care were missing for 7 patients at 3 months and for 18 patients at 9 months.
Numbers of patients are not provided because these percentages are derived from an intent-to-treat, multilevel, repeated-measures logistic regression analysis.
SECONDARY TRIAL OUTCOMES
Depressive symptoms decreased significantly in both the intervention (mean change, −5.7; 95% CI, −7.6 to −3.8) and usual care (mean change, −1.9; 95% CI, −3.8 to −0.1) groups (Table 3). The group difference in depressive symptom decrease was also significant (mean group difference, −3.8; 95% CI, −6.5 to −1.2; t155=2.85 [P=.005]), representing a depression effect size of 0.59 (95% CI, 0.18–1.00). Table 3 also shows that the depressive symptom effects seemed to generalize across men, women, Hispanic patients, and African American patients. In an analysis of the 3-, 5-, 7- and 9-month depressive symptoms, group differences emerged 4 months into the trial (at month 7; t155=2.88 [P=.004]) and remained significant at the end of the trial (t155=2.99 [P=.003]).
Table 3.
Reduction in Depressive Symptoms 3 and 9 Months After ACSa
| Intervention vs Usual Care Group |
||||||
|---|---|---|---|---|---|---|
| Variable | Usual Care Group (n=77) | Intervention Group (n=80) | Between-Group Difference | t Value | P Value | Nondepressed Cohort (n=80) |
| Depressive symptom score at 3 mo | 19.6 (18.2 to 21.1) | 19.0 (17.5 to 20.4) | −0.7 (−2.7 to 1.4) | 0.64 | .52 | 2.8 (2.3 to 3.3) |
| Depressive symptom score at 9 mo | 17.7 (15.6 to 19.7) | 13.2 (11.1 to 15.3) | −4.5 (−7.4 to −1.6) | 3.03 | .003 | 3.4 (2.4 to 4.3) |
| Change in depressive symptom score overall | −1.9 (−3.8 to −0.1) | −5.7 (−7.6 to −3.8) | −3.8 (−6.5 to −1.2) | 2.85 | .005 | 0.5 (−0.3 to 1.4) |
| Men only (n = 73) | −1.2 (−3.9 to −1.5) | −4.8 (−7.6 to −2.0) | −3.6 (−7.5 to 0.3) | 1.83 | .07 | … |
| Women only (n = 84) | −2.6 (−5.1 to 0.0) | −6.5 (−9.1 to −4.0) | −4.0 (−7.6 to −0.3) | 2.16 | .03 | … |
| Hispanic only (n = 68) | −1.6 (−4.4 to 1.3) | −5.1 (−7.9 to −2.2) | −3.5 (−7.6 to 0.5) | 1.71 | .09 | … |
| African American only (n = 29) | −1.5 (−5.3 to −2.4) | −7.9 (−12.7 to −3.1) | −6.4 (−12.6 to −0.2) | 2.05 | .04 | … |
Abbreviations: ACS, acute coronary syndrome; ellipses, not applicable.
Data are presented as mean (95% confidence interval) unless otherwise indicated. Depressive symptom scores were assessed using the Beck Depression Inventory.25 Multilevel linear mixed models were used to estimate a group × time repeated-measures analysis of variance for the Beck Depression Inventory. Significance tests for change in depressive symptom scores are tests of the group × time interaction effect.
Patient-reported adverse events were similar overall between the intervention and usual care groups, except that the usual care patients were significantly more likely to report experiencing a non–depression-related psychiatric problem than those in the intervention group (68 vs 59; [P=.02]).
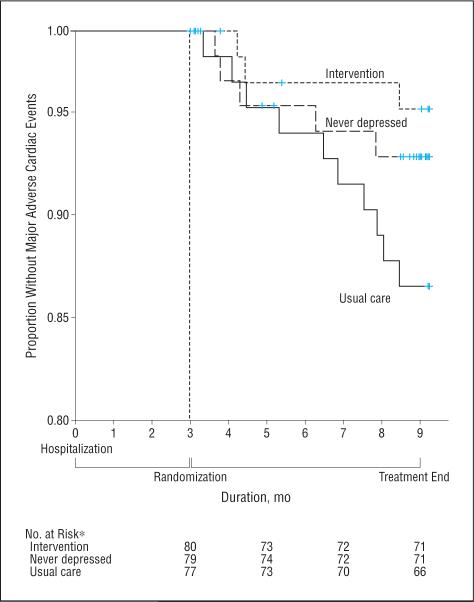
Patients in the intervention group had fewer MACE events (3 events [4%]) than did those in the usual care group (10 [13%]) or the nondepressed observational cohort (5 [6%]). Figure 2 shows the Kaplan-Meier curves for the 3 groups (log-rank test for the usual care vs intervention groups, [P=.047]; for the intervention vs nondepressed group, [P=.049]. No significant site differences were detected for any of the primary or secondary outcomes.
Figure 2.
Kaplan-Meier survival curves for major adverse cardiac events in the Coronary Psychosocial Evaluation Studies for randomized patients and the nondepressed cohort. *The number at risk at 9 months includes those who were evaluated during the ninth month.
COMMENT
In light of the damaging impact of depression on quality of life and prognosis in patients with CHD,1,30 rates of detection and effective treatment for depression remain unacceptably low in this patient population.20 It was in this spirit that the COPES trial was undertaken. The enhanced-care, patient-preference, stepped approach used herein was associated with substantial improvement in satisfaction with depression care and a significant reduction in depressive symptoms. In addition, although the study was not powered for this outcome, the intervention led to a promising difference in MACEs between randomized groups, with the MACE rate in the intervention group resembling that in the nondepressed cohort.
LIMITATIONS
First, patients selected for this trial did not include all patients with ACS. We excluded those with cognitive impairments, other life-threatening conditions, and, most important, other psychiatric conditions such as alcohol or other drug dependence and bipolar disorder. Because these comorbid conditions are highly prevalent in depressed patients, our findings might not be applicable to all patients with ACS and depressive symptoms. Second, we had a relatively small sample size, and the MACE rate was, expectedly, quite small. Thus, further trials of enhanced depression care are required to determine whether this type of treatment can improve post-ACS prognosis. Third, our patients were not blinded to their treatment status. We made every effort to blind the endpoint committee and the outcome assessors by asking patients not to reveal their group and by ensuring that assessors were not in contact with the therapist team, but this is only a single-blind trial. Fourth, we chose usual care as our control condition rather than placebo or another active control, such as clinical management. Thus, we did not account for nonspecific effects of treatment. Fifth, 13 of the 80 patients randomized to treatment never attended a first depression care visit. Another 7 terminated treatment before their care provider advised it, suggesting that, although the acceptance of our depression intervention was more than 50%, there is room for improvement. Recent studies using telephone-delivered cognitive behavioral therapy34 and combined psychotherapy with pharmacotherapy by telephone35,36 provided suggestions for novel delivery methods to further test in patients with ACS. Sixth, we did not collect cost data, which would have aided in the evaluation of this intervention. Finally, our 6-month treatment may have been too brief; we saw significant differences in depressive symptoms only after 4 months of treatment. The American College of Physicians37 recommended that clinicians continue treatment for 4 to 9 months after a satisfactory response in patients with a first episode of major depressive disorder. For patients who have had 2 or more episodes of depression, an even longer duration of therapy may be beneficial. The depression and cardiac outcomes reported herein might be strengthened by longer depression treatment.
COMPARISON OF COPES WITH OTHER DEPRESSION INTERVENTION TRIALS IN PATIENTS AFTER ACS
Although some previous trials have shown statistically significant reductions in depressive symptoms, there were no improvements in cardiovascular outcomes.20 One possible explanation is that the depression treatment effects resulting from the modalities tested were not large enough to alter the increased risk of cardiovascular events and mortality conferred by depression.38 In fact, previous trials had 1 common finding: only clinically modest depression differences between the treatment and control groups.20 One plausible reason for this finding is that the treatments were unacceptable to patients with CHD.17 A patient's willingness to engage in, adhere to, and continue depression treatment can determine whether the treatment succeeds or fails. Most of the depression interventions used in previous trials involving patients with CHD were originally validated with treatment-seeking out-patients with psychiatric problems; therefore, acceptance by the broader population of patients with CHD cannot be assumed.39 Previous studies have shown that fewer patients drop out of PST compared with other psychological therapies.40
Another possible explanation for the lack of improved cardiac prognosis with previously tested depression interventions is that the treatments were not sufficiently powerful.38 Recent systematic reviews of single-modality antidepressant41,42 or psychotherapy43 treatments in other patient populations showed only modest efficacy compared with placebo or usual care. Larger effect sizes have been found with multimodal44,45 or stepped-care22,46 depression treatment interventions. Until this trial, enhanced-care, stepped algorithms had not been tested in patients with CHD, but the results in other medical populations were promising.22,47–49 We thus chose to test this treatment modality in the COPES trial. We found a reasonable depression effect size (0.59) that compares favorably with those of previous interventions designed to reduce depression in patients with CHD (0.20–0.38).20
Large reductions in depressive symptoms in the control group are an issue in trials enrolling depressed patients with and without ACS.38,41 Depression is a relapsingremitting disease50; hence, substantial reductions in symptoms and/or spontaneous remission can occur. Also, medical providers increasingly recognize depressive symptoms in patients with ACS, and some patients' symptoms respond to the conventional depression treatment offered.21 For these reasons, we chose to include a 3-month observation period to identify patients with persistent depressive symptoms and thereby decrease the likelihood of a large reduction in depressive symptoms in the control group. We had a smaller reduction in depressive symptoms in the control group compared with other trials of depressed patients with CHD, possibly as a result of this strategy.
It is not known whether only a subset of patients who are depressed after ACS is at risk for ACS recurrence or mortality.7,51,52 We excluded more patients than we enrolled because of depressive symptom improvement, and this could be viewed as a limitation because we targeted a small sample without psychiatric diagnoses. Most observational cohort studies demonstrating depression-associated risk of ACS recurrence or mortality used a BDI score of 10 or higher to characterize depression4 rather than conventional psychiatric diagnoses.53 Participants with persistently elevated BDI scores (≥10) in these studies were found to be at risk of death.6 In the COPES trial, we similarly targeted patients with a BDI score of 10 or higher rather than just those meeting the diagnostic criteria for a psychiatric disorder. As expected with initial tests of whether reducing a risk factor offsets cardiac event rates, the impact of depression treatments on MACEs and other cardiac risks is disparate among the trials of depression treatment in patients with CHD.54 The results reported herein for the COPES trial offer promising approaches for a larger trial.
Treating depression effectively in patients with CHD may be daunting, but trials to determine the best way to manage these 2 highly prevalent and disabling diseases55 need to continue. In the secondary prevention of cardiovascular disease, stepped-care models of depression treatment with patient preference may offer an effective approach to improve depressive symptoms and satisfaction with care; whether this type of treatment can definitively improve cardiac prognosis awaits a larger trial.
Acknowledgments
Funding/Support: This study was supported by grants HC-25197, HL-76857, and HL-84034 from the National Heart, Lung, and Blood Institute and by grant UL1 RR024156 from the National Center for Research Resources, a component of the National Institutes of Health and National Institutes for Health Roadmap for Medical Research.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00158054
Financial Disclosure: None reported.
Role of the Sponsor: The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
REFERENCES
- 1.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27(23):2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 2.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802–813. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 3.Penninx BW, Beekman AT, Honig A, et al. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58(3):221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Frasure-Smith N, Lespérance F. Reflections on depression as a cardiac risk factor. Psychosom Med. 2005;67(suppl 1):S19–S25. doi: 10.1097/01.psy.0000162253.07959.db. [DOI] [PubMed] [Google Scholar]
- 5.Rosengren A, Hawken S, Ounpuu S, et al. INTERHEART Investigators Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 6.Lespérance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105(9):1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 7.Kaptein KI, de Jonge P, van den Brink RH, Korf J. Course of depressive symptoms after myocardial infarction and cardiac prognosis: a latent class analysis. Psychosom Med. 2006;68(5):662–668. doi: 10.1097/01.psy.0000233237.79085.57. [DOI] [PubMed] [Google Scholar]
- 8.Carney RM, Blumenthal JA, Freedland KE, et al. ENRICHD Investigators Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosom Med. 2004;66(4):466–474. doi: 10.1097/01.psy.0000133362.75075.a6. [DOI] [PubMed] [Google Scholar]
- 9.de Jonge P, Honig A, van Melle JP, et al. MIND-IT Investigators Nonresponse to treatment for depression following myocardial infarction: association with subsequent cardiac events. Am J Psychiatry. 2007;164(9):1371–1378. doi: 10.1176/appi.ajp.2007.06091492. [DOI] [PubMed] [Google Scholar]
- 10.Lane D, Carroll D, Ring C, Beevers DG, Lip GY. In-hospital symptoms of depression do not predict mortality 3 years after myocardial infarction. Int J Epidemiol. 2002;31(6):1179–1182. doi: 10.1093/ije/31.6.1179. [DOI] [PubMed] [Google Scholar]
- 11.Connerney I, Shapiro PA, McLaughlin JS, Bagiella E, Sloan RP. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet. 2001;358(9295):1766–1771. doi: 10.1016/S0140-6736(01)06803-9. [DOI] [PubMed] [Google Scholar]
- 12.van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66(6):814–822. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 13.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22(7):613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 14.Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation. 2005;111(3):250–253. doi: 10.1161/01.CIR.0000154573.62822.89. [DOI] [PubMed] [Google Scholar]
- 15.Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. American Heart Association Prevention Committee of the Council on Cardiovascular Nursing. American Heart Association Council on Clinical Cardiology. American Heart Association Council on Epidemiology and Prevention. American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research. American Psychiatric Association Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118(17):1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 16.Lonn E. Homocysteine-lowering B vitamin therapy in cardiovascular prevention: wrong again? JAMA. 2008;299(17):2086–2087. doi: 10.1001/jama.299.17.2086. [DOI] [PubMed] [Google Scholar]
- 17.Berkman LF, Blumenthal J, Burg M, et al. Enhancing Recovery in Coronary Heart Disease Patients Investigators (ENRICHD) Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 18.van Melle JP, de Jonge P, Honig A, et al. MIND-IT Investigators Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460–466. doi: 10.1192/bjp.bp.106.028647. [DOI] [PubMed] [Google Scholar]
- 19.Glassman AH, O'Connor CM, Califf RM, et al. Sertraline Antidepressant Heart Attack Randomized Trial (SADHEART) Group Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 20.Thombs BD, de Jonge P, Coyne JC, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300(18):2161–2171. doi: 10.1001/jama.2008.667. [DOI] [PubMed] [Google Scholar]
- 21.Taylor CB, Youngblood ME, Catellier D, et al. ENRICHD Investigators Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792–798. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- 22.Unützer J, Katon W, Callahan CM, et al. IMPACT Investigators (Improving Mood-Promoting Access to Collaborative Treatment) Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 23.Burg MM, Lespérance F, Rieckmann N, Clemow L, Skotzko C, Davidson KW. Treating persistent depressive symptoms in post-ACS patients: the project COPES phase-I randomized controlled trial. Contemp Clin Trials. 2008;29(2):231–240. doi: 10.1016/j.cct.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luepker RV, Apple FS, Christenson RH, et al. AHA Council on Epidemiology and Prevention. AHA Statistics Committee. World Heart Federation Council on Epidemiology and Prevention. European Society of Cardiology Working Group on Epidemiology and Prevention. Centers for Disease Control and Prevention. National Heart, Lung, and Blood Institute Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 26.Hegel MT, Imming J, Cyr-Provost M, Noel PH, Arean P, Unützer J. Role of behavioral health professionals in a collaborative stepped care treatment model for depression in primary care: project IMPACT. Fam Syst Health. 2002;20(3):265–277. [Google Scholar]
- 27.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure: the nine-item Patient Health Questionnaire depression scale is a dual-purpose instrument that can establish provisional depressive disorder diagnoses as well as grade depression severity. Psychiatr Ann. 2002;32(9):509–515. [Google Scholar]
- 28.Eagle KA, Lim MJ, Dabbous OH, et al. GRACE Investigators A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291(22):2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 29.Freedland KE, Skala JA, Carney RM, et al. The Depression Interview and Structured Hamilton (DISH): rationale, development, characteristics, and clinical validity. Psychosom Med. 2002;64(6):897–905. doi: 10.1097/01.psy.0000028826.64279.29. [DOI] [PubMed] [Google Scholar]
- 30.Frasure-Smith N, Lespérance F. Recent evidence linking coronary heart disease and depression. Can J Psychiatry. 2006;51(12):730–737. doi: 10.1177/070674370605101202. [DOI] [PubMed] [Google Scholar]
- 31.Davidson KW, Kupfer DJ, Bigger JT, et al. National Heart, Lung, and Blood Institute Working Group Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group report. Psychosom Med. 2006;68(5):645–650. doi: 10.1097/01.psy.0000233233.48738.22. [DOI] [PubMed] [Google Scholar]
- 32.Wothke W. Longitudinal and multigroup modeling with missing data. In: Little TD, Schnabel KU, Baumert J, editors. Modeling Longitudinal and Multilevel Data: Practical Issues, Applied Approaches, and Specific Examples. Lawrence Erlbaum Associates Inc; Mahwah, NJ: 2000. pp. 219–240. [Google Scholar]
- 33.Little RJA, Rubin DB. Statistical Analysis With Missing Data. John Wiley & Sons Inc; New York, NY: 1987. [Google Scholar]
- 34.Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62(9):1007–1014. doi: 10.1001/archpsyc.62.9.1007. [DOI] [PubMed] [Google Scholar]
- 35.Ludman EJ, Simon GE, Tutty S, Von Korff M. A randomized trial of telephone psychotherapy and pharmacotherapy for depression: continuation and durability of effects. J Consult Clin Psychol. 2007;75(2):257–266. doi: 10.1037/0022-006X.75.2.257. [DOI] [PubMed] [Google Scholar]
- 36.Simon GE, Ludman EJ, Tutty S, Operskalski B, Von Korff M. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment: a randomized controlled trial. JAMA. 2004;292(8):935–942. doi: 10.1001/jama.292.8.935. [DOI] [PubMed] [Google Scholar]
- 37.Qaseem A, Snow V, Denberg TD, Forciea MA, Owens DK, Clinical Efficacy Assessment Subcommittee of American College of Physicians Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149(10):725–733. doi: 10.7326/0003-4819-149-10-200811180-00007. [DOI] [PubMed] [Google Scholar]
- 38.Carney RM, Freedland KE. Does treating depression improve survival after acute coronary syndrome? Invited commentary on...effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:467–468. doi: 10.1192/bjp.bp.107.035360. [DOI] [PubMed] [Google Scholar]
- 39.ENRICHD Investigators Enhancing Recovery in Coronary Heart Disease (ENRICHD) study intervention: rationale and design. Psychosom Med. 2001;63(5):747–755. [PubMed] [Google Scholar]
- 40.Cuijpers P, van Straten A, Andersson G, van Oppen P. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J Consult Clin Psychol. 2008;76(6):909–922. doi: 10.1037/a0013075. [DOI] [PubMed] [Google Scholar]
- 41.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5(2):e45. doi: 10.1371/journal.pmed.0050045. doi:10.1371/journal .pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollon SD, DeRubeis RJ, Shelton RC, Weiss B. The emperor's new drugs: effect size and moderation effects. [Accessed January 23, 2010];Prev Treat. 2002 5(1):28. http://psycnet.apa.org /journals/pre/5/1/28c/ [Google Scholar]
- 43.Wilson KC, Mottram PG, Vassilas CA. Psychotherapeutic treatments for older depressed people. Cochrane Database Syst Rev. 2008;(1):CD004853. doi: 10.1002/14651858.CD004853.pub2. [DOI] [PubMed] [Google Scholar]
- 44.de Maat SM, Dekker J, Schoevers RA, de Jonghe F. Relative efficacy of psychotherapy and combined therapy in the treatment of depression: a meta-analysis. Eur Psychiatry. 2007;22(1):1–8. doi: 10.1016/j.eurpsy.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Pampallona S, Bollini P, Tibaldi G, Kupelnick B, Munizza C. Combined pharmacotherapy and psychological treatment for depression: a systematic review. Arch Gen Psychiatry. 2004;61(7):714–719. doi: 10.1001/archpsyc.61.7.714. [DOI] [PubMed] [Google Scholar]
- 46.Gensichen J, Beyer M, Muth C, Gerlach FM, Von Korff M, Ormel J. Case management to improve major depression in primary health care: a systematic review. Psychol Med. 2006;36(1):7–14. doi: 10.1017/S0033291705005568. [DOI] [PubMed] [Google Scholar]
- 47.Hunkeler EM, Katon W, Tang L, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ. 2006;332(7536):259–263. doi: 10.1136/bmj.38683.710255.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61(10):1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 49.Lin EH, Katon W, Von Korff M, et al. IMPACT Investigators Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA. 2003;290(18):2428–2429. doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- 50.Rush AJ, Kraemer HC, Sackeim HA, et al. ACNP Task Force Report by the ACNP Task Force on Response and Remission in Major Depressive Disorder. Neuropsychopharmacology. 2006;31(9):1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 51.de Jonge P, Ormel J. Heterogeneity of patients with coronary artery disease and distress and the need to identify relevant subtypes [letter] Arch Gen Psychiatry. 2008;65(7):851–852. doi: 10.1001/archpsyc.65.7.851. [DOI] [PubMed] [Google Scholar]
- 52.Frasure-Smith N, Lespérance F. Heterogeneity of patients with coronary artery disease and distress and the need to identify relevant subtypes [author reply] Arch Gen Psychiatry. 2008;65(7):852–853. doi: 10.1001/archpsyc.65.7.851. [DOI] [PubMed] [Google Scholar]
- 53.Davidson KW, Rieckmann N, Rapp M. Definitions and distinctions among depressive syndromes and symptoms: implications for a better understanding of the depression–cardiovascular disease association. Psychosom Med. 2005;67(suppl 1):S6–S9. doi: 10.1097/01.psy.0000162257.19266.fc. [DOI] [PubMed] [Google Scholar]
- 54.Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121(11)(suppl 2):S20–S27. doi: 10.1016/j.amjmed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]