Abstract
Little is known about possible interactions between chloroplasts and the Golgi apparatus, although there is increasing evidence for a direct Golgi to chloroplast transport pathway targeting proteins to their destinations within the membranes and stroma of plastids. Here data are presented showing that a blockage of secretion results in a significant increase of starch within plastids. Golgi disassembly promoted either by the secretory inhibitor brefeldin A or through an inducible Sar1-GTP system leads to dramatic starch accumulation in plastids, thus providing evidence for a direct interaction between plastids and Golgi activity. The possibility that starch accumulation is due either to elevated levels of cytosolic sugars because of loss of secretory Golgi activity or even to a blockage of amylase transport from the Golgi to the chloroplast is discussed.
Keywords: Brefeldin A, COP II, Golgi apparatus, plastids, starch
Introduction
An amyloplast is a mature plastid where most of the internal volume is filled with starch. Plastids of this type are typically found in differentiated root cells, particularly in the root cap (Barlow et al., 1984). Amyloplasts are also found in storage tissues, such as cotyledons, endosperm, and tubers. The number, size, and morphology of the starch grains that are present in an amyloplast vary depending on the tissue. Typically the amyloplasts of potato tubers display one very large starch grain, whereas the amyloplasts of root cap cells may contain up to eight tightly packed starch granules. Chloroplasts can also contain starch at varying levels and therefore fulfil a double role as photosynthetically active and starch-storing plastids. Thus, the function of starch-containing plastids is to synthesize and store starch when carbohydrates are available in excess. The stored starch can then be broken down to free sugars or sugar derivatives when the plant has a need for carbohydrates.
Starch and sucrose synthesis are dependent on the rate of CO2 fixation (Smith and Stitt, 2007). One of the key regulators of this mechanism is a triose-phosphate translocator located in the inner chloroplast membrane which imports inorganic phosphate (Pi) from the cytosol in exchange for triose-phosphates. Pi inhibits the plastid enzyme ADP-glucose-pyrophosphorylase, demonstrating that Pi has a direct influence on starch synthesis within the plastid stroma (Lunn, 2007).
Starch breakdown is initially catalysed by two enzymes: the first is a β-amylase and the second a debranching enzyme (e.g.isoamylase or dextrinase) that catalyses subsequent hydrolysis to maltose and maltotriose (Delatte et al., 2006; Zeeman et al., 2007). Starch granules in leaves are thin and disc shaped, allowing for an optimal surface to volume ratio for starch-digesting enzymes. Starch degradation is controlled by a glucan water dikinase (Zeeman et al., 2007) and by a cytosolic transglucosidase (DPE2). In the absence of DPE2 maltose accumulates and starch degradation is blocked (Chia et al., 2004; Lu et al., 2006). Free glucose then is activated to hexose phosphate via hexokinase. These reactions present several possible mechanisms for fine-tuning the rate of starch degradation. Starch accumulation and starch degradation are strongly associated with diurnal metabolism as, after a transition from light to dark, starch breakdown is initiated by a cascade of circadian-regulated enzymes (Zeeman et al., 2007).
Considerably less is known about the relationship between other essential cellular processes such as mitosis, growth, secretion, and starch accumulation. Carbon availability is of major importance for a number of cellular processes such as cell division, cell wall polysaccharide production, and various glycosylation events. For example, carbon starvation can influence the level of transcripts encoding cell wall-producing proteins (Smith and Stitt, 2007). However, a far more important factor is the repression of cell cycle- and cell growth-related proteins and the lack of glycosylation processes (Osuna et al., 2007). A global inhibition of protein synthesis and secretion conserves carbon supply and protects the cells from starvation for a limited time (Smith and Stitt, 2007). Starvation processes are reversible within 30–120 min after restoring a carbon supply. An increase in cytoplasmic sugars could therefore result in a build up of starch in the plastids.
The experiments reported here are based on a stepwise disassembly of the Golgi apparatus using the well-established secretion inhibitor brefeldin A (BFA) and the inhibition of endoplasmic reticulum (ER) export through expression of a mutant version of the GTPase Sar1p responsible for COPII recruitment at ER exit sites (daSilva et al., 2004; Osterrieder et al., 2010). The macrocyclic lactone BFA is an important tool in studying membrane dynamics in the eukaryotic secretory pathway and has been shown to block secretion at the level of the Golgi apparatus (Nebenführ et al., 2002; Langhans et al., 2007; Robinson et al., 2008). Here it is demonstrated that there is a marked increase in starch accumulation in plastids (both with and without chlorophyll) from a number of tissues and cell types when the secretory pathway is blocked by the two different methods. Tissue cultures which are dependent on a sugar source in their media also show the same effect as autotrophic plants.
Material and methods
Alga and culture conditions
Chlamydomonas noctigama (Strain SAG, University of Göttingen) was cultured in TAP medium (Amrhein and Filner, 1973) in 250 ml Erlenmeyer flasks at 25 °C on a rotary shaker (190 rpm). The cultures were subjected to a light (1 kLux)–dark cycle of 16 h:8 h. For inhibitor experiments, log phase cultures (4 d after subculturing) were used. Cell density usually lay between 1.8 and 2×106 cells ml−1 (Hummel et al., 2007).
BY-2 culture conditions and synchronization
Wild-type tobacco BY-2 (Nicotiana tabacum var. Bright Yellow 2) cells were cultivated by shaking (120 rpm) in the dark in Murashige and Skoog's medium (Murashige and Skoog, 1962) at 27 °C. The suspension-cultured cells were maintained in the log-phase, subculturing weekly into fresh medium at a dilution of 1:50. The procedure of Langhans et al. (2007) was used to synchronize cultures. Both synchronized and unsynchronized cells were used to show that starch accumulation in plastids is not due to side effects of synchronization.
Arabidopsis plants
Arabidopsis seeds were surface sterilized. Plants were also grown for some assays on 13 MS agar plates (modified basal medium with Gamborg vitamins; KS) at pH 5.7, normally with 0.5% sucrose, and under constant light (30 μE m2 s1).
BFA treatment
BY-2 cells and C. noctigama cells were treated with 10 μg ml−1 BFA [Sigma Aldrich, stock 10 mg ml−1 in dimethylsulfoxide (DMSO)]. For Arabidopsis a concentration of 50 μg ml−1 was used.
Lugol staining
One drop of Lugol stain [5 g of iodine and 10 g of potassium iodide in 100 ml of distilled water (Bronner, 1975; Hinchman, 1973)] was added to a slide loaded with BY-2 cells. Samples were incubated with the stain for 1 min prior to observation with a Zeiss Axioplan microscope (Carl Zeiss, Welwyn Garden City, UK).
Transient expression in transgenic tobacco plants
Agrobacterium-mediated expression of sialyltransferase (ST)–green fluorescent protein (GFP) in abaxial tobacco leaf epidermal cells was performed as described by Sparkes et al. (2006).
Fluorescent glucose experiments
Fluorescent deoxyglucose {2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose (2-NBDG); Molecular Probes, Eugene, OR, USA} at 5.6 mM in the presence or absence of 10 μg ml−1 BFA was added to 1 ml of BY-2 cells and incubated over a period of 7 h. Samples were taken and observed every hour by confocal microscopy (Etxeberria et al., 2005).
Induction of Sar1-GTP in transgenic tobacco plants
The abaxial leaf sides of stable Sar1-GTP-inducible tobacco plants were painted with a freshly made up solution of 20 μg ml−1 dexamethasone and 0.02% Silwet® Copolymer L77 (Osterrieder et al., 2010).
Confocal imaging
Live cell imaging of GFP expression in tobacco leaf epidermal cells was conducted with a Zeiss LSM 510 confocal laser scanning microscope using a 40×/1.3NA or 63×1.4NA oil immersion lens. GFP was excited using a 488 nm argon laser and emission was detected from 505 nm to 530 nm.
Electron microscopy
Leaf pieces and roots were chemically fixed in 1% glutaraldehyde and 1% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 6.9, washed three times in buffer, and post-fixed in 2% aqueous osmium tetroxide for 90 min (Langhans et al., 2007). Samples were washed four times in water and subsequently block stained overnight in 1% aqueous uranyl acetate (Hayat, 1975). Samples were dehydrated in acetone and embedded in Spurr resin (Spurr, 1969). Post-staining was carried out in 1–3% uranyl acetate in methanol followed by lead citrate for 5–10 min (Robinson et al., 1985).
A fixation protocol similar to that previously described by Zhang and Robinson (1986) was used to fix C. noctigama cells, with the following modifications: the concentrations of the fixatives in the primary fixation solution were 1% glutaraldehyde and 1% osmium tetroxide, and the buffer used throughout was 0.1 M cacodylate pH 7.0 (Hummel et al., 2007). Fixed cells were dehydrated and embedded as in Zhang and Robinson (1986).
Starch assay
The starch assay followed the protocol of Smith and Zeeman (2006) with the following adaptations. A 0.5 g aliquot of BY-2 cells (3–5 d old) was harvested every hour and samples were pelleted by centrifugation at 600 g for 2 min. Pellets were frozen in liquid nitrogen and stored at –80 °C. Then 5 ml of 80% ethanol was added to the pellets and boiled for 3 min prior to centrifugation for 10 min at 10 000 g. This process was repeated three times. Ethanol was allowed to evaporate after the final wash and 2 ml of water was added to the pellet. Samples were sonicated to obtain a non-particulate homogeneous solution. Tubes were filled to 5 ml with water and aliquoted into 4× 0.5 ml samples. Samples were boiled for 10 min to gelatinize starch, and 0.5 ml of sodium acetate (pH 5.5) was added to each tube. Amyloglucosidase (10 U; Sigma, UK) and α-amylase (5 U; Sigma) were added to two of the tubes and an equal amount of enzyme-free water was added to the other two control samples. All samples (four from each time point) were incubated for 4 h at 37 °C and frozen overnight at –20 °C. Samples were centrifuged for 10 min at 15 000 g and analysed with an NADH assay containing hexokinase and glucose-6-phosphate dehydrokinase (Sigma). The starch content of the samples was measured at OD 340 nm and calculated with the extinction coefficient for NADH+H+: change in OD/6.22. The mean value of A for control samples Ac was subtracted from the mean value of the samples treated with enzymes. The quantity of starch was obtained in μmol and converted to starch per g (Smith and Zeeman, 2006)
Results
Starch accumulation in BY-2 cells after BFA treatment
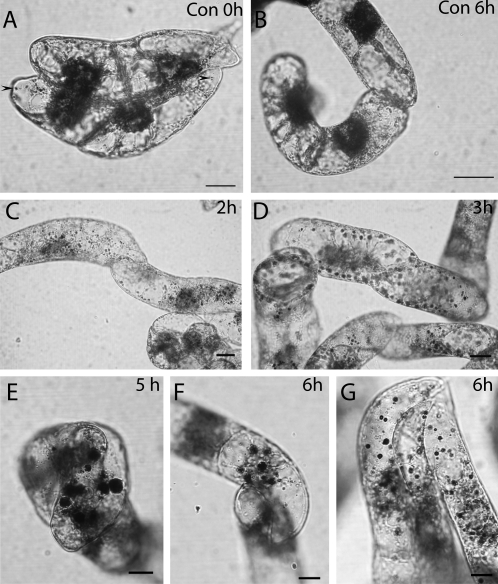
Interphase cells, both synchronized and non-synchronized, 3 d or 5 d after subculturing were treated with BFA. In BY-2 cells relatively low concentrations of BFA (10 μg ml−1) result in complete Golgi disassembly within 2 h. Prolonged incubation with BFA (over a period of up to 8 h) is possible without causing cell death. Control cells without BFA treatment contain few small starch granules when observed after iodine staining (Fig. 1A, arrows). The starch content does not increase in untreated cells after 6 h of incubation during the same experiment (Fig. 1B). After 1 h of BFA treatment, no significant increase in starch content was observed (data not shown), but after 2 h there is a clear change in size and number of granules (Fig. 1C). At this time point most Golgi stacks had disappeared and the Golgi-associated enzymes were relocated into the ER (data not shown; see Saint-Jore et al., 2002; Langhans et al., 2007). In non-synchronized cultures, 3 h of BFA treatment were required to remove the Golgi apparatus completely. Further treatment led to continued growth of starch granules which were significantly larger after 3 h of incubation in BFA (Fig. 1D). Small isodiametric cells tended to show larger starch granules (Fig. 1E, F) than larger elongated cells (Fig. 1G).
Fig. 1.
Lugol staining shows starch accumulation in BY-2 cells after BFA treatment. Scale bars=10 μm. (A and B) Control cells. Nuclei are stained brown by iodine. Only a few starch granules are visible (A, arrows). Control cells after 6 h show no starch signal at all (B). (C) Two hours after adding BFA the number of iodine-positive starch granules increases. (D) Three hours after adding the inhibitor the black starch granules are easy to observe. (E) Four hours after adding BFA in younger cells large starch granules are visible. (F and G) The size of starch granules in BY-2 cells after adding BFA increases continuously up to 6 h of treatment.
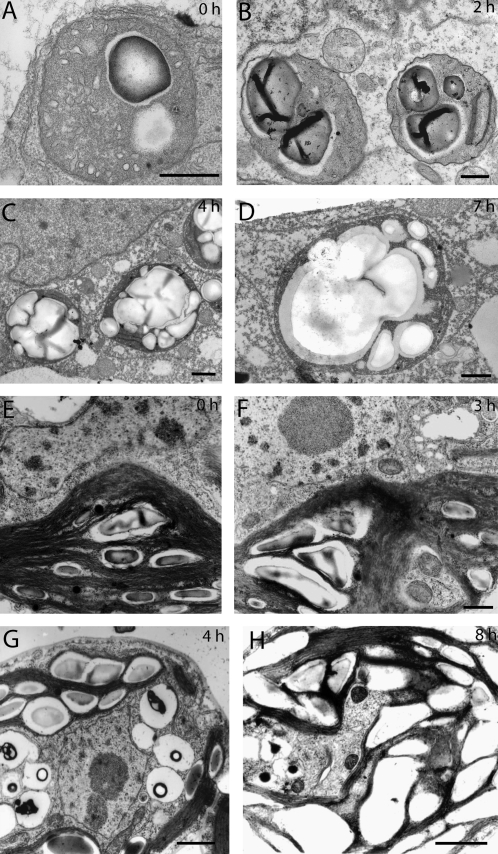
Transmission electron microscopy confirmed the results obtained through Lugol staining. Plastids in BY-2 cells displayed typical proplastid features, with only one or two thylakoids present. In proplastids without thylakoids, undefined membrane structures within the stroma were occasionally observed (Fig. 2A). Prior to BFA treatment, starch granules were frequently present, with a maximum of three grains filling not more than half of the plastid. In agreement with the results obtained at the light microscope level, a significant increase in the amount of starch within a plastid was observed after 2 h BFA treatment (Fig. 2B). After 4 h most of the plastid stroma was filled with starch grains (Fig. 2C). Prolonged treatment resulted in a further increase in the quantity and size of starch grains, and after 7 h of BFA treatment grains of up to 3 μm in diameter dominate (Fig. 2D). Untreated cells incubated for 6 h under the same conditions showed no significant difference in the amount of starch in the plastids (data not shown).
Fig. 2.
Electron micrographs showing starch accumulation in BY-2 cells (A–F, scale bars=500 nm) and Chlamydomonas noctigama plastids (E–G, scale bars=1000 nm) treated with BFA (10 μg ml−1). (A) Untreated BY-2 cells show typical patterns of proplastids. Undefined membranes are present in the stroma. Plastids can accumulate starch in the matrix typical of amyloplasts. (B) After 2 h BFA treatment nearly all plastids contain starch granules. (C) At 4 h after BFA treatment starch grains occupy most of the plastid volume. (D) After 9 h, fusion of the starch grains is more or less complete and the plastid matrix is completely filled with starch. (E) A typical Chlamydomonas chloroplast with a number of small starch granules located in the interthylakoid spaces. (F) At 3 h after adding BFA, starch seems to accumulate. Starch granules are considerably larger compared with the control. (G) A 4 h BFA treatment leads to a significant increase of starch within the plastid. (H) After 8 h the interthylakoidal spaces are filled with a number of large starch grains.
Accumulation of starch in C. noctigama has similar advantages to BY-2 cells regarding suitability for BFA treatment. Complete disassembly of the Golgi apparatus was observed in Chlamydomonas within 4 h in response to BFA (Hummel et al., 2007). Plastids in this single-cell green alga are morphologically different from those in BY-2 cells and photosynthetically active tissues in higher plants, in the sense that Chlamydomonas has only one cup-shaped plastid per cell occupying more than half of the intracellular space (Proschold et al., 2001). Starch deposits are usually found in interthylakoid spaces and, depending on the day–night cycle, Chlamydomonas plastids can either contain up to eight small starch grains within their stroma (Fig. 2E) or show no starch deposits, as is the case at the end of the night cycle. In the present experiments, BFA was always added during the light cycle.
As described for BY-2 cells, an increase in plastid starch also correlated with the disappearance of Golgi membranes in Chlamydomonas. An increase in starch was first observed after 3 h of BFA treatment (Fig. 2F) and most of the Golgi bodies disappeared after 4 h of treatment (Hummel et al., 2007), when large starch grains dominated the plastid morphology (Fig. 2G). Further treatment led to a continuous increase in the number of starch granules. Due to the fact that thylakoids appeared to limit the growth and fusion of starch granules, Chlamydomonas showed separate starch granules at all time points. However, when starch grains occurred within the same interthylakoidal space, they tended to fuse (Fig. 2H). Control cells incubated for 6 h in the absence of BFA showed a slight increase in starch, but not to the extent seen in BFA-treated samples (data not shown).
Starch accumulation in A. thaliana
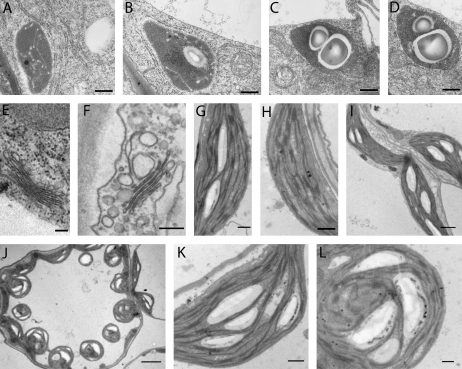
In Arabidopsis roots, plastids show the same proplastid features as described for BY-2 cells. Some plastids were starch free (Fig. 3A), whilst others contained small starch grains (Fig. 3B) which differ in size depending on their proximity to the root cap. After 2 h of BFA treatment (50 μg ml−1) most of the observed plastids contained starch, which filled up more than half of the plastid volume (Fig. 3C, D). The starch content increased continuously during further treatment with BFA, even though BFA did not completely disassemble the Golgi (Robinson et al., 2008). Similar results were obtained in roots of maize treated with BFA (data not shown).
Fig. 3.
Starch accumulation after brefeldin A treatment in Arabidopsis thaliana roots (A–D, scale bars=500 nm) and cotyledonary leaves (E–K, scale bars E–F=100 nm; G–L=500 nm). (A and B) Root meristem plastids may be starch free or contain only small starch grains. (C and D) At 2 h after treatment nearly all plastids are showing large starch granules. (E) Golgi in untreated cotyledons with an approximate size of 600–800 nm. (F) Golgi bodies are still present after 2 h BFA treatment but are much smaller (∼300 nm in diameter) than control Golgi bodies. (G and H) Untreated cotyledonary leaves showing similar patterns to roots. Plastids with and without starch grains are present. (I) After 3 h of BFA (50 μg ml−1) treatment most of the plastids show starch granules. (J) After 4 h in BFA nearly all plastids within a cell show large starch granules. (K) A leaf treated for 5 h with starch granules within the interthylakoidal spaces. (L) Chloroplasts 6 h after adding BFA.
Cotyledonary leaves of Arabidopsis showed a different response in terms of BFA sensitivity, as Golgi bodies slowly disassembled (Fig. 3E, F) as described for Chlamydomonas and BY-2 cells (Hummel et al., 2007; Langhans et al., 2007; Robinson et al., 2008). A 3 h after the initial treatment, cisternal length decreased from between 800 nm and 600 nm to ∼300 nm, with considerable vesiculation (Fig. 3F) before the Golgi stacks eventually disappeared. From this time on the starch content in chloroplasts increased significantly (Fig. 3L) as compared with untreated cells (Fig. 3G, H) which had no or only small starch grains. After 4 h treatment with the inhibitor nearly all plastids exhibited large starch grains (Fig. 3J). Starch accumulated during further BFA treatment up to 6 h, although within a single sample the number and size of starch granules differed (Fig. 3K, L).
Golgi disassembly and starch accumulation in Sar1-GTP plants
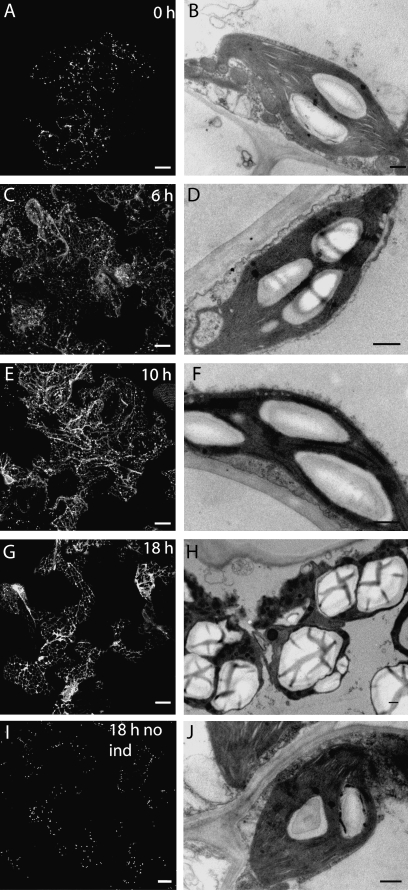
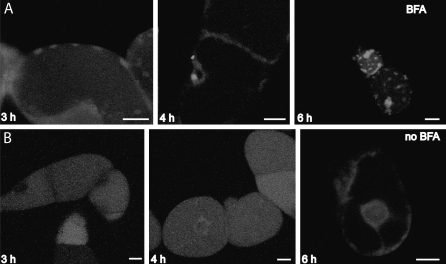
As an alternative strategy to disrupt the Golgi apparatus, a GTP-locked dominant-negative mutant version of the small GTPase Sar1p (Sar1-GTP) was utilized. Expression of Sar1-GTP results in blockage of COPII-mediated ER to Golgi protein transport and redistribution of Golgi markers into the ER (Andreeva et al., 2000; Takeuchi et al., 2000; daSilva et al., 2004). Transgenic tobacco plants containing Sar1-GTP under the control of a dexamethasone-inducible promoter (Craft et al., 2005; Samalova et al., 2005) were created and characterized (Osterrieder et al., 2010). When analysing the effects of Sar1-GTP expression on the Golgi stacks at the ultrastructural level, a significant increase in the starch content of plastids during the Golgi disassembly process was also discovered, similar to the experiments with BFA described above. To monitor the disappearance of the Golgi apparatus during Sar1-GTP induction, the well-established Golgi marker ST–GFP was used (Boevink et al., 1998), and the sequential disappearance of Golgi fluorescence coupled with an appearance of ER fluorescence was monitored over 18 h (Fig. 4A, C, E, G). Before Sar1-GTP induction, many plastids already contained small starch grains (Fig. 4B). Basal starch levels depend on the level of light that the leaves were exposed to, but usually starch granules did not fill more than a third of the plastid as seen in thin sections. At 6 h after Sar1-GTP induction, the ST–GFP signal became weaker (Fig. 4C), and at the ultrastructural level a decrease in Golgi cisternal diameter was observed (data not shown, but see Osterrieder et al., 2010). At this time point, a build up of starch in plastids was already seen (Fig. 4D). After 10 h, the ST–GFP signal was observed not only in Golgi bodies but also in the ER, reflecting a redistribution of the Golgi marker after blockage of ER to Golgi transport (Fig. 4E). The amount of starch within the plastids increased significantly at this time point (Fig. 4F). At 18 h after induction when ST–GFP was detected only in ER membranes (Fig. 4G), plastids contained few but very large starch granules which almost filled the whole plastid (Fig. 4 H). A significant increase in starch was not observed in the NII control plants (which only contain the first vector of the two-vector inducible system but not the inducible Sar1-GTP vector), even after 18 h treatment with dexamethasone (Fig. 4J).
Fig. 4.
(A, C, E, and G) Micrographs showing a time course of Sar1-GTP induction in stable inducible tobacco leaf epidermal cells expressing ST–GFP (A, C, E, G, scale bars=20 μm). (A) Control cells show a typical fluorescent punctate Golgi body signal. (C) At 6 h after induction some ER fluorescence can be seen. (E) At 10 h after induction the GFP signal in ER membranes becomes clear and with a faint Golgi signal. (G) At 18 h after induction almost all the Golgi signal is relocated to the ER. Electron micrographs show cells fixated at the same time points (B, D, F, H, scale bars=500 nm). (B) Mesophyll cell before induction. Many plastids contain starch granules. (D) After 6 h a slight increase in the amount of starch in induced plants can be observed. (F) At 10 h after induction there is a further increase in starch content and size of grains. (H) After 18 h large starch granules almost fill the plastid volume and cells begin to die. Golgi labelling and starch content were unaffected in NII control plants without the inducible Sar1-GTP vector (I, J, scale bars=20 μm). (I) The ST–GFP signal is unaffected after 18 h dexamethasone treatment under the same conditions. (J) No significant increase of starch can be observed.
Starch assays in BY-2 cells
By histology and electron microscopy it was demonstrated that starch accumulates in plastids upon Golgi disassembly. To confirm this phenomenon biochemically, an assay described by Smith and Zeeman (2006) was used, which was adapted to the BY-2 cell system. For these experiments, 3- and 5-day-old BY-2 cell cultures were used. In both cases starch content was measured for up to 7 h after addition of BFA (10 μg ml−1). Starch content in μg g−1 BY-2 cells was calculated by measuring change in absorption by NADH.
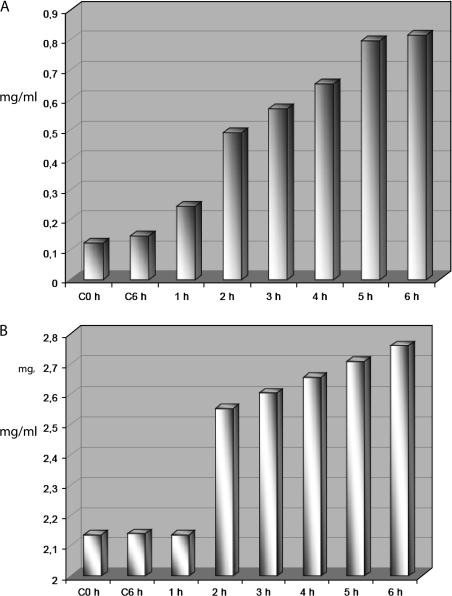
A continuous increase in starch was measured over the 7 h time period (Fig. 5). Three-day-old cultures (Fig. 5A) exhibited a higher initial starch content compared with 5-day-old cultures (Fig. 5B), but both showed dramatic increases after 2 h incubation in BFA, with levels stabilizing after 6–7 h. This corroborates the data from histochemical staining and electron microscopy. The biochemical assay carried out in BY-2 cells did not show any significant increase in starch during 6 h in non-BFA-treated cells (Fig. 5).
Fig. 5.
Starch assays of BY-2 cells before and after BFA. Starch content measured in mg per g of cells. (A) Three-day-old synchronized cell culture in interphase. At time zero the starch level in plastids is low. During BFA treatment the starch content in cells increases and reaches the maximum 6 h after adding the inhibitor. (B) Starch assay in a 5-day-old unsynchronized culture. Again starch levels increase dramatically over the 6 h incubation period. C0h=control no treatment; C6h=control cells after 6 h.
Uptake of fluorescent glucose in plastids during BFA treatment
Figure 6 shows the uptake of fluorescent glucose with and without BFA treatment in BY-2 cells. During BFA treatment the fluorescent glucose analogue (2-NDBG) accumulated in spherical bodies. Plastid-like fluorescent features were observed after 3 h (Fig. 6A). Prolonged treatment led to a continued increase in fluorescence in these structures (Fig. 6B). After 6 h the number of 2-NDBG-positive cells increased and a large number of positive stained putative plastids were observed (Fig. 6C). It would appear that fluorescent glucose is integrated into the starch molecules during treatment with BFA. Controls taken at the same time point and under the same culture conditions clearly show the presence of fluorescent glucose in the cytoplasm (Fig. 6D, E). Even after 6 h of treatment no significant increase in fluorescent bodies was observed (Fig. 6F). These results strongly suggest that blockage of Golgi secretion stimulates starch synthesis, since the integration of fluorescent glucose in plastid-like structures was not seen in control cells.
Fig. 6.
Uptake of fluorescent glucose (2-NDBG) in BFA-treated and non-BFA-treated BY-2 cells. (A) Fluorescent glucose in plastids 3 h after adding BFA. The 2-NDBG signal becomes more intensive after 4 h. The intensity of fluorescent glucose and the number of 2-NDBG-positive structures becomes more prominent up to 6 h BFA treatment. (B) Untreated control cells show uptake of fluorescent glucose but no compartmentalization into punctae.
Discussion
The data presented here show that an increase in the starch level in plastids including chloroplasts, in a range of tissues and species, appears to correlate with an inhibition of secretion. Significantly, this cannot be attributed directly to a secondary effect of BFA treatment as the same result was obtained when ER to Golgi transport was inhibited by expression of a GTP-locked form of the Sar1p GTPase. This treatment has also been shown to result in a complete disassembly of the Golgi apparatus (Andreeva et al., 2000; Takeuchi et al., 2000; Osterrieder et al., 2010).
The prime targets of BFA are guanine nucleotide exchange factors (GEFs), involved in the activation of ADP ribosylation factors (ARFs) which are small GTPases primarily involved in the initiation of the coatomer complex around Golgi-derived COPI vesicles (Robinson et al., 2008). Disruption of the vesicle coat results in resorption of Golgi membrane into the ER as observed through live cell imaging of GFP-tagged Golgi proteins (Saint-Jore et al., 2002). In Arabidopsis the first ARF-GEF to be described, GNOM, localizes to components of the endocytic pathway and is BFA sensitive, and loss of function results in aggregation of the trans-Golgi network and late endosomal membranes (Geldner et al., 2003; Grebe et al., 2003). Interestingly, it has been suggested that Arabidopsis Golgi stacks themselves are not affected by BFA as the Golgi-located ARF-GEF GNL1 (GNOM-like1) is resistant to the drug (Richter et al., 2007). However, Robinson et al. (2008) have suggested that in Arabidopsis roots BFA does in fact have a morphological effect inducing Golgi stack aggregation and curvature, whilst in leaves it induces complete Golgi disassembly. These authors postulate that BFA is tissue and not plant specific (Robinson et al., 2008). The results presented here, based on an increase in plastid starch in response to BFA, suggest that in Arabidopsis roots secretion is compromised by the drug, even though the Golgi apparatus may not be completely destroyed by BFA treatment.
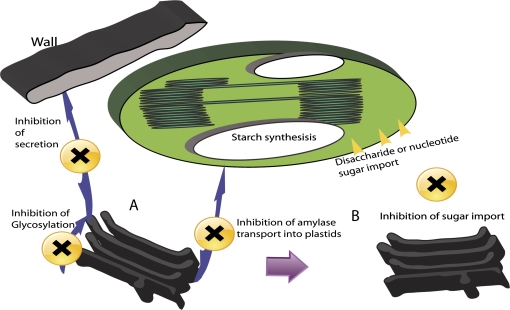
If compromising Golgi activity results in loss of secretory activity, then it is not unreasonable to presume that the data presented here provide strong evidence for the hypothesis that Golgi activity has a direct effect on the carbohydrate status of the cytoplasm and that, under particular circumstances, free carbohydrate may be redirected to the plastids for conversion into starch (Fig. 7). As the Golgi apparatus plays a mayor role in the biosynthesis of cell wall material (Reyes and Orellana, 2008), and in protein glycosylation (Steinkellner and Strasser, 2003), a steady supply of nucleotide sugars for import across cisternal membranes is required and disruption and/or loss of the cisternal stacks could result in a build up of cytoplasmic carbohydrate. A new study on the red microalga Porphyridium sp. which does not store starch in its plastids has shown that BFA leads to a change in cell wall composition and also to a build up of cytoplasmic starch (Keidan, 2009). This supports the model of free cytosolic sugars being converted into starch and, along with the significantly higher accumulation of starch in dividing BY-2 cells in the present experiments, supports the direct linkage between cell wall production and cytoplasmic sugar contents. The results using fluorescent glucose loading of BY-2 cells indicate that blockage of Golgi function leads to an accumulation of fluorescence in punctate structures in the cytoplasm which could equate to plastid starch granules (Fig. 7). These results support the contention that carbohydrate partitioning into plastids and starch synthesis is stimulated after secretion is inhibited.
Fig. 7.
Possible relationship between plastids and Golgi secretion. (A) Loss of Golgi function and cisternal membranes will result in a build up of cytoplasmic sugars which are imported into plastids for storage as starch. (B) Inhibition of secretion may result in a loss of amylases within the plastid which directly inhibits starch degradation.
Smith and Stitt (2007) considered the possible relationship between carbon balance and growth processes as an additional factor involved in starch metabolism. It has been shown how carbon supply, and therefore the balance between starch degradation and synthesis, influences cellular processes involved in division and growth (Li et al., 2003; Osuna et al., 2007; Reyes and Orellana, 2008). However, to date there is no direct evidence of a direct relationship between plastids and Golgi stacks in terms of sugar and carbohydrate supply. Starch synthesis and degradation is strongly related to the diurnal metabolism of photosynthesis. Mutant studies have provided significant insight into the proteins and signal molecules that are involved in controlling starch metabolism (Zeeman et al., 2004, 2007; Smith and Zeeman, 2006; Smith and Stitt, 2007). The key function of ADP-glucose-pyrophosphorylase is to convert glucose-1-phosphate and ATP into ADP-glucose. Mutations in enzymes involved in this pathway have a major influence on starch synthesis and degradation (Caspar et al., 1991; Lytovchenko et al., 2002). Both genetic and biochemical evidence suggest a strong relationship between these pathways and the plastid lumen. However, despite the strong genetic evidence supporting plastid-localized ADP-glucose production, this is not the only pathway of starch synthesis. Cytosolic enzymes appear to be involved in starch regulation (Baroja-Fernandez et al., 2004; Munoz et al., 2005), although models involving cytosolic enzymes are highly controversial and further evidence is required for them to be included in the current model of starch metabolism.
Another possible explanation for an increase in starch during inhibition of secretion is a block in starch degradation or turnover (Fig. 7). A major enzyme needed during starch degradation, α-amylase, has been suggested to be transported in the secretory pathway via Golgi membranes to its destinations in plastids and the extracellular space (Asatsuma et al., 2005; Kitajima et al., 2009). Translocation across the outer membrane of chloroplasts could be achieved by fusion of post-Golgi vesicles with the outer chloroplast membrane. Via this pathway α-amylase is released into the space between the inner and outer membrane (Asatsuma et al., 2005). Evidence is now growing in support of a direct transport route for ER-synthesized proteins from the Golgi to plastids and therefore Golgi disassembly should influence this arm of the secretory pathway (Villarejo et al., 2005; Nanjo et al., 2006; Radhamony and Theg, 2006). It has been suggested that α-amylase is unnecessary for transitory starch breakdown in Arabidopsis leaves (Yu et al., 2005). However, the main candidate for starch degradation in Arabidopsis appears to be a β-amylase, with four of the nine members of the family being located to chloroplasts.
Recently, it has been shown that in rice an α-amylase (Amyl-1) is transported to plastids via the Golgi apparatus and is involved in degradation of starch granules (Fulton et al., 2008; Kitajima et al., 2009). In this study, AmyI-1–GFP labelled the ER network upon co-expression with the dominant-negative Sar1-GTP mutant. The present results open up the possibility that starch turnover is also blocked in such a manner, but obviously further studies need to be carried out to investigate the secretion of the different amylase homologues to plastids in the different cell types (Ziegler and Beck, 1986; Ziegler, 1988; Schupp and Ziegler, 2004).
In conclusion, the present results suggest a strong influence of Golgi-based secretion on the regulation of the carbohydrate supply within a plant cell (Fig. 7). It is a well known fact that photosynthetic active tissues such as leaves and Chlamydomonas are difficult systems with which to investigate the relationship between starch accumulation and Golgi secretion due to the light-induced accumulation of starch during the light period. For this reason, results could differ due to the fact that starch uptake might be simultaneously induced through photosynthesis. However, BY-2 cells are grown constantly in the dark and are not able to produce starch as products of the photosynthesis. A sugar source has to be added to maintain growth. Likewise, plastids in root tissue contain no chlorophyll.
Various techniques have been used over the years to assay secretion such as radiolabelling of precursor sugars or amino acids prior to measurement of wall polysaccharides or secreted proteins (Driouich et al., 1993), or the transfection of protoplasts to express the enzyme being assayed, for example α-amylase (Phillipson et al., 2001) or secretory phaseolin (Frigerio et al., 1998). The present results offer the potential to develop a new and perhaps more rapid assay measuring quantitatively starch accumulation related to inhibition of secretion using BY-2 cells as a model. With such an assay it might be possible to investigate the influence which Golgi proteins or other potential regulators of the secretory pathway have on secretory activity simply by measuring plastid starch accumulation.
Acknowledgments
We would like to thank Anne Kearns for maintaining BY-2 cultures, Steffi Gold and Barry Martin for help with electron microscopy, and Maria Shvedunova for growing Arabidopsis seedlings for our experiments. Special thanks to Professor David Fell, Dr Mark Poolman (Oxford Brookes), and Dr Kay Denyer (John Innes Institute) for fruitful discussions about the role of starch metabolism. Also we thank Professor Samuel Zeeman for helpful advice and for help in establishing the starch assays. Part of this work was supported by a BBSRC grant BB/D001080/1 awarded to CH, and a grant from the DFG to DGR.
References
- Amrhein N, Filner P. Sensitization of colchicine binding protein to ultraviolet light by bound colchicine. FEBS Letters. 1973;33:139–142. doi: 10.1016/0014-5793(73)80178-4. [DOI] [PubMed] [Google Scholar]
- Andreeva AV, Zheng H, Saint-Jore CM, Kutuzov MA, Evans DE, Hawes CR. Organization of transport from endoplasmic reticulum to Golgi in higher plants. Biochemical Society Transactions. 2000;28:505–512. [PubMed] [Google Scholar]
- Asatsuma S, Sawada C, Itoh K, Okito M, Kitajima A, Mitsui T. Involvement of alpha-amylase I-1 in starch degradation in rice chloroplasts. Plant and Cell Physiology. 2005;46:858–869. doi: 10.1093/pcp/pci091. [DOI] [PubMed] [Google Scholar]
- Barlow PW, Hawes CR, Horne JC. Structure of amyloplasts and endoplasmic reticulum in the root caps of Lepidium sativum and Zea mays observed after selective membrane staining and by high-voltage electron microscopy. Planta. 1984;160:363–371. doi: 10.1007/BF00393418. [DOI] [PubMed] [Google Scholar]
- Baroja-Fernandez E, Munoz FJ, Zandueta-Criado A, Moran-Zorzano MT, Viale AM, Alonso-Casajus N, Pozueta-Romero J. Most of ADP×glucose linked to starch biosynthesis occurs outside the chloroplast in source leaves. Proceedings of the National Academy of Sciences, USA. 2004;101:13080–13085. doi: 10.1073/pnas.0402883101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. The Plant Journal. 1998;15:441–447. doi: 10.1046/j.1365-313x.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- Bronner R. Simultaneous demonstration of lipids and starch in plant tissues. Stain Technology. 1975;50:1–4. doi: 10.3109/10520297509117023. [DOI] [PubMed] [Google Scholar]
- Caspar T, Lin TP, Kakefuda G, Benbow L, Preiss J, Somerville C. Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiology. 1991;95:1181–1188. doi: 10.1104/pp.95.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia T, Thorneycroft D, Chapple A, Messerli G, Chen J, Zeeman SC, Smith SM, Smith AM. A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. The Plant Journal. 2004;37:853–863. doi: 10.1111/j.1365-313x.2003.02012.x. [DOI] [PubMed] [Google Scholar]
- Craft J, Samalova M, Baroux C, Townley H, Martinez A, Jepson I, Tsiantis M, Moore I. New pOp/LhG4 vectors for stringent glucocorticoid-dependent transgene expression in Arabidopsis. The Plant Journal. 2005;41:899–918. doi: 10.1111/j.1365-313X.2005.02342.x. [DOI] [PubMed] [Google Scholar]
- daSilva LL, Snapp EL, Denecke J, Lippincott-Schwartz J, Hawes C, Brandizzi F. Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. The Plant Cell. 2004;16:1753–1771. doi: 10.1105/tpc.022673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte T, Umhang M, Trevisan M, Eicke S, Thorneycroft D, Smith SM, Zeeman SC. Evidence for distinct mechanisms of starch granule breakdown in plants. Journal of Biological Chemistry. 2006;281:12050–12059. doi: 10.1074/jbc.M513661200. [DOI] [PubMed] [Google Scholar]
- Driouich A, Zhang GF, Staehelin LA. Effect of brefeldin A on the structure of the Golgi apparatus and on the synthesis and secretion of proteins and polysaccharides in sycamore maple (Acer pseudoplatanus) suspension-cultured cells. Plant Physiology. 1993;101:1363–1373. doi: 10.1104/pp.101.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxeberria E, Gonzalez P, Tomlinson P, Pozueta-Romero J. Existence of two parallel mechanisms for glucose uptake in heterotrophic plant cells. Journal of Experimental Botany. 2005;56:1905–1912. doi: 10.1093/jxb/eri185. [DOI] [PubMed] [Google Scholar]
- Frigerio L, de Virgilio M, Prada A, Faoro F, Vitale A. Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. The Plant Cell. 1998;10:1031–1042. doi: 10.1105/tpc.10.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton DC, Stettler M, Mettler T, et al. beta-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active beta-amylases in Arabidopsis chloroplasts. The Plant Cell. 2008;20:1040–1058. doi: 10.1105/tpc.107.056507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jurgens G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112:219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Grebe M, Xu J, Mobius W, Ueda T, Nakano A, Geuze HJ, Rook MB, Scheres B. Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Current Biology. 2003;13:1378–1387. doi: 10.1016/s0960-9822(03)00538-4. [DOI] [PubMed] [Google Scholar]
- Hayat MA. Positive staining for electron microscopy. New York: Van Nostrand Rheinold; 1975. [Google Scholar]
- Hinchman RR. A permanent iodine stain–mountant combination for starch in plant tissues. Stain Technology. 1973;48:344–346. doi: 10.3109/10520297309116653. [DOI] [PubMed] [Google Scholar]
- Hummel E, Schmickl R, Hinz G, Hillmer S, Robinson DG. Brefeldin A action and recovery in Chlamydomonas are rapid and involve fusion and fission of Golgi cisternae. Plant Biology (Stuttgart) 2007;9:489–501. doi: 10.1055/s-2006-924759. [DOI] [PubMed] [Google Scholar]
- Keidan M, Friedlander M, Arad SM. Effect of Brefeldin A on cell wall polysaccharide production in the red microalga Porphyridium sp. (Rhodophyta) through its effect on the Golgi apparatus. Journal of Applied Phycology. 2009;21:707–717. [Google Scholar]
- Kitajima A, Asatsuma S, Okada H, et al. The rice alpha-amylase glycoprotein is targeted from the Golgi apparatus through the secretory pathway to the plastids. The Plant Cell. 2009;21:2844–2858. doi: 10.1105/tpc.109.068288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans M, Hawes C, Hillmer S, Hummel E, Robinson DG. Golgi regeneration after brefeldin A treatment in BY-2 cells entails stack enlargement and cisternal growth followed by division. Plant Physiology. 2007;145:527–538. doi: 10.1104/pp.107.104919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Burton RA, Harvey AJ, Hrmova M, Wardak AZ, Stone BA, Fincher GB. Biochemical evidence linking a putative callose synthase gene with (1→3)-beta-d-glucan biosynthesis in barley. Plant Molecular Biology. 2003;53:213–225. doi: 10.1023/B:PLAN.0000009289.50285.52. [DOI] [PubMed] [Google Scholar]
- Lu Y, Steichen JM, Yao J, Sharkey TD. The role of cytosolic alpha-glucan phosphorylase in maltose metabolism and the comparison of amylomaltase in Arabidopsis and Escherichia coli. Plant Physiology. 2006;142:878–889. doi: 10.1104/pp.106.086850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JE. Compartmentation in plant metabolism. Journal of Experimental Botany. 2007;58:35–47. doi: 10.1093/jxb/erl134. [DOI] [PubMed] [Google Scholar]
- Lytovchenko A, Bieberich K, Willmitzer L, Fernie AR. Carbon assimilation and metabolism in potato leaves deficient in plastidial phosphoglucomutase. Planta. 2002;215:802–811. doi: 10.1007/s00425-002-0810-9. [DOI] [PubMed] [Google Scholar]
- Munoz FJ, Baroja-Fernandez E, Moran-Zorzano MT, Viale AM, Etxeberria E, Alonso-Casajus N, Pozueta-Romero J. Sucrose synthase controls both intracellular ADP glucose levels and transitory starch biosynthesis in source leaves. Plant and Cell Physiology. 2005;46:1366–1376. doi: 10.1093/pcp/pci148. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for growth and rapid bioassays with tobacco tissue culture. Physiologia Plantarum. 1962;15:473–494. [Google Scholar]
- Nanjo Y, Oka H, Ikarashi N, Kaneko K, Kitajima A, Mitsui T, Munoz FJ, Rodriguez-Lopez M, Baroja-Fernandez E, Pozueta-Romero J. Rice plastidial N-glycosylated nucleotide pyrophosphatase/phosphodiesterase is transported from the ER–Golgi to the chloroplast through the secretory pathway. The Plant Cell. 2006;18:2582–2592. doi: 10.1105/tpc.105.039891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A, Ritzenthaler C, Robinson DG. Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiology. 2002;130:1102–1108. doi: 10.1104/pp.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieder A, Hummel E, Carvalho CM, Hawes C. Golgi membrane dynamics after induction of a dominant-negative mutant Sar1 GTPase in tobacco. Journal of Experimental Botany. 2010;61:405–422. doi: 10.1093/jxb/erp315. [DOI] [PubMed] [Google Scholar]
- Osuna D, Usadel B, Morcuende R, et al. Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. The Plant Journal. 2007;49:463–491. doi: 10.1111/j.1365-313X.2006.02979.x. [DOI] [PubMed] [Google Scholar]
- Phillipson BA, Pimpl P, daSilva LL, Crofts AJ, Taylor JP, Movafeghi A, Robinson DG, Denecke J. Secretory bulk flow of soluble proteins is efficient and COPII dependent. The Plant Cell. 2001;13:2005–2020. doi: 10.1105/TPC.010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proschold T, Marin B, Schlosser UG, Melkonian M. Molecular phylogeny and taxonomic revision of Chlamydomonas (Chlorophyta). I. Emendation of Chlamydomonas Ehrenberg and Chloromonas gobi, and description of Oogamochlamys gen. nov. and Lobochlamys gen. nov. Protist. 2001;152:265–300. doi: 10.1078/1434-4610-00068. [DOI] [PubMed] [Google Scholar]
- Radhamony RN, Theg SM. Evidence for an ER to Golgi to chloroplast protein transport pathway. Trends in Cell Biology. 2006;16:385–387. doi: 10.1016/j.tcb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Reyes F, Orellana A. Golgi transporters: opening the gate to cell wall polysaccharide biosynthesis. Current Opinion in Plant Biology. 2008;11:244–251. doi: 10.1016/j.pbi.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Richter S, Geldner N, Schrader J, Wolters H, Stierhof YD, Rios G, Koncz C, Robinson DG, Jurgens G. Functional diversification of closely related ARF-GEFs in protein secretion and recycling. Nature. 2007;448:488–492. doi: 10.1038/nature05967. [DOI] [PubMed] [Google Scholar]
- Robinson DG, Ehlers U, Herken R, Hermann B, Mayer F, Schuermann FW. Praeparationsmethodik in der Elektronenmikroskopie. Berlin: Springer; 1985. [Google Scholar]
- Robinson DG, Langhans M, Saint-Jore-Dupas C, Hawes C. BFA effects are tissue and not just plant specific. Trends in Plant Science. 2008;13:405–408. doi: 10.1016/j.tplants.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Saint-Jore CM, Evins J, Batoko H, Brandizzi F, Moore I, Hawes C. Redistribution of membrane proteins between the Golgi apparatus and endoplasmic reticulum in plants is reversible and not dependent on cytoskeletal networks. The Plant Journal. 2002;29:661–678. doi: 10.1046/j.0960-7412.2002.01252.x. [DOI] [PubMed] [Google Scholar]
- Samalova M, Brzobohaty B, Moore I. pOp6/LhGR: a stringently regulated and highly responsive dexamethasone-inducible gene expression system for tobacco. The Plant Journal. 2005;41:919–935. doi: 10.1111/j.1365-313X.2005.02341.x. [DOI] [PubMed] [Google Scholar]
- Schupp N, Ziegler P. The relation of starch phosphorylases to starch metabolism in wheat. Plant and Cell Physiology. 2004;45:1471–1484. doi: 10.1093/pcp/pch170. [DOI] [PubMed] [Google Scholar]
- Smith AM, Stitt M. Coordination of carbon supply and plant growth. Plant, Cell and Environment. 2007;30:1126–1149. doi: 10.1111/j.1365-3040.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- Smith AM, Zeeman SC. Quantification of starch in plant tissues. Nature Protocols. 2006;1:1342–1345. doi: 10.1038/nprot.2006.232. [DOI] [PubMed] [Google Scholar]
- Sparkes IA, Runions J, Kearns A, Hawes C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nature Protocols. 2006;1:2019–2025. doi: 10.1038/nprot.2006.286. [DOI] [PubMed] [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Steinkellner H, Strasser R. Glycosyltransferases in the plant Golgi. In: Robinson DG, editor. The Golgi apparatus and the plant secretory pathway. Oxford: Blackwell; 2003. pp. 181–192. [Google Scholar]
- Takeuchi M, Ueda T, Sato K, Abe H, Nagata T, Nakano A. A dominant negative mutant of Sar1 GTPase inhibits protein transport from the endoplasmic reticulum to the Golgi apparatus in tobacco and Arabidopsis cultured cells. The Plant Journal. 2000;23:517–525. doi: 10.1046/j.1365-313x.2000.00823.x. [DOI] [PubMed] [Google Scholar]
- Villarejo A, Buren S, Larsson S, et al. Evidence for a protein transported through the secretory pathway en route to the higher plant chloroplast. Nature Cell Biology. 2005;7:1224–1231. doi: 10.1038/ncb1330. [DOI] [PubMed] [Google Scholar]
- Yu TS, Zeeman SC, Thorneycroft D, et al. Alpha-Amylase is not required for breakdown of transitory starch in Arabidopsis leaves. Journal of Biological Chemistry. 2005;280:9773–9779. doi: 10.1074/jbc.M413638200. [DOI] [PubMed] [Google Scholar]
- Zeeman SC, Smith SM, Smith AM. The diurnal metabolism of leaf starch. Biochemical Journal. 2007;401:13–28. doi: 10.1042/BJ20061393. [DOI] [PubMed] [Google Scholar]
- Zeeman SC, Thorneycroft D, Schupp N, Chapple A, Weck M, Dunstan H, Haldimann P, Bechtold N, Smith AM, Smith SM. Plastidial alpha-glucan phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress. Plant Physiology. 2004;135:849–858. doi: 10.1104/pp.103.032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-H, Robinson DG. The endomembranes of Chlamydomonas rheinhardii: a comparison of the wildtype with wall mutants CW2 and CW15. Protoplasma. 1986;133:186–194. [Google Scholar]
- Ziegler P. Partial purification and characterization of the major endoamylase of mature pea leaves. Plant Physiology. 1988;86:659–666. doi: 10.1104/pp.86.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler P, Beck E. Exoamylase activity in vacuoles isolated from pea and wheat leaf protoplasts. Plant Physiology. 1986;82:1119–1121. doi: 10.1104/pp.82.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]