Abstract
The spontaneous translocation of the short arm of chromosome 1 of rye (1RS) in bread wheat is associated with higher root biomass and grain yield. Recent studies have confirmed the presence of QTL for different root morphological traits on the 1RS arm in bread wheat. This study was conducted to address two questions in wheat root genetics. First, does the presence of the 1RS arm in bread wheat affect its root anatomy? Second, how does root morphology and anatomy of bread wheat respond to different dosages of 1RS? Near-isogenic plants with a different number (0 to 4 dosages) of 1RS translocations were studied for root morphology and anatomy. The F1 hybrid, with single doses of the 1RS and 1AS arms, showed heterosis for root and shoot biomass. In other genotypes, with 0, 2, or 4 doses of 1RS, root biomass was incremental with the increase in the dosage of 1RS in bread wheat. This study also provided evidence of the presence of gene(s) influencing root xylem vessel number, size, and distribution in bread wheat. It was found that root vasculature follows a specific developmental pattern along the length of the tap root and 1RS dosage tends to affect the transitions differentially in different positions. This study indicated that the inherent differences in root morphology and anatomy of different 1RS lines may be advantageous compared to normal bread wheat to survive under stress conditions.
Keywords: 1RS translocation, dosage effect, heterosis, root anatomy, root morphology, stress adaptation
Introduction
All higher plants have roots and the root fraction of the plant's total mass varies widely, even within the same species. Although, roots encounter many fluctuations in their external environments that affect their growth, it is their tendency to accommodate and survive these as a whole system that makes them strongly homoeostatic (Barlow, 1986). Knowledge of these modifications in the root system at a morphological and anatomical level, whether due to environmental response or genetic control, is of importance (Weaver, 1926).
Root morphological traits and root growth response to the external environment have been studied widely in a number of crop species. In a greenhouse study, drought-stressed Lolium perenne L. plants increased the number and growth of lateral roots (Jupp and Newman, 1987). In barley (Hordeum vulgare L.), potassium deficiency was associated with the reduced length of laterals and larger root diameter compared with phosphorus deficiency under greenhouse conditions (Hackett, 1968). In wheat (Triticum aestivum L.), temperature had a profound effect on dry weight and root length under greenhouse environments (Bowen and Rovira, 1971; Huang, 1991). Besides root morphology, anatomical traits are also greatly influenced by the surrounding environment and have been widely studied in different crop species such as maize (Zea mays L.) (Hose et al., 2001), rice (Oryza sativa L.) (Uga et al., 2008), and other cereals (Aloni and Griffith, 1991; Liljeroth, 1995). In controlled environmental growth chamber-grown winter and spring wheat, chilling and high temperature decreases the diameter of the central metaxylem vessel (Huang et al., 1991; Terzioglu and Ekmekci, 1995).
There are reports which suggest that specific morphological and anatomical root traits help stress-tolerant plants survive a particular stress condition. In rice, traits such as deep root-to-shoot ratio and deep specific root length were found to contribute to drought avoidance in the field (Yoshida and Hasegawa, 1982; Fukai and Cooper, 1995). Drought-tolerant wheat cultivars have smaller xylem vessel diameters (Yang et al., 2007). This anatomical adaptation of the tolerant wheat genotypes proved to be an advantage for the survival and higher grain yield under water stress (Richards and Passioura, 1989; Huang et al., 1991; Huang and Taylor, 1993; Ou et al., 2005).
The genetic control of root characteristics is poorly understood especially in bread wheat. Robertson et al. (1979) characterized genetic variability for seedling root number within the genus Triticum to examine its value in a breeding programme and found this trait to be positively correlated with seed weight. In a field study in winter wheat, post-anthesis root growth was investigated and cultivars were found to be significantly different in root length and mass especially below 30 cm (Ford et al., 2006). O'Brien (1979) examined different wheat genotypes for root development in glass-fronted growth containers in the greenhouse and reported that the variability in seminal roots was sufficient for the root morphology to be altered by hybridization and selection. In bread wheat, xylem vessel diameter was found to have more useful genetic variation and higher heritability than the number of seminal axes in the roots and they showed a significant response to selection (Richards and Passioura, 1989). In a wheat backcross breeding programme, lines selected for reduced xylem vessel diameter yielded 3–11% more grain in the driest environments than unselected controls, depending on the genetic background (Richards and Passioura, 1989). Besides these findings, there is still no report in bread wheat of the chromosomal localization of genes that affect root anatomy.
Weaver (1926) compared the root systems of rye and bread wheat under natural field conditions and reported that rye had deeper seminal roots. The spontaneous translocation of the short arm of chromosome 1 of rye (1RS) (Secale cereale L.) to the long arm of chromosome 1B (1BL) in bread wheat (Triticum aestivum L.) was first identified in the late 1930s (Kattermann, 1938; Mettin et al., 1973). Over the past few decades, there have been several reports of higher grain yield of 1RS translocated wheats over other commonly grown wheat genotypes (Rajaram et al., 1983; Villareal et al., 1991; Owuoche et al., 2003; Kim et al., 2004). In other studies, an increase in grain yield among 1RS wheats was found to be positively correlated with higher root biomass (Ehdaie et al., 2003) while there were no significant differences found for shoot traits (Kim et al., 2004). Roots of 1RS.1BL translocation wheats were thinner and there was a higher root length density when grown in acid soils and this enhanced the root surface area (Manske and Vlek, 2002).
Lukaszewski (1993) reconstructed the complete chromosomes of 1B and 1R from a 1RS.1BL translocation and, later, produced three new centric translocations namely, 1RS.1AL, 1RS.1BL, and 1RS.1DL in ‘Pavon 76’ (Lukaszewski, 1997). Each of three translocations had the same 1RS arm but in different locations in the genome and each was mitotically stable. All three translocations had greater root biomass and higher grain yield under irrigated and water-stressed greenhouse conditions and under irrigated field conditions (Ehdaie et al., 2003; Kumlay et al., 2003). These translocation lines were ranked for root biomass as Pavon 1RS.1AL>Pavon 1RS.1DL>Pavon 1RS.1BL>Pavon 76 (Ehdaie et al., 2003). Recently, a genetic map was generated using 1RS-1BS recombinant breakpoints in Pavon 76 wheat and their genetic analysis indicated the distal 15% of the physical length of chromosome 1RS may carry the gene(s) for better rooting ability and root morphological traits (Sharma et al., 2009).
The present study was conducted to address the dosage effect of the 1RS translocation in bread wheat. Wheat genotypes were used that differ in their number of the 1RS translocations in a spring bread wheat ‘Pavon 76’ genetic background. For generating F1 seeds, Pavon 1RS.1AL was the preferred parent of choice due to its better performance for root biomass than other 1RS lines (Ehdaie et al., 2003). The dosage effect of a 1RS chromosome arm on different root traits including root biomass, root diameter, stele diameter, central metaxylem (CMX) vessel diameter, CMX vessel area, CMX vessel number, and number of xylem poles of wheat roots is reported here. The results from this study may validate previous results of the presence of genes for rooting ability on the 1RS chromosome arm. This study also provides evidence for the presence of genes affecting root anatomy on the 1RS arm.
Materials and methods
Plant material
Five genotypes, Pavon 76, F1 (Pavon 1RS.1AL×Pavon 76), Pavon 1RS.1AL, Pavon 1RS.1DL, and Pavon 1RS.1AL-Pavon 1RS.1DL were used. They were coded as R0, RA1, RA2, RD2, and RAD4, respectively. Here, ‘R’ denoted the dosage of chromosome arm 1RS, the second letter A and D denoted the chromosome 1 of the respective genome of wheat to which 1RS is translocated and numbers in the subscripts are dosage number of 1RS chromosome arms present in the genotype. Pavon 76 is a spring bread wheat from the breeding programme of Centro Internacional de Mejoramiento de Maíz y Trigo (CIMMYT), Mexico. For a single dose of 1RS, crosses were made between Pavon 1RS.1AL and Pavon 76 and F1 seed was used as RA1. Seeds of RA2, RD2, and RAD4 genotypes were provided by Dr AJ Lukaszewski, University of California, Riverside.
Experimental set-up
To study the effect of different dosages of the 1RS chromosome arm of rye in a wheat background and their effect on root morphology, an experiment was set up in a randomized complete block design with six replicates per genotype during January 2007, April 2007, January 2008, and January 2009. The genotypes were grown under a natural photoperiod in a temperature controlled greenhouse (20–30 °C and 50–90% relative humidity). Anatomy of the primary seminal roots or specifically the primary axile roots (Watt et al., 2008) was studied for two of these seasons. Seminal roots and primary-axile roots are mentioned from here onwards as tap root in accordance with root nomenclature guidelines (http://rootrap.boku.ac.at/index.php?id=28). Seeds from the above-mentioned five genotypes were surface-sterilized with 5% commercial bleach for 5 min, washed for 10 min in distilled water, soaked in water for 24 h, and then germinated on wet filter paper in Petri dishes. 3–5-d-old seedlings were transplanted to 7.5 l cylindrical pots (21×21.5 cm diameter), lined with a plastic bag containing 6.5 kg of washed silica sand no. 30 with bulk density of 1.42 g ml−1 and 24% field capacity (w/w). Small holes were made at the bottom of the plastic bags to allow drainage of excess water. Plants were watered, when necessary, with half-strength Hoagland's solution to ensure that nutrients and water were not limiting.
Phenotypic data
Plants were harvested 45 d after germination at the mid-to-late tillering stage or Decimal code of 28–29 for growth stages (Zadoks et al., 1974) and pots containing roots in polythene bags were stored at 4 °C until processed. Each polythene bag was laid out on a screen frame in a tub half-filled with water and a cut was made on the side of the bag. The frame was moved gently in the tub to separate sand from the roots. Roots were recovered without damage using the floatation technique (Böhm, 1979) and attached sand was removed by hand. Different plant characters were measured including plant height (PH), number of tillers (NT), longest leaf length (LLL), maximum width of the longest leaf (LLW), leaf area of the longest leaf (LA), longest root length (LRL), root biomass (RB), shoot biomass (SB), and root biomass to shoot biomass ratio (R/S). Root and shoot biomass was determined from dry matter after drying to constant weight in a forced-air drier for 96 h at 65 °C. Leaf area of the longest leaf was calculated by LLL×LLW×0.78 for each genotype (Quarrie and Jones, 1977).
Heterosis
Mid-parent heterosis (MPH) and best-parent heterosis (BPH) of the F1 hybrid (RA1) over its parents (R0 and RA2) for root biomass and shoot biomass were calculated as:
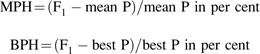 |
Root anatomy
After washing, root samples were collected in two different seasons from the tap roots of six plants of each genotype at three different locations, namely root tip, middle of the root, and 1 cm from the base of the root adjacent to the seed. Root samples were immersed in half-strength Karnovosky's fixative (2.5% gluteraldehyde and 4% formaldehyde in 50 mM phosphate buffer, pH 7.2). The roots were left in the fixative at 4 °C for 24 h. The roots were dehydrated by passing through a graded ethanol series of 10%, 35%, 50%, and 70% and then left overnight in 70% ethanol followed by another 2 h treatment in 85%, 90%, and 100% and then subsequently overnight in 100% with 1% Erythrosine B (C.I. 11A). Then the samples were transferred through an ethanol/xylene series of 3/1, 1/1, and 1/3 for 30 min to 1 h each. Tissues were then placed in pure xylene for 2 h followed by overnight treatment. Infiltration and embedding was done in paraffin.
Paraffin blocks containing tissues were sectioned at 10 μm thick using an A/O rotary Microtome and stainless steel knives. Root tips were serially sectioned in the transverse plane starting at the tip of the root cap. Sections were mounted on slides and stained with Safranin O (C.I. 50240) and Fast Green FCF (C.I.# 19) using Sass's procedure (Ruzin, 1999). Samples were observed on a Zeiss standard compound brightfield microscope. Digital images were taken using an Infinity 1 digital camera (Luminere Inc, Canada). Data were recorded for the different root anatomical features such as root diameter, stele diameter, central metaxylem (CMX) vessel diameter, CMX vessel area, CMX vessel number, and number of xylem poles from different regions of the roots of all the above-mentioned five genotypes. Data on CMX vessel diameter and area were made on transverse sections of root tips at a distance of 1370 μm from the tip for all genotypes. At this position the CMX vessel was clearly evident and there was no significant difference in the position of its appearance from the root tip between the genotypes. Calibrations and measurements were carried out by camera compliant software ‘Analyse Infinity’ (Luminere Inc, Canada).
Statistical analysis
The morphological data were subjected to analysis of variance (ANOVA) for each year (Steel et al., 1997). The combined ANOVA across seasons was performed for each measured and calculated trait using a split plot design in time. Season was treated as the main plot and genotype as a sub-plot. In combined ANOVA, all the factors were considered fixed. The anatomical data were also subjected to ANOVA to determine the genetic differences among genotypes. Simple correlation coefficients were calculated to determine the relationship among different anatomical traits. Linear regression analysis was carried out for different anatomical root traits to assess the relationship between dosage of the 1RS chromosome arm and the root anatomical traits in bread wheat. Statistix 8 program (Analytical Software, Tallahassee FL) was used to carry out correlation and regression analysis.
Results
Phenotypic study
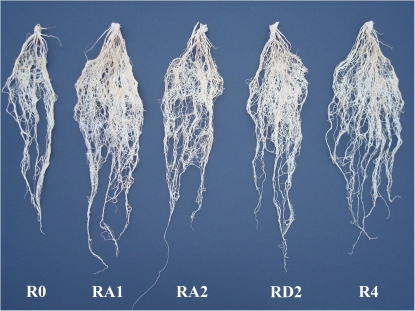
There was lesser total root biomass in Pavon 76 (R0) than any of its 1RS sister lines. Among the 1RS lines, F1 (RA1) and RAD4 had the highest root biomass (Fig. 1). There were significant differences among genotypes for plant height, number of tillers, length of longest leaf, maximum width of longest leaf, root biomass, shoot biomass, and root to shoot ratio (P <0.05) (Table 1). For leaf area, differences among genotypes were not significant. All the genotypes showed significant difference for all the phenotypic traits across the seasons (P <0.05). Genotype × season interactions (P <0.01) were not significant for leaf area and root-to-shoot ratio. The RAD4 genotype had the highest number of tillers, longest leaf length, and maximum root-to-shoot ratio but the shortest plant height, minimum width of longest leaf measured at its widest point, and minimum leaf area of the longest leaf. The RA1 hybrid performed significantly differently from other genotypes for root biomass and shoot biomass (P <0.05). Mean values of RA2 were higher for maximum width of the longest leaf and leaf area. For root biomass, RA1 and RAD4 were significantly different from RA2, RD2, and check R0 (P <0.05) (Fig. 1; Table 1). RA2 was significantly different from RD2, and check R0 only at P <0.01 data not shown). All the genotypes were significantly similar for shoot biomass except RA1 which was significantly different from them (P <0.05) (Table 1).
Fig. 1.
Roots of different wheat genotypes with different number of 1RS translocations in spring bread wheat ‘Pavon 76’ background harvested 45 d after germination (grown in pots). Pavon 76 (R0)=0 dose of 1RS, Pavon 1RS.1AL×Pavon 76 (RA1)=1 dose of 1RS, Pavon 1RS.1AL (RA2)=2 doses of 1RS on 1AL, Pavon 1RS.1DL (RD2)=2 doses of 1RS on 1DL, and Pavon 1RS.1AL- Pavon 1RS.1DL (RAD4) = 4 doses of 1RS on 1AL and 1DL. (This figure is available in colour at JXB online.)
Table 1.
Summary of combined ANOVA and mean values of plant height (PH), number of tillers (NT), longest leaf length (LLL), maximum width of the longest leaf (LLW), leaf area of the longest leaf (LA), root biomass per plant (RB), shoot biomass per plant (SB), and root to shoot biomass ratio (R/S) for bread wheat Pavon 76 (R0), F1, Pavon 1RS.1AL×Pavon 76 (RA1), Pavon 1RS.1AL (RA2), Pavon 1RS.1DL (RD2), and Pavon 1RS.1AL-Pavon 1RS.1DL (RAD4) grown in sand pots for 45 d (mid to late tillering stage) averaged across four seasons
| Genotype | PHa | NTa | LLLa | LLWa | LAa | RBa | SBa | R/Sa |
| (cm) | (no.) | (cm) | (cm) | (cm2) | (mg) | (mg) | ||
| R0 | 54 b | 11 b | 35 c | 1.38 ab | 38.1 ab | 1073 bc | 3692 b | 0.33 bc |
| RA1 | 55 b | 11 b | 37 b | 1.31 b | 37.7 ab | 1549 a | 4319 a | 0.36 ab |
| RA2 | 58 a | 11 b | 37 b | 1.42 a | 40.9 a | 1232 b | 3802 b | 0.36 ab |
| RD2 | 58 a | 9 c | 36 bc | 1.35 ab | 38.3 ab | 1087 b | 3637 b | 0.31 c |
| RAD4 | 52 c | 13 a | 39 a | 1.20 c | 37.1 b | 1461 a | 3743 b | 0.38 a |
| Season | ** | ** | ** | ** | ** | ** | ** | ** |
| Genotype | ** | ** | ** | ** | NS | ** | ** | ** |
| Genotype×Season | ** | ** | ** | ** | NS | ** | ** | NS |
| CV (%)b | 7 | 15 | 7 | 10 | 14 | 24 | 17 | 16 |
Means followed by the same small letter within a column are not significantly different at P <0.05 according to LSD test; **=significant (P <0.01); NS=not significant (P >0.05).
CV=Coefficient of variation.
Mid-parent and best-parent heterosis
Mean values of the F1 hybrid (RA1) were significantly different from both of its parents (R0 and RA2) for root biomass and shoot biomass (P <0.05) (Table 1). MPH and BPH for root biomass were calculated to be 34.4% and 25.7%, respectively (Table 2). For shoot biomass, heterotic gain of F1 over mid-parent and best-parent was 15.3% and 13.6%, respectively (Table 2).
Table 2.
Mid-parent heterosis (MPH) and best-parent heterosis (BPH) for root and shoot biomass (per plant) of bread wheat hybrid F1 - Pavon 1RS.1AL×Pavon 76 (RA1) over parents Pavon 1RS.1AL (RA2) and Pavon 76 (R0) grown in sand pots for 45 d (mid-tillering stage).
| Trait | Mean | MPH (%) | BPH (%) | ||
| Hybrid (RA1) | Mid-parent | Best-parent (RA2) | |||
| (mg) | (mg) | (mg) | |||
| Root biomass | 1549 | 1153 | 1232 | 34.4 | 25.7 |
| Shoot biomass | 4319 | 3747 | 3802 | 15.3 | 13.6 |
Mean values are averaged across four seasons.
Root anatomy along the root
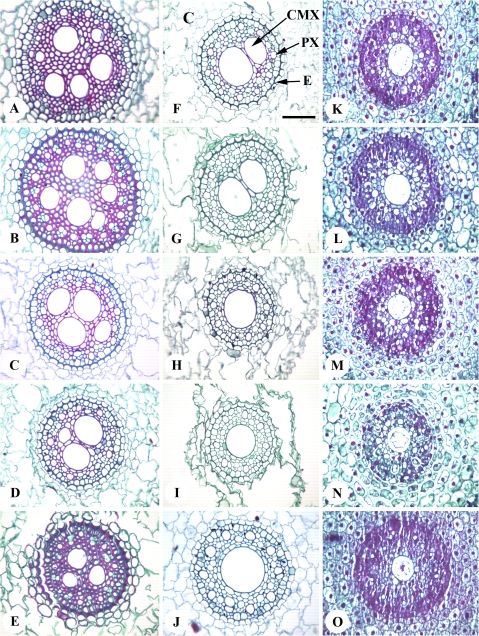
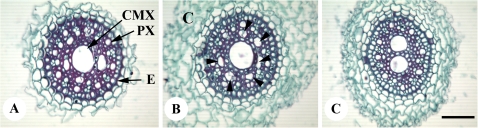
To study root anatomy, the two seasons selected were representative of the four seasons used for the study. For most of the phenotypic traits, including root and shoot biomass, genetic differences observed across four years were also statistically significant when the data across the two years (in which the microscopy was done) were analysed separately. The size, number, and arrangement of metaxylem vessels varied along the length of the tap root from the top to the tip region for each genotype. In the top region, there were 3–6 large metaxylem vessels that were not necessarily central in position. More of these were present in the transverse sections of R0, RA1, and RA2 than in RD2 and RAD4 (Fig. 2A–E). In the latter genotypes, especially RAD4, there was variation for number and arrangement of CMX vessels in the basal portion transverse sections of the tap root (Fig. 3A, B, C). A partial ring of smaller metaxylem vessels surrounding the bigger CMX vessel was observed in some of the tap roots where there was only a single CMX vessel. Similar variation was also observed in RD2 (data not shown). The diameter of the stele was larger in R0 and RA1 than RA2, RD2, and RAD4 (Fig. 2A–E).
Fig. 2.
Stained transverse sections of different regions (A–E top, F–J middle, K–0 tip) of roots of wheat genotypes. (A, F, K) Pavon 76 (R0); (B, G, L) F1 of Pavon 1RS.1AL×Pavon 76 (RA1); (C, H, M) Pavon 1RS.1AL (RA2); (D, I, N) Pavon 1RS.1DL (RD2); (E, J, O) Pavon 1RS.1AL-Pavon 1RS.1DL (RAD4); (F) is labelled with C, cortex; CMX, central metaxylem vessel; E, endodermis; PX, protoxylem; Scale bar, 100 μm.
Fig. 3.
(A, B, C) Examples of patterns seen in the number of CMX vessels in the top region of tap root of the RAD4 genotype (four doses of 1RS chromosome arm). (B) is labelled with arrowheads, inner xylem vessels; C, cortex; CMX, central metaxylem vessel; E, endodermis; PX, protoxylem; Scale bar, 100 μm.
In the middle region of the root, there were two centrally located metaxylem vessels in the stele of R0 and RA1 and a single CMX vessel in RD2 and RAD4 (Fig. 2F–J). The diameter of the stele was smaller in the middle than in the top region of the root for most of the genotypes (data not shown). In the root tip region, there was a single differentiating CMX vessel in all the genotypes which was narrower in RA2, RD2, and RAD4 genotypes (Fig. 2M, N, O). There was not much variation for other anatomical traits in the root tip region among the genotypes (Fig. 2K–O).
Top region of the root (TOP)
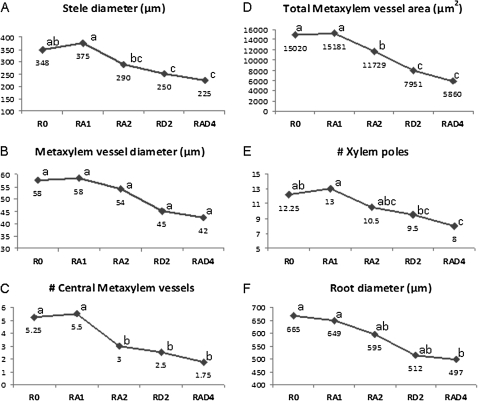
There were significant differences among genotypes for stele diameter, total metaxylem vessel area, number of CMX vessels, and number of xylem poles in the top region of the tap root. Differences among genotypes were not significant for xylem diameter, CMX number, and root diameter but all genotypes showed the same trend in their performance for all the root anatomical traits measured (Fig. 4A–F). Hybrid RA1 was the largest among genotypes, except for root diameter, followed by R0, for all the traits in the top region of the tap root while RAD4 was the smallest.
Fig. 4.
Graphical representation of mean performances of anatomical traits: (A) stele diameter; (B) metaxylem vessel diameter; (C) number of central metaxylem vessels; (D) total metaxylem vessel area; (E) number of xylem poles (protoxylem); and (F) root diameter measured from top region of the roots of each wheat genotype with different number of 1RS translocation arms in the genetic background of spring bread wheat ‘Pavon 76’. The same small letters within a graph are not significantly different. X-axis, bread wheat genotypes namely Pavon 76 (R0)=0 dose of 1RS, Pavon 1RS.1AL×Pavon 76 (RA1)=1 dose of 1RS, Pavon 1RS.1AL (RA2)=2 doses of 1RS on 1AL, Pavon 1RS.1DL (RD2)=2 doses of 1RS on 1DL, and Pavon 1RS.1AL- Pavon 1RS.1DL (RAD4)=4 doses of 1RS on 1AL and 1DL; Y axis, scale for root trait.
The calculation of correlation coefficients (r) revealed a strong degree of association among traits of root anatomy in the top region of the root (Table 2). Stele diameter showed a positive correlation with CMX vessel area (0.82), number of xylem poles (0.81), and root diameter (0.84). CMX vessel area was further associated highly with CMX vessel diameter (0.77), number of xylem poles (0.71)), and root diameter (0.86). There was also a high correlation between root diameter and number of xylem poles (0.79) (Table 3).
Table 3.
Simple correlation coefficients between different root anatomical traits in different root regions in the genetic background of spring bread wheat ‘Pavon 76’ with different number of 1RS translocation arms; n=20 for TOP and TIP regions, n=25 for MID regions
| Root traits | Root regions | Root traitsa | ||||
| Stele diameter | CMX vessel area | CMX vessel diameter | CMX vessel number | Xylem poles | ||
| CMX vessel area | TOP | 0.82** | ||||
| MID | 0.96** | |||||
| TIP | 0.68** | |||||
| CMX vessel diameter | TOP | 0.46* | 0.77** | |||
| MID | 0.96** | 0.96** | ||||
| TIP | 0.73** | 0.99** | ||||
| CMX vessel number | TOP | 0.86** | 0.85** | 0.68** | ||
| MID | 0.29 | 0.19 | 0.21 | |||
| TIP | – | – | – | |||
| Xylem poles | TOP | 0.81** | 0.71** | 0.53* | 0.84** | |
| MID | 0.91** | 0.88** | 0.89** | 0.27 | ||
| TIP | 0.14 | –0.02 | –0.01 | – | ||
| Root diameter | TOP | 0.84** | 0.86** | 0.56* | 0.84** | 0.79** |
| MID | 0.83** | 0.75** | 0.78** | 0.08 | 0.84** | |
| TIP | 0.75** | 0.59** | 0.64** | – | –0.08 | |
** Significant (P ≤0.01); * significant (P ≤0.05 >0.01).
Linear regression analysis showed significant R2 values for all anatomical root traits measured in this study. The number of CMX vessels in the top of the root was the best explained trait (51%) due to 1RS dosage. Forty-seven per cent of the variation of two root traits, CMX area and number of xylem poles, was explained by the linear regression on the number of 1RS dosages in a genotype (Table 4). The number of 1RS dosages explained 45% and 38% of the variation in stele diameter and root diameter, respectively. The negative slope of the regression equation showed a negative relationship between root anatomical traits in the top region and number of 1RS dosages (Table 4). This is also evident from the negative trends in Fig. 4.
Table 4.
Summary of regression analysis
| Dependent variable | Intercept | Slope | R2 | F | P |
| Root top | |||||
| Stele diameter (μm) | 363.4 | –36.5 | 0.45 | 14.66 | 0.00 |
| Total CMX area (μm2) | 15720.0 | –2540.0 | 0.47 | 16.01 | 0.00 |
| CMX diameter (μm) | 59.0 | –4.2 | 0.21 | 4.65 | 0.04 |
| CMX (number) | 5.4 | –1.0 | 0.51 | 18.63 | 0.00 |
| Xylem pole (number) | 12.9 | –1.2 | 0.47 | 15.78 | 0.00 |
| Root diameter (μm) | 667.7 | –44.5 | 0.38 | 6.60 | 0.03 |
| Root tip | |||||
| Stele (μm) | 250.8 | –5.4 | 0.10 | 2.00 | 0.17 |
| CMX area (μm2) | 6161.4 | –773.5 | 0.54 | 21.30 | 0.00 |
| CMX diameter (μm) | 87.7 | –6.0 | 0.52 | 19.69 | 0.00 |
| Xylem pole (number) | 8.8 | 0.1 | 0.01 | 0.21 | 0.65 |
| Root diameter (μm) | 655.8 | –15.1 | 0.06 | 1.19 | 0.29 |
The independent variable is number of 1RS translocation arms in the spring bread wheat ‘Pavon 76’ genetic background (n=20)
Mid-region of the root (MID)
ANOVA showed no significant differences among genotypes for any of the anatomical root traits in the mid-region of the tap root except number of xylem poles (data not shown). However, all the traits showed stronger association among themselves except CMX vessel number (Table 3). Regression analysis showed a negligible dependence of root traits on the number of 1RS dosages (data not shown).
Tip of the root (TIP)
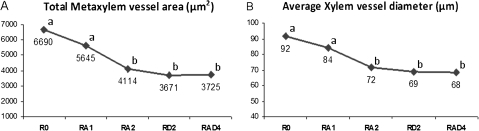
In root tips of the tap roots, there were significant differences among genotypes for CMX vessel area and CMX vessel diameter. For both the traits, R0 and RA1 showed significantly higher mean values than 1RS double dosage genotypes (RA2 and RD2) and quadruple dosage of 1RS in RAD4 genotype (Fig. 5A, B). Correlation coefficient values of both the above traits also showed a highly positive association with each other followed by stele and root diameter (Table 3). Again in the regression analysis, both the traits, CMX vessel area and CMX vessel diameter, were significantly influenced by different 1RS dosages in the genotypes. Their regression equation showed negative slope and the variation explained for CMX vessel area and CMX vessel diameter was 54% and 52%, respectively (Table 4).
Fig. 5.
Graphical representation of mean performances of (A) total metaxylem vessel diameter; and (B) average metaxylem diameter measured in root tips of the roots of different wheat genotypes with different number of 1RS translocation arms in the genetic background of spring bread wheat ‘Pavon 76’. The same small letters within a graph are not significantly different. X- axis, bread wheat genotypes, namely Pavon 76 (R0)=0 dose of 1RS, Pavon 1RS.1AL×Pavon 76 (RA1)=1 dose of 1RS, Pavon 1RS.1AL (RA2)=2 doses of 1RS on 1AL, Pavon 1RS.1DL (RD2)=2 doses of 1RS on 1DL, and Pavon 1RS.1AL - Pavon 1RS.1DL (RAD4)=4 doses of 1RS on 1AL and 1DL; Y -axis, scale for root trait.
Discussion
From previous studies (Ehdaie et al., 2003; Sharma et al., 2009), it was clear that there was a gene (or genes) present on the 1RS chromosome arm which affects root traits in bread wheat. These authors did not attempt to determine if this chromosomal region affects any root anatomical traits in bread wheat. The purpose of this study was to look for variation in root morphology and anatomy among different wheat genotypes and then to determine how these differences are related to different dosages of 1RS in bread wheat. During this study, we came to some interesting conclusions: (i) F1 hybrids showed a heterotic effect for root biomass and there was an additive effect of the 1RS arm number on root morphology of bread wheat; (ii) there was a specific development pattern in the root vasculature from top to tip in wheat roots and 1RS dosage tended to affect root anatomy differently in different regions of the tap root. Further, the differences in root biomass, and traits associated with CMX of the different genotypes may have specific bearing on their ability to tolerate water and heat stress.
The results in this study were obtained at an early stage of growth in the greenhouse environment. Although the positive correlation of higher root biomass of 1RS wheats in the greenhouse and their yield performance in field conditions was demonstrated (Ehdaie et al., 2003), it may be difficult to translate the observed effects of this study to wheat root systems in the field at later stages, especially the anatomical traits on xylem vessel number, size etc which were observed on only one tap root per plant.
Effect of 1RS dosage on root morphology
The effect of number of 1RS translocation arms in bread wheat was clearly evident from their averaged mean values for root biomass. RA1 and RAD4 were ranked highest while R0 ranked at the bottom (Fig. 1; Table 1). Genotype RD2 performed only slightly better than R0 for root biomass because of its poor performance in one season, otherwise it showed better rooting ability in the other three seasons. Otherwise, all the genotypes with 1RS translocations showed higher root biomass than R0 which carried a normal 1BS chromosome arm. Data in this study suggested two types of effects of 1RS on wheat roots. First, an additive effect of 1RS, there was increase in root biomass with the increase in 1RS dosage from zero (R0) to two (RA2 and RD2) and then to four (RAD4).
A second major effect noted was a heterotic effect of 1RS and 1AS on root biomass and shoot biomass. MPH and BPH of the F1 hybrid (RA1) were higher for root biomass than for shoot biomass (Table 2). This further explained the more pronounced effect of 1RS on root biomass than shoot biomass. Significant positive heterosis was observed for root traits among wheat F1 hybrids and 27% of the genes were differentially expressed between hybrids and their parents (Wang et al., 2006). The possible role of differential gene expression was suggested to play a role in root heterosis of wheat and other cereal crops (Wang et al., 2006). In a recent molecular study of heterosis, it was speculated that up-regulation of TaARF, an open reading frame (ORF) encoding a putative wheat ARF protein, might be contributing to heterosis observed in wheat root and leaf growth (Yao et al., 2009).
Root anatomy in three dimensions in different doses of 1RS arms
There is a large void in root research involving the study of root anatomy in wheat as well as other cereal crops. Most of the anatomical literature is either limited to root anatomy near the base of the root (Aloni and Griffith, 1991; Watt et al., 2008) or near the root tip (Huang et al., 1991) in young seedlings (Richards and Passioura, 1989). There is still a general lack of knowledge about the overall structure and pattern of whole root vasculature during the later stages of growth in cereals, especially in wheat. Recently, a study was conducted on root anatomy of various classes of winter wheat, barley, and triticale roots, including primary axile (tap) roots, scutellar, coleoptile, and leaf node axile roots, as well as branch roots of various orders (Watt et al., 2008). These authors sampled axile (tap) roots at 3–5 cm from the base of young plants grown in the greenhouse and other branch roots from field-grown plants at flowering.
In the present study, root anatomical traits were studied in the tap roots of different spring wheat genotypes containing different dosages of 1RS translocation arms harvested during the late-tillering stage. Root sections were made from three regions along the length of the root, namely top of the root, middle of the root, and root tip, to get an overview of the complete structure and pattern of root anatomy relative to differences in 1RS dosage. A comparison of the different regions of the root of a genotype showed a transition for CMX vessel number and CMX area from higher in the top region of the root to a single CMX vessel in the root tip. In barley, a similar pattern was reported only in the nodal roots retaining the central pith (Luxová, 1989, 1991), but the author also observed a few instances of more than one CMX vessel in the base of the seminal (tap) root. Watt et al. (2008) reported one to three CMX vessels in the primary axile (tap) roots of hexaploid winter wheat and tritcale (Triticale hexaploid). The variation that was found along a single tap root of spring wheat spans the differences between different root types in winter wheat observed by Watt et al. (2008). In the root tip only CMX vessel diameter and area were traceable as other cell types were still differentiating. This developmental pattern was consistent across the different wheat genotypes used in this study. Interestingly, there was variation in timing for the transitions in root anatomy among genotypes and this variation was explained by dosage of the 1RS arm in bread wheat. RD2 and RAD4 transitioned earlier, from having multiple metaxylem vessels and a larger stele to a single, CMX vessel and smaller stele, than did R0 and RA1.
In the top region, all the root traits were significantly different among genotypes except average CMX vessel diameter and CMX vessel number (Fig. 4A–F). The average CMX diameter was calculated from the average of diameters of all the CMX number of that subsequent genotype and, hence, the number of CMX vessels was different in each genotype and so was the total CMX vessel area. Interestingly, all the root traits in the top region showed negative slope in regression analysis and most of them were significant, especially stele diameter, total CMX vessel area, and peripheral xylem pole number. Variation in all the traits was explained by number of 1RS dosages in wheat genotypes and root anatomical traits were smaller with a higher number of 1RS dosages (Fig. 2A–E). Significant positive correlation among almost all the root traits from the top region and mid-region of the roots (except CMX vessel number) suggested their inter-dependences in growth and development. Root diameter could not be measured for all the replicates of each genotype because of the degeneration and mechanical damage to the cortex and epidermis. Earlier, a study on the rate of cortical death in seminal (tap) roots was investigated in different cereals. It was found that rate of cortical death was faster in hexaploid wheat and was positively associated with root age (Liljeroth, 1995). Our results suggest there are genes on the 1RS arm that affect xylem anatomy which is further affected by 1RS dosage.
Structural adaptation to stress
Root morphology and root architecture are responsible for both water and nutrient uptake while in root anatomy, xylem vessels are essential for their transportation to the shoots to allow continued photosynthesis and growth. Variations in xylem anatomy and hydraulic properties occur at interspecific, intraspecific, and intraplant levels (Zimmermann, 1983; Sperry and Saliendra, 1994; Jackson et al., 2000). Variations in xylem vessel diameter can drastically affect the axial flow because of the fourth-power relationship between radius and flow rate through a capillary tube, as described by the Hagen–Poiseuille law (Zimmermann, 1983; Tyree and Ewers, 1991). Thus, even a small increase in mean vessel diameter would have exponential effects on specific hydraulic conductivity (Ks) for the same pressure difference across a segment (McElrone et al., 2004). Xylem diameters tend to be narrower in drought-tolerant genotypes (Ou et al., 2005; Yang et al., 2007), and at higher temperature (Huang et al., 1991). Smaller xylem diameters pose higher flow resistance and slower water flow which may help the wheat plant to survive water-stressed conditions. Richards and Passioura (1989) reported increased grain yield of two Australian wheat cultivars by selecting for narrow xylem vessels in seminal (tap) roots. Reduced water flow in seminal axile (tap) roots may be most important at the early stages of plant development since they are the first to emerge and seedling survival is often a critical stage, especially in rainfed environments, where seedling survival solely depends on the available sub-soil moisture.
A model for water and nitrogen capture in cereal root systems predicted greater economic returns from crops with greater partitioning of dry matter to the roots (King et al., 2003). The results in this study generally showed more allocation of dry matter to roots in the 1RS genotypes. It was observed that partitioning of dry matter to roots was incremental with an increase in dosage of the 1RS arm excluding the F1 hybrid genotype (data not shown). The non-significant difference in R/S ratio between RD2 (=0.31) and R0 (=0.33) may be due to the poor performance of RD2 during the 2008 season. Ehdaie et al. (2003) observed a better R/S ratio in RD2 genotype than Pavon 76 (R0) in a greenhouse experiment grown to maturity. These authors also studied resource capture involving genotypes RA2 and RD2 which showed better water use efficiency (Ehdaie et al., 2003) and more nutrient uptake with less leachate concentration than check R0 (B Ehdaie and JG Waines, unpublished data). These authors reported a larger and well-branched root system in 1RS wheats which is further positively correlated with an increase in grain yield and its components (B Ehdaie and JG Waines, unpublished data). The model for resource capture (King et al., 2003) also predicted maximum returns from crops with smaller specific root weight, i.e. finer roots. It was suggested to be advantageous for the plants to invest in more surface area available for resource capture at lower depths.
The results of this study also showed that the presence of 1RS arm in bread wheat increased root biomass (Fig. 1; Table 1) and reduced the dimensions of some root parameters, especially the CMX vessel area and diameter in the root tips (Fig. 2A–E, Fig. 5A, B) as well as in the top of the root (Figs 2K–O, 4 B, D). Future experiments warrant inclusion of traits such as root number, extensiveness of root branching, and root surface area along with resource capture studies to address the effect of 1RS wheats. The present study also suggests the possibility of different genes present on the 1RS arm affecting root biomass and anatomy in wheat as dosage of the 1RS arm affects these traits differently. Manske and Vlek (2002) reported that wheat genotypes with the 1RS translocated chromosome arm had thinner roots and higher root-length density compared with normal wheat with a 1BS chromosome arm under field conditions. Similar differences were also observed for root length density, diameters of stele and CMX vessel between winter wheat and triticale (genome formula=AABBRR) (Watt et al., 2008). Among 1RS translocation wheats, significant association was observed between root biomass and grain yield under well-watered and droughted environments (Ehdaie et al., 2003). Narrow metaxylem vessels and higher root biomass may provide 1RS translocation wheats with better adaptability to water stress and make them better performers for grain yield.
Conclusion
In a recent study on rice root anatomy, QTL for metaxylem vessels were identified on the distal end of the long arm of chromosome 10 of rice (Uga et al., 2008) which showed synteny to the 1RS chromosome arm of rye (Hackauf et al., 2009). These reports also support the present findings and may validate the purpose of the current study on wheat root anatomy. This study suggests that the short arm of chromosome 1 of rye carries important genes affecting root morphology and root anatomy in bread wheat. The 1RS arm showed heterotic effect and significant dosage effect on root biomass and root anatomy in bread wheat. Thus, higher root biomass and narrow metaxylem vessels in 1RS wheat strongly advocate their inclusion as a selection criterion in wheat breeding programmes.
Acknowledgments
This research was supported by United States Department of Agriculture–New Mexico State Southwest Consortium on Plant Genetics and Water Resources, New Mexico, USA Project No. 064N09/07R09. SS would like to thank Dr David Carter for providing technical help for microscopy and useful discussions.
References
- Aloni R, Griffith M. Functional xylem anatomy in root-shoot junctions of six cereal species. Planta. 1991;184:123–129. doi: 10.1007/BF00208245. [DOI] [PubMed] [Google Scholar]
- Barlow PW. The cellular organization of roots and its response to the physical environment. In: Gregory PJ, Lake JV, Rose DA, editors. Root development and function. Cambridge: Cambridge University Press; 1987. pp. 1–26. [Google Scholar]
- Böhm W. 1979. Methods of studying root systems. Ecological Studies Vol. 33. Wallingford: CAB International. [Google Scholar]
- Bowen GD, Rovira AD. Recent advances in plant nutrition. Vol. 1. New York: Gordon and Breach Science Publishers Inc. USA; 1971. Relationship between root morphology and nutrient uptake; pp. 293–305. [Google Scholar]
- Ehdaie B, Whitkus RW, Waines JG. Root biomass, water-use efficiency, and performance of wheat-rye translocations of chromosomes 1 and 2 in spring bread wheat ‘Pavon’. Crop Science. 2003;43:710–717. [Google Scholar]
- Ford KE, Gregory PJ, Gooding MJ, Pepler S. Genotype and fungicide effects on late-season root growth of winter wheat. Plant and Soil. 2006;284:33–44. [Google Scholar]
- Fukai S, Cooper M. Development of drought-resistant cultivars using physio-morphological traits in rice. Field Crops Research. 1995;40:67–86. [Google Scholar]
- Hackauf B, Rudd S, Voort JRvd, Miedaner T, Wehling P. Comparative mapping of DNA sequences in rye (Secale cereale L.) in relation to the rice genome. Theoretical and Applied Genetics. 2009;118:371–384. doi: 10.1007/s00122-008-0906-0. [DOI] [PubMed] [Google Scholar]
- Hackett C. A study of the root system of barley. 1. Effects of nutrition on two varieties. New Phytologist. 1968;67:287–299. [Google Scholar]
- Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W. The exodermis: a variable apoplastic barrier. Journal of Experimental Botany. 2001;52:2245–2264. doi: 10.1093/jexbot/52.365.2245. [DOI] [PubMed] [Google Scholar]
- Huang B. Wheat root morphology, root anatomy, and hydraulic conductivity as affected by temperature. Dissertation Abstracts International. B, Sciences and Engineering. 1991;52:2358B. [Google Scholar]
- Huang BR, Taylor HM. Morphological development and anatomical features of wheat seedlings as influenced by temperature and seeding depth. Crop Science. 1993;33:1269–1273. [Google Scholar]
- Huang BR, Taylor HM, McMichael BL. Effects of temperature on the development of metaxylem in primary wheat roots and its hydraulic consequence. Annals of Botany. 1991;67:163–166. [Google Scholar]
- Jackson RB, Sperry JS, Dawson TE. Root water uptake and transport: using physiological processes in global predictions. Trends in Plant Science. 2000;5:482–488. doi: 10.1016/s1360-1385(00)01766-0. [DOI] [PubMed] [Google Scholar]
- Jupp AP, Newman EI. Morphological and anatomical effects of severe drought on the roots of Lolium perenne L. New Phytologist. 1987;105:393–402. doi: 10.1111/j.1469-8137.1987.tb00876.x. [DOI] [PubMed] [Google Scholar]
- Kattermann G. On constant strains with pubescent stems from wheat–rye crosses with 2n=42 chromosomes. Zeitschrift für induktive Abstammungs- und Vererbungslehre. 1938;74:354–375. [Google Scholar]
- Kim W, Johnson JW, Baenziger PS, Lukaszewski AJ, Gaines CS. Agronomic effect of wheat–rye translocation carrying rye chromatin (1R) from different sources. Crop Science. 2004;44:1254–1258. [Google Scholar]
- King J, Gay A, Sylvester-Bradley R, Bingham I, Foulkes J, Gregory P, Robinson D. Modelling cereal root systems for water and nitrogen capture: towards an economic optimum. Annals of Botany. 2003;91:383–390. doi: 10.1093/aob/mcg033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumlay AM, Baenziger PS, Gill KS, Shelton DR, Graybosch RA, Lukaszewski AJ, Wesenberg DM. Understanding the effect of rye chromatin in bread wheat. Crop Science. 2003;43:1643–1651. [Google Scholar]
- Liljeroth E. Comparisons of early root cortical senescence between barley cultivars, Triticum species and other cereals. New Phytologist. 1995;130:495–501. doi: 10.1111/j.1469-8137.1995.tb04326.x. [DOI] [PubMed] [Google Scholar]
- Lukaszewski AJ. Reconstruction in wheat of complete chromosomes 1B and 1R from the 1RS.1BL translocation of ‘Kavkaz’ origin. Genome. 1993;36:821–824. doi: 10.1139/g93-109. [DOI] [PubMed] [Google Scholar]
- Lukaszewski AJ. Further manipulation by centric misdivision of the 1RS.1BL translocation in wheat. Euphytica. 1997;94:257–261. [Google Scholar]
- Luxová M. The vascular system in the roots of barley and its hydraulic aspects. Structural and functional aspects of transport in roots. 1989 Third international symposium on structure and function of roots, Nitra, Czechoslovakia, 3–7 Aug. 1987, 15–20. [Google Scholar]
- Luxová M. Mechanisms of reduction of the stelar pattern along barley roots. Botanica Acta. 1991;104:163–168. [Google Scholar]
- Manske GGB, Vlek PLG. Root architecture: wheat as a model plant. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots: the hidden half. New York USA: Marcel Dekker Inc.; 2002. pp. 249–259. [Google Scholar]
- McElrone AJ, Pockman WT, Martinez-Vilalta J, Jackson RB. Variation in xylem structure and function in stems and roots of trees to 20 m depth. New Phytologist. 2004;163:507–517. doi: 10.1111/j.1469-8137.2004.01127.x. [DOI] [PubMed] [Google Scholar]
- Mettin D, Bluthner WD, Schlegel G. Additional evidence on spontaneous 1B/1R wheat-rye substitutions and translocations. Proceedings of the fourth international wheat genetics symposium. Alien genetic material. 1973:179–184. [Google Scholar]
- O'Brien L. Genetic variability of root growth in wheat (Triticum aestivum L.) Australian Journal of Agricultural Research. 1979;30:587–595. [Google Scholar]
- Ou Q, Ni J, Ma R. Relationship between root xylem vessels and drought resistance in spring wheat. Journal of Triticeae Crops. 2005;25:27–31. [Google Scholar]
- Owuoche JO, Sears RG, Brown-Guedira GL, Gill BS, Fritz AK. Heterotic effects of wheat–rye chromosomal translocations on agronomic traits of hybrid wheat (Triticum aestivum L.) under an adequate moisture regime. Euphytica. 2003;132:67–77. [Google Scholar]
- Quarrie SA, Jones HG. Effects of abscisic acid and water stress on development and morphology of wheat. Journal of Experimental Botany. 1977;28:192–203. [Google Scholar]
- Rajaram S, Mann CE, Ortiz-Ferrara G, Mujeeb-Kazi A. Adaptation, stability and high yield potential of certain 1B/1R CIMMYT wheats. In: Sakamoto S, editor. Sixth international wheat genetic symposium. Kyoto, Japan: Plant Germ-Plasm Institute; 1983. pp. 613–621. [Google Scholar]
- Richards RA, Passioura JB. A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Australian Journal of Agricultural Research. 1989;40:943–950. [Google Scholar]
- Robertson BM, Waines JG, Gill BS. Genetic variability for seedling root numbers in wild and domesticated wheats. Crop Science. 1979;19:843–847. [Google Scholar]
- Ruzin SE. Staining. In: Ruzin SE, editor. Plant microtechnique and microscopy. New York, NY: Oxford University Press Inc.; 1999. pp. 87–116. [Google Scholar]
- Sharma S, Bhat P, Ehdaie B, Close T, Lukaszewski A, Waines J. Integrated genetic map and genetic analysis of a region associated with root traits on the short arm of rye chromosome 1 in bread wheat. Theoretical and Applied Genetics. 2009;119:783–793. doi: 10.1007/s00122-009-1088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Saliendra NZ. Intra- and inter-plant variation in xylem cavitation in Betula occidentalis. Plant, Cell and Environment. 1994;17:1233–1241. [Google Scholar]
- Steel RGD, Torrie JH, Dickey DA. Principles and procedures of statistics: a biometrical approach. New York, NY USA: McGraw-Hill Inc; 1997. [Google Scholar]
- Terzioglu S, Ekmekci YT. Effects of chilling on seminal root central metaxylem diameter in some wheat (Triticum aestivum L.) cultivars. Turkish Journal of Botany. 1995;19:1–5. [Google Scholar]
- Tyree MT, Ewers FW. Tansley review No. 34. The hydraulic architecture of trees and other woody plants. New Phytologist. 1991;119:345–360. [Google Scholar]
- Uga Y, Okuno K, Yano M. QTLs underlying natural variation in stele and xylem structures of rice root. Breeding Science. 2008;58:7–14. [Google Scholar]
- Villareal RL, Rajaram S, Mujeeb-Kazi A, Toro ED. The effect of chromosome 1B/1R translocation on the yield potential of certain spring wheats (Triticum aestivum L.) Plant Breeding. 1991;106:77–81. [Google Scholar]
- Wang Z, Ni Z, Wu H, Nie X, Sun Q. Heterosis in root development and differential gene expression between hybrids and their parental inbreds in wheat (Triticum aestivum L.) Theoretical and Applied Genetics. 2006;113:1283–1294. doi: 10.1007/s00122-006-0382-3. [DOI] [PubMed] [Google Scholar]
- Watt M, Magee LJ, McCully ME. Types, structure and potential for axial water flow in the deepest roots of field-grown cereals. New Phytologist. 2008;178:135–146. doi: 10.1111/j.1469-8137.2007.02358.x. [DOI] [PubMed] [Google Scholar]
- Weaver EJ. Root habits of rye. New York: McGraw-Hill Book Company Inc; 1926. [Google Scholar]
- Yang X, Zhang S, Liu X, Mu Z. Relationship between roots hydraulic conductivity and root anatomy of winter wheat (T. aestivum) Journal of Northwest A and F University - Natural Science Edition. 2007;35:160–164. [Google Scholar]
- Yao Y, Ni Z, Du J, Han Z, Chen Y, Zhang Q, Sun Q. Ectopic overexpression of wheat adenosine diphosphate-ribosylation factor, TaARF, increases growth rate in Arabidopsis. Journal of Integrative Plant Biology. 2009;51:35–44. doi: 10.1111/j.1744-7909.2008.00792.x. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Hasegawa S. The rice root system: its development and function. Drought resistance in crops with emphasis on rice. 1982:97–114. [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. A decimal code for the growth stages of cereals. Weed Research. 1974;14:415–421. [Google Scholar]
- Zimmermann MH. Xylem structure and the ascent of sap. Berlin German Federal Republic: Springer-Verlag; 1983. [Google Scholar]