Abstract
Woody plants native to mesic habitats tend to be more vulnerable to drought-induced cavitation than those in xeric habitats. Cavitation resistance in herbaceous plants, however, is rarely studied and whether or not annual plants in arid habitats conform to the trends observed in woody plants is unknown. This question is addressed by comparing the hydraulic properties of annual plants endemic to relatively mesic and seasonally xeric habitats in the Great Basin Desert, in both native and experimental settings. Vulnerability to cavitation between species differed as predicted when vulnerability curves of similar-sized native individuals were compared. Contrary to expectations, Helianthus anomalus from the relatively mesic dune sites, on average, exhibited higher native embolism, lower soil-to-leaf hydraulic conductance (kL) and lower transpiration rates, than its xeric analogue, H. deserticola. In transplant gardens, H. anomalus’ vulnerability to cavitation was unaffected by transplant location or watering treatment. In H. deserticola, however, vulnerability to cavitation varied significantly in response to watering in transplant gardens and varied as a function of stem water potential (Ψstem). H. deserticola largely avoided cavitation through its higher water status and generally more resistant xylem, traits consistent with a short life cycle and typical drought-escape strategy. By contrast, H. anomalus’ higher native embolism is likely to be adaptive by lowering plant conductance and transpiration rate, thus preventing the loss of root-to-soil hydraulic contact in the coarse sand dune soils. For H. anomalus this dehydration avoidance strategy is consistent with its relatively long 3–4 month life cycle and low-competition habitat. We conclude that variance of hydraulic parameters in herbaceous plants is a function of soil moisture heterogeneity and is consistent with the notion that trait plasticity to fine-grained environmental variation can be adaptive.
Keywords: Adaptation, arid habitats, safety margin, sand dunes, sunflowers, water potential, xylem cavitation
Introduction
For woody species, the observation that plants from mesic habitats are more vulnerable to drought-induced xylem cavitation than plants from xeric habitats holds at broad spatial scales and in evolutionary frameworks (Alder et al., 1996; Mencuccini and Comstock, 1997; Davis et al., 1999; Kolb and Sperry, 1999; Pockman and Sperry, 2000; Maherali et al., 2004). However, recent work by Jacobsen et al. (2007) showed that aridity does not always predict cavitation resistance in woody shrubs, and while some species in a semi-arid climate had high cavitation resistance, the dominant species in an arid desert community were, on average, less resistant to drought-induced cavitation. In annual and herbaceous plants, drought-induced xylem cavitation is poorly described and whether or not herbaceous annuals from mesic habitats are more vulnerable to cavitation than those from xeric habitats is an open question. This is surprising given that many annual crops are grown in non-irrigated and dryland ecosystems. Of the studies that have examined vulnerability to drought-induced xylem embolism and cavitation in herbaceous species (Milburn and McLaughlin, 1974; Tyree et al., 1986; Neufeld et al., 1992; McCully et al., 1998; Buchard et al., 1999; Stiller and Sperry, 2002; Kocacinar and Sage, 2003; Stiller et al., 2003; Lo Gullo et al., 2004; Li et al., 2009) few have been completed in the field, particularly in arid ecosystems, where water availability varies in space and time.
Because vulnerability to cavitation has a genetic basis, species and population differences in cavitation resistance can be due to adaptive or ecotypic differentiation (Neufeld et al., 1992; Kavanagh et al., 1999; Kolb and Sperry, 1999; Maherali et al., 2004). In addition, gene flow or phenotypic plasticity among populations may alter adaptive or ecotypic differentiation (Maherali et al., 2002). Vulnerability to cavitation is also an environmentally plastic trait such that drought induced stress can temporarily diminish or ‘weaken’ xylem resistance to cavitation (i.e. a rapid loss of xylem conductivity at relatively high water potentials). This ‘cavitation fatigue’ phenomenon, which has been demonstrated in a few genera including Helianthus (Hacke et al., 2001), complicates interpretations of field-based measures of cavitation resistance. Evidence suggests that plants may take several days to regain previous cavitation resistance levels following drought stress (Stiller and Sperry, 2002). The implication is that, in desert habitats which have spatially and temporally heterogeneous moisture availability, vulnerability to cavitation could change seasonally for a given individual. Recently, Jacobsen et al. (2007) demonstrated that cavitation resistance changed in ‘wet’ versus ‘dry” seasons in several woody species in the Chaparral, Coastal scrub, and Mojave Desert scrub habitats. Therefore both genetic and environmental effects need to be considered when comparing vulnerability to cavitation within and among species.
In order to clarify how variation in water availability affects drought-induced cavitation in herbaceous taxa, two species of wild desert sunflowers, Helianthus anomalus and Helianthus deserticola, were studied. They are an excellent species pair to investigate the relationship between hydraulic parameters and habitat water status in desert annuals because they are closely related (Rieseberg, 1991; Rieseberg et al., 1991) and can be found in adjacent but divergent habitats that are well characterized (Schwarzbach et al., 2001; Rosenthal et al., 2005; Donovan et al., 2007). The species are locally endemic to active sand dunes (H. anomalus) and the adjacent stabilized dunes and desert floor (H. deserticola) in the Great Basin Desert, USA. Helianthus anomalus is relatively long-lived for a desert annual, germinating in the early spring and remaining active until the first frost in late autumn, presumably an adaptation to the relatively mesic dune habitat. However, in the stabilized off-dune habitat, plant water availability decreases rapidly during summer droughts (Rosenthal et al., 2005) and H. deserticola completes its life cycle within 2 months.
The sand dunes habitat of H. anomalus has significantly coarser textured soils than the adjacent stabilized dunes where H. deserticola thrives (Rosenthal et al., 2005). Soil texture is relevant here since hydraulic failure can occur in the soil before xylem cavitation, either due to coarse-textured soil or to the low surface area of the absorbing roots (Sperry et al., 1998; Hacke et al., 2000). Therefore, edaphic differences between these habitats may differentially affect soil and plant water status (Rosenthal et al., 2005) and this, in turn, would affect plant hydraulic properties. In general, the dune habitat of H. anomalus is considered to be relatively mesic for the entire growing season since soil moisture is available to plants throughout the summer provided their roots are deep enough (Rosenthal et al., 2005). In addition, nutrient availability is also significantly lower in H. anomalus (Ludwig et al., 2006). While the species in our study have identical hybrid origins, different suites of traits have facilitated ecological transitions in these sunflowers (Rieseberg et al., 2003) and it is unknown how hydraulic parameters may contribute to the ecological differentiation.
In the present study, the hypothesis that the ‘on-dune’ species H. anomalus is more vulnerable to drought-induced xylem cavitation, than the ‘off-dune’ species H. deserticola was tested. This was done by measuring vulnerability to cavitation, native embolism, transpiration rate (E), predawn (Ψpd) and midday (Ψmd) water potential, soil-to-leaf hydraulic conductance (kL), and related hydraulic parameters in native plants during two summer growth seasons. In a second experiment, seedlings of each species were removed from native populations and reciprocally transplanted into common gardens in each of their respective habitats. Within each transplant garden, half the plants received additional water. It was hypothesized that resistance to cavitation would vary in response to water availability. If variance in vulnerability to cavitation for either species is an environmentally plastic trait driven by water availability then cavitation was expected to differ in watered versus unwatered treatments.
Materials and methods
Study site
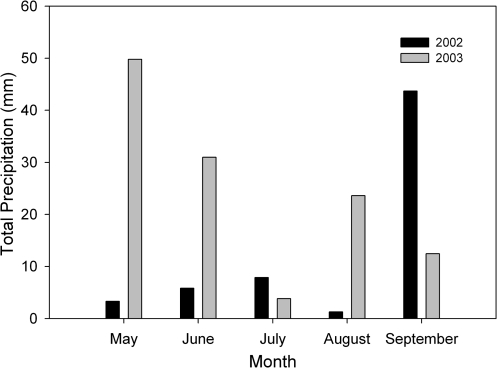
The study was conducted in the Little Sahara Recreation Area, Juab County, Utah (latitude 39o44' N, longitude 112o18' W). The climate is typical of the Great Basin Desert with the majority of precipitation occurring as snow or rain during the cold winter and cool spring months. The summers are characterized by a significant drought usually beginning in June and July. Mean annual precipitation is 312 mm, making this an arid zone according to the UNESCO (1977) classification. During the two years preceding the common garden study, annual precipitation was well below average (2002, 110 mm; 2001, 191 mm) compared to 317 mm in an average year (Rosenthal et al., 2005), reflecting a significant long-term drought in the area. Annual precipitation was slightly below average (287 mm) in 2003, the year of the common garden study, with 20% (58 mm) falling during the summer study period (June-August) (Fig. 1).
Fig. 1.
Mean monthly precipitation at Little Sahara Sand Dunes for May through September of 2002 and 2003.
The dominant vegetation at this site has been described previously (Rosenthal et al., 2005). Briefly, cover is significantly lower on-dunes (12.3%) than off-dunes (67.8%) the most common species on dunes are Psoralidium lanceolatum (Pursh) Rydb. (Dune scurfpea), Salsola tragus L. (Russian thistle), and Achnatherum hymenoides (Roem. & Schult.) Barkworth (Indian ricegrass). Common taxa off the dunes are Bromus tectorum, Artemisia tridentata var. tridentata, and several Agropyron sp. The proportion of soil covered by litter is also far lower on-dunes than off (6.4% versus 45.5%, respectively). The vast majority of off-dune litter was identifiable as B. tectorum (Rosenthal et al., 2005).
Sampling of native plants
In the first year of our study, native plants of both species were collected simultaneously on two dates (July 2002 and August 2002). In the second year, plants of similar sizes and growth stages (i.e. at anthesis) were collected on two separate dates. H. deserticola plants were collected in July 2003 and H. anomalus plants were collected in August 2003 to coincide with measurements taken in the reciprocal transplant gardens as described below.
Reciprocal transplant experiment
Reciprocal transplant gardens were established in the H. anomalus and H. deserticola habitats in the Little Sahara Recreation Area, Juab County, UT, USA (latitude 39°44' N, longitude 112°18' W) (hereafter referred to as ‘on-dune’ and ‘off-dune’, respectively). One-hundred-and-twenty-five H. anomalus and H. deserticola seedlings were collected between 25 May and 27 May 2003 from naturally occurring populations at Little Sahara Sand Dunes. Individual plants were placed in temporary pots and 48 seedlings per species were randomly selected for the experiment. Half of the selected seedlings from each species were planted in the on-dune garden and off-dune garden, respectively. Within each transplant garden there were two irrigation treatments, watered (+H2O) and unwatered (NO H2O). The +H2O and NO H2O plants were in separate plots so that water additions would not affect control plants. To account for any heterogeneity within treatments, 2 blocks were nested within each treatment plot.
All plants were watered every other day for 14 d following the transplant. Previous studies indicated that this initial period of heavy watering would be necessary to minimize transplanting shock and facilitate seedling establishment (Ludwig et al., 2004). Four weeks after transplanting, the +H2O plants were supplemented with water equivalent to 33 mm of precipitation. Since it was not possible to know in advance what precipitation would occur, the average precipitation expected for that seasonal period (ten year average=35 mm) was added. The actual precipitation during the study was 22.4 mm. Therefore +H2O plants received 55.4 mm precipitation (about 20.4 mm more than the average for that period), which was administered four times at two-week intervals.
Helianthus deserticola blooms, sets seed, and frequently senesces before H. anomalus begins flowering. In order to compare cavitation resistance at similar growth stages in the experimental garden plants, H. deserticola was harvested and measured on 17 July and H. anomalus on 28 August. At these dates, both plants had terminated height growth and had initiated flowering.
Cavitation resistance and native embolism measurements
Vulnerability to xylem cavitation was quantified from curves based on the relationship between xylem pressure and the percentage loss of hydraulic conductivity (PLC) (Sperry et al., 1988; Alder et al., 1997). Hydraulic conductivity of stem segments was measured using a modification of the method of Sperry et al. (1988) from the flow rate of deionized and filtered (0.2 μm) water onto an electronic balance (BA210S, Sartorius, Goettingen, Germany). Whole plants were collected in the field, immediately sealed into humid plastic bags to minimize desiccation, and placed in coolers for the 2 h journey to the laboratory at the University of Utah. Several studies have shown that this treatment neither affects the level of native embolism nor the plants’ vulnerability to xylem cavitation. In the laboratory, 0.14 m stem segments were cut from the main stem of each plant underwater and then the native hydraulic conductivity (knative) of each stem was measured using a pressure head of 4–6 kPa. The stems were flushed at 100 kPa for 45 min to refill air-filled conduits to determine the stem maximum hydraulic conductivity (kmax). Native embolism or native per cent loss of conductivity (PLC native) is given by:
Once knative and kmax were known, the stem segments were mounted in custom-built centrifuge rotors and spun for 4 min in a Sorval RC5C centrifuge to generate xylem embolisms. Xylem pressure is a function of the angular velocity and the distance from the centre of rotation to the stem ends, with the lowest pressures at the centre of the stem (Alder et al., 1997). After spinning, stem hydraulic conductivity was remeasured. This process was repeated several times, spinning at incrementally higher speeds. Vulnerability curves were then derived from the plots of PLC versus xylem pressure.
Differences in cavitation resistance are frequently presented as the pressure required to cause a 50% loss of hydraulic conductivity (P50). However, sunflowers have been shown to suffer from ‘cavitation fatigue’ (Hacke et al., 2001; Stiller and Sperry, 2002) and minor xylem tensions of only –1.0 MPa can cause a dramatic decrease in hydraulic conductivity. This shift is particularly prominent at the less negative (–1 or greater MPa) pressure end of the vulnerability curve. In order to correct vulnerability curves for cavitation fatigue it is not sufficient to use the conductivity at –0.5 MPa as the kmax. Therefore, to avoid confounding the effects of xylem weakening with inherent cavitation resistance, cavitation resistance at 75 PLC (P75) was compared as well as at 50 PLC (P50) (Sperry and Hacke, 2002).
Water relations and gas exchange measurements
Prior to harvesting plants for hydraulic measurements, plant predawn (Ψpd) and midday (Ψmd) water potentials were determined with a pressure chamber (PMS instruments, Corvallis, Oregon, USA). Only fully expanded, mature, non-senescent leaves were used. Although soil Ψ and plant Ψpd may not equilibrate for some plants (Donovan et al., 2001, 2003), night-time transpiration is similar and relatively low in H. anomalus and H. deserticola (Howard and Donovan, 2007). Therefore any deviation from equilibrium with soil Ψ would have small and similar effects on plant Ψ. Immediately following or preceding Ψmd, transpiration rate (E) was measured on mature non-senescent leaves using a Li-Cor 6400 portable photosynthesis system (CO2 concentration 360 ppm, ambient air temperature, photosynthetically active radiation 1800 μmol m−2 s−1). Transpiration measurements were done on clear days between 11.00 h and 13.00 h at similar air temperatures and VPD. Prior to measurement, ambient relative humidity and temperature were recorded and chambers were set to mimic ambient conditions.
Soil-to-leaf hydraulic conductance (kL) was calculated using the following equation:
where kL is defined by the ratio between flow rate (i.e. transpiration) and the ‘driving force’ (Kolb and Davis, 1994) defined by the difference between soil and leaf water potential. Field stem xylem pressure (Ψstem) was estimated as the midpoint between (Ψpd) and (Ψmd):
which corresponds to the xylem pressure at the midpoint of the soil-to-leaf continuum (Linton et al., 1998) and is considered a better estimate of the xylem pressure in the shoots used to measure cavitation resistance.
After determining Ψstem, the safety margin against hydraulic failure was calculated. Plant Ψcrit is defined here as a conservative estimate of the minimum Ψstem allowing xylem transport (see also Pockman and Sperry, 2000). If Ψstem were to reach Ψcrit, all hydraulic transport would cease (i.e. 100% loss of hydraulic conductivity). The difference between the Ψmin and Ψcrit is considered the margin of safety against hydraulic failure (Sperry et al., 1998). The safety margin for mesic species is generally small because water availability is usually high and predictable in those habitats. This margin is wider for species in arid habitats, presumably allowing plants to extract soil water at lower water potentials (Sperry, 1995). Ψ at P75 was conservatively selected as Ψcrit and Ψmd as Ψmin. The safety margin (Ψmargin) was calculated as the difference between Ψcrit and Ψmd.
Sampling and statistical methods
The pressure causing 50% and 75% loss in xylem conductivity (P50 and P75, respectively) was estimated for native and experimental populations by fitting a Weibull function to loss of hydraulic conductivity versus xylem tension for each individual stem (Neufeld et al., 1992). Both, P75 and P50 were estimated since the former is less likely to be affected by drought-induced cavitation fatigue than the latter (Sperry and Hacke, 2002).
To test for overall differences in vulnerability curves between species in the native habitat, the response of per cent loss of stem conductivity (PLC) to change in xylem pressure (MPa) was modelled using a repeated measures analysis of variance (SAS, PROC GLM) with species as a fixed effect and xylem pressure as the repeated effect. For the reciprocal transplant experiment the ANOVA was done by species as we were primarily interested in the species response to watering, treatment (i.e. +H2O versus NO H2O) and habitat (i.e. on-dune and off-dune) were treated as fixed effects, and block nested within habitat was treated as a random effect (SAS, PROC MIXED). To compare between species across all experiments species summary statistics were generated and tested for significance of species level differences by comparing the measured values of Ψpd, Ψmd , Ψstem, E, kL,native embolism, P50, P75, and Ψmargin between species with a MANOVA (PROC GLM option MANOVA).
Results
Native plants
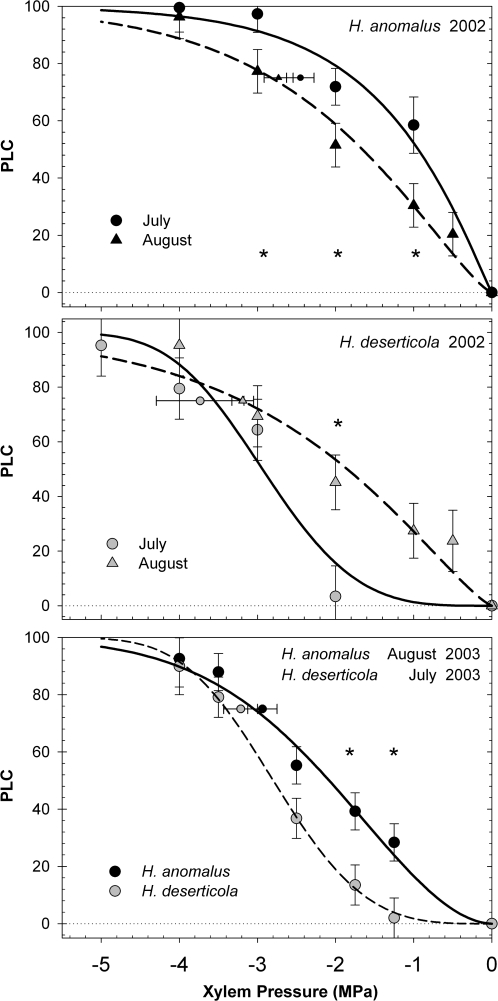
In 2002, vulnerability curves were measured for both species in July and then again in August to assess seasonal variation. Within each species, entire vulnerability curves differed between sampling dates in 2002 when compared using repeated measures ANOVA (PROC MIXED, (H. deserticola F1,37=10.6, P <0.01; H. anomalus; F1,29=5.38, P <0.05) (Fig. 2). Within sampling dates in 2002, species vulnerability curves differed significantly in July (F1,26=20.86, P <0.001) but not August. In 2003, native plant vulnerability curves differed significantly (F1,48=12.01, P <0.005) (Fig. 2), but these were sampled at different times. Curves did not differ between years for a species, based on a comparison of the August data for H. anomalus and the July data for H. deserticola. The P75 was also less negative for H. anomalus than H. deserticola, but only significantly so in 2002. Overall, H. deserticola exhibited greater variability and range in susceptibility to xylem cavitation than did H. anomalus (Table 1). H. anomalus tended to be more vulnerable to cavitation and its water potential dropped much more during the summer (Ψmd below –2 MPa) than H. deserticola (Ψmd near –1.5 MPa; Fig. 3). Consequently, H. anomalus developed higher native embolism values at the end-of-summer August sampling dates in 2002 (39.2±8.9%) than H. deserticola (19.0±12.0%).
Fig. 2.
Percent loss of hydraulic conductivity (PLC) as a function of xylem pressure for H. anomalus and H. deserticola plants collected in native habitats in 2002 and 2003 (Experiment 1). Sampling dates in 2003 were determined by the plants’ growth stage (July for H. deserticola and August for H. anomalus). Larger symbols (n=4–7 stems at each xylem pressure ±SE) are adjusted means from repeated measure ANOVA. Smaller symbols with horizontal error bars represent mean P75 (+SE) as estimated from Weibull curves for each stem. Curves are Weibull functions fitted to the entire data set of each species at that time point. Asterisks denote means that are significantly different following post hoc tests.
Table 1.
Mean and range of xylem pressure at 75% loss of hydraulic conductivity (P75)
| Species (year) | n | Mean | Standard error | Minimum | Maximum | Variance |
| 2002 (*) | ||||||
| H. anomalus | 9 | –2.663 | 0.133 | –3.45 | –2.15 | 0.142 |
| H. deserticola | 9 | –3.421 | 0.250 | –4.80 | –2.90 | 0.438 |
| 2003 (ns) | ||||||
| H. anomalus | 8 | –2.838 | 0.189 | –3.65 | –2.15 | 0.287 |
| H. deserticola | 12 | –2.817 | 0.173 | –3.90 | –2.00 | 0.359 |
P75 was estimated for each individual then pooled for all native H. anomalus and H. deserticola collected in 2002 and 2003. Species means differ significantly in 2002 but are not significant in 2003. Note that native plants were sample at the same time in 2002 but not in 2003. See Materials and methods for details.
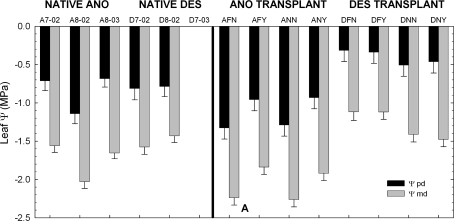
Fig. 3.
Summary of leaf Ψ for H. anomalus and H. deserticola. Data for native plants Experiment (1), and the two transplant garden experiments (Experiment 2) are separated by a solid black line. The first letter of all abbreviations refer to species (H. anomalus=A and H. deserticola=D) and for Experiment 1 the month and year are noted. For Experiment 2, the second and third letters refer to treatments: off-dune and NO H2O (=FN); off-dune and +H2O (=FY); on-dune and +H2O (=NY); on-dune and NO H2O (=NN). Bars are means (n=4–7 for Experiment (1) and n=4 for Experiment (2) ±SE). Standard errors are based on a pooled estimate of the error variance for a one-way ANOVA. Note that H. deserticola transplant data were collected in July 2003 and H. anomalus transplant data were collected in Augusr 2003.
Reciprocal transplants
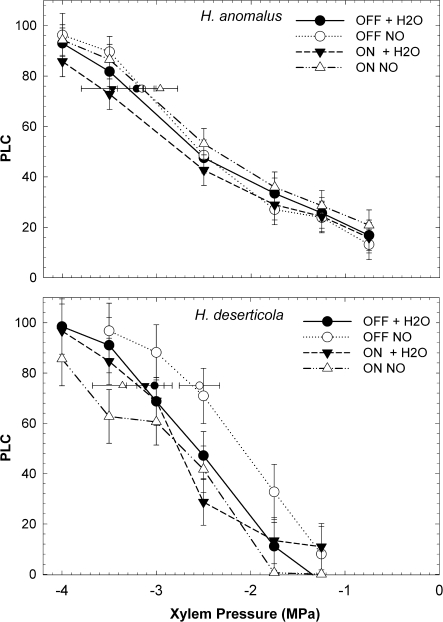
Helianthus anomalus cavitation resistance in experimental gardens did not differ significantly by garden (on-dune and off-dune), treatment (+H2O or No H2O) or their interaction when entire vulnerability curves were compared by repeated measures ANOVA (Table 2; Fig. 4). Nor were there any differences in P75. However, on-dune and off-dune, H. anomalus +H2O (Fig. 3; ANY and AFY) had significantly greater Ψpd and Ψmd than H. anomalus No H2O (ANN and AFN) indicating their water status improved with additional watering (Table 3; Fig. 3).
Table 2.
Repeated measures analysis of variance for the effects of habitat (on-dune and off-dune) and treatments (+H2O and NO H2O) and xylem pressure (MPa) on per cent loss of hydraulic conductivity (PLC) in common garden transplants of H. anomalus and H deserticola
| Effect | Ndf |
H. anomalus |
H. deserticola |
||||
| Ddf | F | P | Ddf | F | P | ||
| Habitat | 1 | 70 | 0.06 | 0.8082 | 61 | 12.09 | 0.0009 |
| Treatment | 1 | 70 | 2.63 | 0.109 | 61 | 0.36 | 0.5505 |
| Habitat×Treatment | 1 | 70 | 2.57 | 0.1135 | 61 | 7.56 | 0.0078 |
| MPa | 5 | 70 | 99.08 | <0.0001 | 61 | 57.64 | <0.0001 |
| MPa×Garden | 5 | 70 | 0.39 | 0.8555 | 61 | 0.66 | 0.6515 |
| MPa×Treatment | 5 | 70 | 0.46 | 0.8019 | 61 | 0.88 | 0.5019 |
| MPA×Garden×Treatment | 5 | 70 | 0.06 | 0.9974 | 61 | 0.22 | 0.9523 |
Note that H. deserticola plants were sampled in July and H. anomalus plants were sampled in August 2003.
Fig. 4.
Percent loss of hydraulic conductivity as a function of xylem pressure for H. anomalus and H. deserticola in Experiment (2) in on-dune (circles) and off-dune gardens (triangles) subjected to additional water (+H2O) or no additional water (NO H2O). Curve data were collected in July 2003 for H. deserticola and in August 2003 for H. anomalus when plants were at a comparable growth stage. Smaller symbol with horizontal error bars represent mean P75 (+SE) as estimated from Weibull curves for each stem.
Table 3.
ANOVA results for leaf predawn (Ψpd) and midday (Ψmd) water potential for the same plants that were used to generate vulnerability curves in the common garden 2003 (see Fig. 2)
| H. anomalus | Ψpd |
Ψmd_ |
||||
| df | F | P | df | F | P | |
| Habitat | 1 | 0.17 | 0.69 | 1 | 0.72 | 0.42 |
| Treatment | 1 | 23.28 | <0.005 | 1 | 35.31 | <0.001 |
| Habitat×Treatment | 1 | 0.01 | 0.94 | 1 | 0.2 | 0.67 |
| Block (Habitat×Treatment) | 4 | 6.9 | <0.05 | 4 | 8.23 | <0.01 |
| Error | 8 | 8 | ||||
| H. deserticola | Ψpd |
Ψmd_ |
||||
| df | F | P | df | F | P | |
| Habitat | 1 | 18.08 | <0.005 | 1 | 11.4 | <.01 |
| Treatment | 1 | 0.53 | 0.49 | 1 | 0.40 | 0.55 |
| Habitat×Treatment | 1 | 0.1 | 0.76 | 1 | 1.72 | 0.23 |
| Block (Habitat×Treatment) | 4 | 0.34 | 0.84 | 4 | 1.30 | 0.36 |
| Error | 7 | 7 | ||||
H. deserticola individuals were sampled in July and H. anomalus in August, habitat (on-dune versus off-dune) and treatments (+H2O and NO H2O)
In contrast to the low variability in cavitation resistance for H. anomalus in the gardens, H. deserticola's resistance to cavitation in transplant gardens differed significantly between on-dune and off-dune gardens based on whole-curve ANOVA comparisons and P75 data. The magnitude of the difference varied depending on watering treatment (Table 2; Fig. 4). In the off-dune transplant garden H. deserticola was significantly less vulnerable to cavitation only when watered (Fig. 4) and both Ψpd and Ψmd were significantly greater in the off-dune gardens (Table 3; Fig. 3).
Species trends
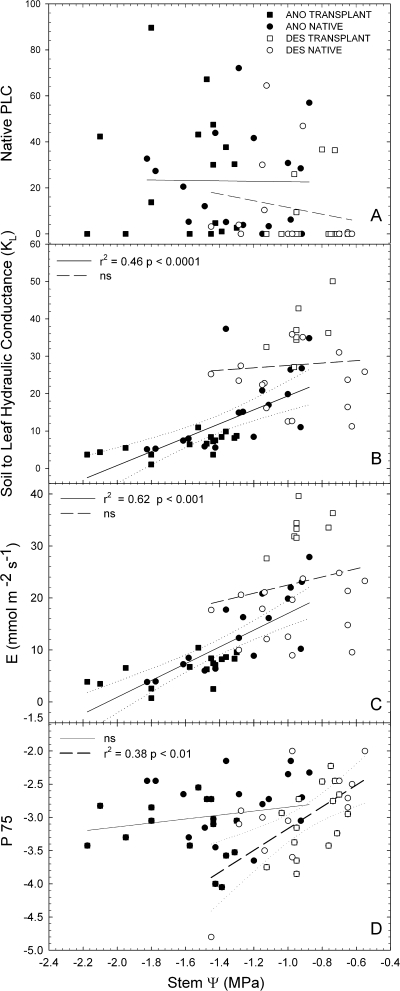
To compare species' hydraulic parameters response to plant water status in the broadest sense, data from native and experimental garden plants were combined and compared to Ψstem (i.e. [(Ψpd +Ψmd)/2], Fig. 5). Overall, H. anomalus developed significantly lower water potentials and higher native embolism than H. deserticola (Fig. 5A; Table 4). Consequently, H. anomalus had, on average, an approximately three times lower soil-to-leaf hydraulic conductance (kL) than H. deserticola and a similarly lower average transpiration rate (Fig. 5B, C; Table 4).
Fig. 5.
Native embolism (PLC) (A), soil-to-leaf hydraulic conductance (B), transpiration (C), and P75 (D) for native and transplanted H. anomalus (black symbols, solid lines) and H. deserticola (white symbols, dashed lines) as a function of stem Ψ. Symbols are values for individual plants and 95% confidence intervals are only shown for regressions that are significant.
Table 4.
Species summary statistics of hydraulic parameters measured in both native and field-grown H. anomalus and H. deserticola
| Variables |
H. anomalus |
H. deserticola |
df | P | ||
| Mean | SE | Mean | SE | |||
| Ψpd (MPa) | –0.97 | 0.052 | –0.54 | 0.06 | 1,52 | <0.0001 |
| Ψmd (MPa) | –1.90 | 0.05 | –1.37 | 0.06 | 1,52 | <0.0001 |
| Stem Ψ (MPa) | –1.44 | 0.05 | –0.96 | 0.06 | 1,52 | <0.0001 |
| E (mmol m−2 s−1) | 10.06 | 1.170 | 24.27 | 2.428 | 1,48 | <0.0001 |
| kL (mmol m−2 s−1 MPa−1) | 11.33 | 1.560 | 30.36 | 2.311 | 1,48 | <0.0001 |
| Native embolism (%) | 24.24 | 3.69 | 10.16 | 3.87 | 1,52 | <0.01 |
| P50 (MPa) | –2.16 | 0.10 | –2.67 | 0.10 | 1,52 | <0.01 |
| P75 (MPa) | –2.98 | 0.09 | –3.17 | 0.10 | 1,52 | ns |
| Ψ margin (MPa) | –1.08 | 0.09 | –1.79 | 0.11 | 1,52 | <0.0001 |
Several H. deserticola individuals had missing data for E and kL (see Fig. 3). Means and standard errors are from ANOVAs. Significant differences between species were calculated with a MANOVA testing for the effect of species for all variables.
The P75 exhibited a significant relationship with Ψstem in H. deserticola, such that plants exposed to more negative xylem pressure tended to be more resistant to cavitation. There was no correlation between Ψstem and P75 in H. anomalus in spite of its much wider range of Ψstem (Fig. 5D). When all native and experimental plants were pooled, the mean P50 was significantly higher (more vulnerable) in H. anomalus (2.16±0.1 versus 2.67±0.1 MPa; P <0.01) than in H. deserticola (Table 4). Differences in P75 followed similar trends with H. anomalus being more vulnerable than H. deserticola, but these differences were not statistically significant (2.98±0.09 versus 3.17±0.1; P >0.05).
Discussion
In support of our hypothesis native H. anomalus plants were significantly more vulnerable to cavitation than H. deserticola when similar-sized native plants were sampled early and late in the growth season of 2002. This trend held for P50 and P75 but was not significant for the latter when all plants were pooled. Helianthus anomalus plants have a smaller margin of safety (i.e. P75–Ψmd) than H. deserticola (1.08 MPa versus 1.79 MPa) consistent with the notion that the active on-dune habitat is mesic relative to off-dune habitats (Rosenthal et al., 2005). A wider safety margin is more typical of xeric species as a hedge against variable soil moisture (Sperry, 1995). By contrast, and contrary to our expectations, H. anomalus experienced more than twice the native embolism of H. deserticola (25% versus 10%) which caused H. anomalus to operate at lower Ψ, and to have roughly a three times lower soil-to-leaf hydraulic conductance than H. deserticola. This was especially true when the native embolism of native plants was compared at similar times in 2002 (39.2±8.9% versus 19.0±12.0%) for H. anomalus and H. deserticola, respectively.
Why would H. anomalus maintain a relatively high native embolism in spite of being in the putatively more mesic habitat? It is well known that the root–soil interface is especially vulnerable to severe desiccation in coarse soils where water is easily displaced by air in the large pore spaces (Bristow et al., 1984; Hacke et al., 2000). Previously, it was demonstrated that soil Ψ decreases precipitously as a function of water content in these dune habitats (Rosenthal et al., 2005). In spite of this, native plant water status remains high in this dune habitat relative to adjacent off-dune habitats (Rosenthal et al., 2005) presumably because plants in coarser soils have deep roots that are able to mine soil moisture. This has been shown for woody species, which developed deeper roots in coarse textured soils to reach wetter soils (Hacke et al., 2000; Jackson et al., 2000; Sperry and Hacke, 2002). We have previously argued that H. anomalus can maximize access to soil moisture by having deep roots (Ludwig et al., 2004; Donovan et al., 2007, 2009). However, a recent study has shown that, while native H. anomalus did have some deep roots (>130 cm deep), more than 88% of its root biomass can be in the top 25 cm of soil (Ludwig et al., 2006). The significant increases in Ψpd in the watered (+H2O) H. anomalus transplant garden are consistent with this observation and suggest that H. anomalus may not always have the capacity to develop a sufficiently extensive deep root system to meet its water requirements. As a result, higher native embolism in H. anomalus would decrease the rate of water consumption by lowering hydraulic conductivity and transpiration, extending the time of hydraulic contact between water in the coarse soil and the root system (Sperry et al., 1998). This makes adaptive sense on the dunes because low plant cover means inter-specific competition is minimal. This resource conservation strategy is consistent with the notion that H. anomalus’ adaptation to the dune habitat consists of a dehydration avoidance and stress tolerating strategy (Brouillette et al., 2006; Donovan et al., 2009).
Interestingly, our results revealed that both species suffered from ‘cavitation fatigue’ (Hacke et al., 2001; Stiller and Sperry, 2002). However, this apparent increase in cavitation vulnerability at less negative xylem pressures was much more pronounced in H. anomalus, which also was able to recover from it (compare 60% embolism at –1 MPa in July 2002 versus 30% embolism at –1 MPa in August 2002; Fig. 2). In order to avoid confounding the effects of cavitation fatigue with inherent cavitation resistance, cavitation resistance at 75% embolism (P75) as well as at 50% embolism (P50) were compared (Sperry and Hacke, 2002).
A growing body of work suggests that temporal or ontogenetic changes in physiology or morphology in response to fine grained environmental variation can be adaptive (Winn, 1999; Miner and Vonesh, 2004; Picotte et al., 2007; Maherali et al., 2009). H. deserticola showed significantly more variability both within and between curves than H. anomalus, evidence of a greater plasticity in cavitation resistance. The variance in H. deserticola’s hydraulic parameters was correlated with Ψstem, such that plants experiencing lower Ψstem also had lower P75 (Fig. 5). For instance, in 2002 with a meagre 9 mm of precipitation in July and August, H. deserticola had significantly lower P75 than H. anomalus but this was not the case in 2003 when July and August precipitation was much greater (27.4 mm). This makes adaptive sense for a cavitation-avoiding species like H. deserticola. Avoidance of cavitation by H. deserticola by maintaining a more cavitation-resistant xylem in drier times would contribute to its higher soil-to-leaf hydraulic conductance and transpiration rates. These traits may be beneficial in its higher-cover habitat with greater potential for inter-specific competition, higher soil moisture heterogeneity, and, where finer-textured soils permit, a higher rate of water uptake per root area. The drought-escaping strategy is also consistent with its short 1–2 month life cycle. In sharp contrast, H. anomalus did not show any adjustment in P75 with Ψstem, consistent with a strategy of maintaining a consistently higher level of native embolism regardless of stem Ψ.
Three commonly described general drought adaptations are dehydration tolerance, dehydration avoidance, and drought escape (used here as defined by Ludlow, 1989). So what drought-adaptation strategies apply to these desert annuals? If H. deserticola is a cavitation-avoiding drought escapist, then H. anomalus is an embolism-maintaining dehydration avoider. Desert annuals are frequently characterized as drought escapists, however, a growing number of studies of functional traits in annuals have shown that annual species exhibit a range of adaptations from dehydration avoidance to dehydration escape (Geber and Dawson, 1990, 1997; Stanton et al., 2000; McKay et al., 2003; Heschel and Riginos, 2005; Sherrard and Maherali, 2006). Helianthus deserticola’s increasing resistance to xylem cavitation in response to decreasing water availability ensures that it will maintain hydraulic continuity at a greater range of soil water potentials even at higher transpiration rates. Thus, H. deserticola maximizes the length of time that resources are favourable consistent with the idea that annual drought-escapers may only reap fitness benefits when resources are favourable (Sherrard and Maherali, 2006). This is consistent with Donovan et al. (2007) in that higher fitness is not always associated with direct selection for lower water use efficiency in native H. deserticola populations (Donovan et al., 2007, 2009). By contrast, H. anomalus persists throughout the summer growing season and continues flowering until late in the autumn. If, as mentioned earlier, H. anomalus does not always have an extensive deep root system it may avoid dehydration by maintaining high native embolism and low transpiration rates. Indeed, a recent study suggested that nutrient limitation, not water use efficiency, appears to have been the driving selective force on H. anomalus populations (Donovan et al., 2009).
There still remains unexplained variation in cavitation resistance in these species, suggesting other factors beyond the scope of this study, such as root cavitation, variance in growth rate, and nutrient status, all with potential influences on the cavitation phenotype. Ultimately, the cavitation phenotype in herbaceous and woody species appears to be a complex function of inter-vessel pit structure and number (Hacke et al., 2004; Sperry and Hacke, 2004) the details of which need to be more fully studied.
Acknowledgments
We thank Jim wheeler who helped with vulnerability measurements on several occasions. We also thank Jennifer Lance, Fulco Ludwig, Maria Sanchez, and Rebecca Jewitt for assistance in the laboratory and in the field and Ferris Clegg and Richfield, Utah BLM for allowing us access to field sites at Little Sahara Sand Dunes, Utah. The authors thank two anonymous reviewers, whose thoughtful comments helped us improve the manuscript. This work was supported by NSF grant IBN-0131078 to LAD.
References
- Alder NN, Pockman WT, Sperry JS, Nuismer S. Use of centrifugal force in the study of xylem cavitation. Journal of Experimental Botany. 1997;48:665–674. [Google Scholar]
- Alder NN, Sperry JS, Pockman WT. Root and stem xylem embolism, stomatal conductance, and leaf turgor in Acer grandidentatum populations along a soil moisture gradient. Oecologia. 1996;105:293–301. doi: 10.1007/BF00328731. [DOI] [PubMed] [Google Scholar]
- Bristow KL, Campbell GS, Calissendorff C. The effects of texture on the resistance to water movement within the rhizosphere. Soil Science Society of America Journal. 1984;48:266–270. [Google Scholar]
- Brouillette LC, Gebremedhin M, Rosenthal DM, Donovan L. Testing hypothesized evolutionary shifts toward stress tolerance in hybrid Helianthus species. Western North American Naturalist. 2006;66:409–419. [Google Scholar]
- Buchard C, McCully M, Canny M. Daily embolism and refilling of root xylem vessels in three dicotyledonous crop plants. Agronomie. 1999;19:97–106. [Google Scholar]
- Davis SD, Ewers FW, Wood J, Reeves JJ, Kolb KJ. Differential susceptibility to xylem cavitation among three pairs of Ceanothus species in the Transverse mountain ranges of southern California. Ecoscience. 1999;6:180–186. [Google Scholar]
- Donovan LA, Dudley SA, Rosenthal DM, Ludwig F. Phenotypic selection on leaf water use efficiency and related ecophysiological traits for natural populations of desert sunflowers. Oecologia. 2007;152:13–25. doi: 10.1007/s00442-006-0627-5. [DOI] [PubMed] [Google Scholar]
- Donovan LA, Linton MJ, Richards JH. Predawn plant water potential does not necessarily equilibrate with soil water potential under well-watered conditions. Oecologia. 2001;129:328–335. doi: 10.1007/s004420100738. [DOI] [PubMed] [Google Scholar]
- Donovan LA, Ludwig F, Rosenthal DM, Rieseberg LH, Dudley SA. Phenotypic selection on leaf ecophysiological traits in Helianthus. New Phytologist. 2009;183:868–879. doi: 10.1111/j.1469-8137.2009.02916.x. [DOI] [PubMed] [Google Scholar]
- Donovan LA, Richards JH, Linton MJ. Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology. 2003;84:463–470. [Google Scholar]
- Geber MA, Dawson TE. Genetic variation in and covariation between leaf gas exchange, morphology, and development in Polygonum arenastrum, an annual plant. Oecologia. 1990;85:153–158. doi: 10.1007/BF00319396. [DOI] [PubMed] [Google Scholar]
- Geber MA, Dawson TE. Genetic variation in stomatal and biochemical limitations to photosynthesis in the annual plant, Polygonum arenastrum. Oecologia. 1997;109:535–546. doi: 10.1007/s004420050114. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Ewers BE, Ellsworth DS, Schafer KVR, Oren R. Influence of soil porosity on water use in Pinus taeda. Oecologia. 2000;124:495–505. doi: 10.1007/PL00008875. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Pittermann J. Analysis of circular bordered pit function. II. Gymnosperm tracheids with torus-margo pit membranes. American Journal of Botany. 2004;91:386–400. doi: 10.3732/ajb.91.3.386. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Stiller V, Sperry JS, Pittermann J, McCulloh KA. Cavitation fatigue. Embolism and refilling cycles can weaken the cavitation resistance of xylem. Plant Physiology. 2001;125:779–786. doi: 10.1104/pp.125.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heschel MS, Riginos C. Mechanisms of selection for drought stress tolerance and avoidance in Impatiens capensis (Balsaminacea) American Journal of Botany. 2005;92:37–44. doi: 10.3732/ajb.92.1.37. [DOI] [PubMed] [Google Scholar]
- Howard AR, Donovan LA. Helianthus night-time conductance and transpiration respond to soil water but not nutrient availability. Plant Physiology. 2007;143:145–155. doi: 10.1104/pp.106.089383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RB, Sperry JS, Dawson TE. Root water uptake and transport: using physiological processes in global predictions. Trends in Plant Science. 2000;5:482–488. doi: 10.1016/s1360-1385(00)01766-0. [DOI] [PubMed] [Google Scholar]
- Jacobsen AL, Pratt RB, Davis SD, Ewers FW. Cavitation resistance and seasonal hydraulics differ among three arid Californian plant communities. Plant, Cell and Environment. 2007;30:1599–1609. doi: 10.1111/j.1365-3040.2007.01729.x. [DOI] [PubMed] [Google Scholar]
- Kavanagh KL, Bond BJ, Aitken SN, Gartner BL, Knowe S. Shoot and root vulnerability to xylem cavitation in four populations of Douglas-fir seedlings. Tree Physiology. 1999;19:31–37. doi: 10.1093/treephys/19.1.31. [DOI] [PubMed] [Google Scholar]
- Kocacinar F, Sage RF. Photosynthetic pathway alters xylem structure and hydraulic function in herbaceous plants. Plant, Cell and Environment. 2003;26:2015–2026. [Google Scholar]
- Kolb KJ, Davis SD. Drought tolerance and xylem embolism in co-occurring species of coastal sage and chaparral. Ecology. 1994;75:648–659. [Google Scholar]
- Kolb KJ, Sperry JS. Differences in drought adaptation between subspecies of sagebrush (Artemisia tridentata) Ecology. 1999;80:2373–2384. [Google Scholar]
- Li YY, Sperry JS, Shao MA. Hydraulic conductance and vulnerability to cavitation in corn (Zea mays L.) hybrids of differing drought resistance. Environmental and Experimental Botany. 2009;66:341–346. [Google Scholar]
- Linton MJ, Sperry JS, Williams DG. Limits to water transport in Juniperus osteosperma and Pinus edulis: implications for drought tolerance and regulation of transpiration. Functional Ecology. 1998;12:906–911. [Google Scholar]
- Lo Gullo MA, Noval LC, Salleo S, Nardini A. Hydraulic architecture of plants of Helianthus annuus L. cv. Margot: evidence for plant segmentation in herbs. Journal of Experimental Botany. 2004;55:1549–1556. doi: 10.1093/jxb/erh169. [DOI] [PubMed] [Google Scholar]
- Ludlow MM. Strategies of response to water stress. In: Kreeb KH, Richter H, Hinckley TM, editors. Structural and functional responses to water stress. The Hague: SPB Academic Press; 1989. [Google Scholar]
- Ludwig F, Jewitt RA, Donovan LA. Nutrient and water addition effects on day- and night-time conductance and transpiration in a C3 desert annual. Oecologia. 2006;148:219–225. doi: 10.1007/s00442-006-0367-6. [DOI] [PubMed] [Google Scholar]
- Ludwig F, Rosenthal DM, Johnston JA, Kane N, Gross BL, Lexer C, Dudley SA, Rieseberg LH, Donovan LA. Selection on leaf ecophysiological traits in a desert hybrid Helianthus species and early-generation hybrids. Evolution. 2004;58:2682–2692. doi: 10.1111/j.0014-3820.2004.tb01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali H, Caruso CM, Sherrard ME. The adaptive significance of ontogenetic changes in physiology: a test in Avena barbata. New Phytologist. 2009;183:908–918. doi: 10.1111/j.1469-8137.2009.02845.x. [DOI] [PubMed] [Google Scholar]
- Maherali H, Pockman WT, Jackson RB. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology. 2004;85:2184–2199. [Google Scholar]
- Maherali H, Williams BL, Paige KN, Delucia EH. Hydraulic differentiation of Ponderosa pine populations along a climate gradient is not associated with ecotypic divergence. Functional Ecology. 2002;16:510–521. [Google Scholar]
- McCully ME, Huang CX, Ling LEC. Daily embolism and refilling of xylem vessels in the roots of field-grown maize. New Phytologist. 1998;138:327–342. doi: 10.1046/j.1469-8137.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- McKay JK, Richards JH, Mitchell-Olds T. Genetics of drought adaptation in Arabidopsis thaliana. I. Pleiotropy contributes to genetic correlations among ecological traits. Molecular Ecology. 2003;12:1137–1151. doi: 10.1046/j.1365-294x.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Mencuccini M, Comstock J. Vulnerability to cavitation in populations of two desert species, Hymenoclea salsola and Ambrosia dumosa, from different climatic regions. Journal of Experimental Botany. 1997;48:1323–1334. [Google Scholar]
- Milburn JA, McLaughlin ME. Studies of caviation in isolated vascular bundles and whole leaves of Plantago major L. New Phytologist. 1974;73:861–871. [Google Scholar]
- Miner BG, Vonesh JR. Effects of fine grain environmental variability on morphological plasticity. Ecology Letters. 2004;7:794–801. [Google Scholar]
- Neufeld HS, Grantz DA, Meinzer FC, Goldstein G, Crisosto GM, Crisosto C. Genotypic variability in vulnerability of leaf xylem to cavitation in water-stressed and well-irrigated sugarcane. Plant Physiology. 1992;100:1020–1028. doi: 10.1104/pp.100.2.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picotte JJ, Rosenthal DM, Rhode JM, Cruzan MB. Plastic responses to temporal variation in moisture availability: consequences for water use efficiency and plant performance. Oecologia. 2007;153:821–832. doi: 10.1007/s00442-007-0794-z. [DOI] [PubMed] [Google Scholar]
- Pockman WT, Sperry JS. Vulnerability to xylem cavitation and the distribution of Sonoran desert vegetation. American Journal of Botany. 2000;87:1287–1299. [PubMed] [Google Scholar]
- Rieseberg LH. Homoploid reticulate evolution in Helianthus (Asteraceae): evidence from ribosomal genes. American Journal of Botany. 1991;78:1218–1237. [Google Scholar]
- Rieseberg LH, Beckstromsternberg SM, Liston A, Arias DM. Phylogenetic and systematic inferences from chloroplast DNA and isozyme variation in Helianthus Sect. Helianthus (Asteraceae). Systematic Botany. 1991;16:50–76. [Google Scholar]
- Rieseberg LH, Raymond O, Rosenthal DM, Lai Z, Livingstone K, Nakazato T, Durphy JL, Schwarzbach AE, Donovan LA, Lexer C. Major ecological transitions in wild sunflowers facilitated by hybridization. Science. 2003;301:1211–1216. doi: 10.1126/science.1086949. [DOI] [PubMed] [Google Scholar]
- Rosenthal DM, Ludwig F, Donovan LA. Plant responses to an edaphic gradient across an active sand dune/desert boundary in the great basin desert. International Journal of Plant Sciences. 2005;166:247–255. [Google Scholar]
- Schwarzbach AE, Donovan LA, Rieseberg LH. Transgressive character expression in a hybrid sunflower species. American Journal of Botany. 2001;88:270–277. [PubMed] [Google Scholar]
- Sherrard ME, Maherali H. The adaptive significance of drought escape in Avena barbata, an annual grass. Evolution. 2006;60:2478–2489. [PubMed] [Google Scholar]
- Sperry JS. Limitations on stem water transport and their consequences. In: Gartner B, editor. Plant stems: physiology and functional morphology. New York: Academic Press; 1995. pp. 105–124. [Google Scholar]
- Sperry JS, Adler FR, Campbell GS, Comstock JP. Limitation of plant water use by rhizosphere and xylem conductance: results from a model. Plant, Cell and Environment. 1998;21:347–359. [Google Scholar]
- Sperry JS, Hacke UG. Desert shrub water relations with respect to soil characteristics and plant functional type. Functional Ecology. 2002;16:367–378. [Google Scholar]
- Sperry JS, Hacke UG. Analysis of circular bordered pit function. I. Angiosperm vessels with homogenous pit membranes. American Journal of Botany. 2004;91:369–385. doi: 10.3732/ajb.91.3.369. [DOI] [PubMed] [Google Scholar]
- Sperry JS, Donnely JR, Tyree MT. A method for measuring hydraulic conductivity and embolism in xylem. Plant, Cell and Environment. 1988;11:35–40. [Google Scholar]
- Stanton ML, Roy BA, Thiede DA. Evolution in stressful environments. I. Phenotypic variability, phenotypic selection, and response to selection in five distinct environmental stresses. Evolution. 2000;54:93–111. doi: 10.1111/j.0014-3820.2000.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Stiller V, Lafitte HR, Sperry JS. Hydraulic properties of rice and the response of gas exchange to water stress. Plant Physiology. 2003;132:1698–1706. doi: 10.1104/pp.102.019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller V, Sperry JS. Cavitation fatigue and its reversal in sunflower (Helianthus annuus L.) Journal of Experimental Botany. 2002;53:1155–1161. doi: 10.1093/jexbot/53.371.1155. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Fiscus EL, Wullschleger SD, Dixon MA. Detection of xylem cavitation in corn under field conditions. Plant Physiology. 1986;82:597–599. doi: 10.1104/pp.82.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNESCO. World map of arid regions. United Nations Educational, Scientific, and Cultural Organization. 1977 [Google Scholar]
- Winn AA. Is seasonal variation in leaf traits adaptive for the annual plant. Dicerandra linearifolia? Journal of Evolutionary Biology. 1999;12:306–313. [Google Scholar]