Abstract
The connexin43 (Cx43)-interacting protein of 85 kDa CIP85 has been identified as an interacting partner for the cytoplasmically located, carboxyl-terminal tail of Cx43. Further characterization has shown that the interaction between Cx43 and CIP85 is associated with increased turnover of Cx43 that may be lysosome-mediated. This suggests that CIP85 may regulate the endocytic trafficking of Cx43 from the plasma membrane and its degradation, and thus, indirectly influence gap junction function. This study reports the first successful production of monoclonal antibodies (MAbs) against CIP85. These antibodies are useful in detecting CIP85 expressed in several species in immunoblotting, immunoprecipitation, and immunofluorescence microscopy experiments. These MAbs will assist in defining the functional roles of CIP85, including its influence on Cx43 trafficking and intercellular communication through Cx43-containing gap junctions.
Introduction
Gap junctions are transmembrane channels that are essential for the control of physiological events, such as cell differentiation, growth, organ function, and certain developmental processes.(1,2) These channels are composed of connexin proteins, which form small pores in the plasma membrane, allowing direct exchange of small molecules (<1 kDa) between cells.(1,3) Connexin43 (Cx43) is the most ubiquitously expressed connexin protein and is critical in maintaining normal cellular functions.(2,4) CIP85 was initially identified in a yeast two-hybrid screen as an in vivo binding partner using the C-terminal region of Cx43 as bait.(5,6) Mouse CIP85 and its human homologue are ubiquitously expressed in mouse and human tissues and are conserved in human, D. melanogaster, and C. elegans.(7) CIP85 contains conserved SH3, RUN, and TBC domains as well as a short coiled-coil protein-binding motif of unknown function.(8) Src homology 3 (SH3) domains are known to bind to proline-rich regions of target proteins.(9,10)
Previously we have demonstrated that the CIP85 SH3 domain is necessary for its interaction with a proline-rich region of Cx43 and have shown that this interaction appears to induce the turnover of Cx43 through the lysosomal pathway.(11) CIP85 also contains a RUN domain, which may play a role in Ras-like GTPase signaling.(12) The CIP85 TBC domain is also found in the Tre-2, BUB2p, and Cdc16p proteins and is known to exhibit Rab GTPase-activating activity.(13–16) The human CIP85 homologue RabGAP5 has been shown to function as a Rab5 GAP through the activity of its TBC domain, and it is involved in the endocytic trafficking of epidermal growth factor from the plasma membrane to lysosomes.(17,18)
Cx43 can be internalized from the cell surface in either endosomes or connexosomes and is degraded in both lysosomes and proteosomes.(19–22) Based upon the demonstration of the Rab5 GAP activity of CIP85 and the association of CIP85′s interaction with Cx43, which appears to be associated with the increased turnover of Cx43, we propose that CIP85 may be involved in the endocytic trafficking of Cx43 from the plasma membrane to lysosomes where it is degraded.
We have generated monoclonal antibodies against the SH3 domain and N-terminal region of the mouse CIP85 protein to allow for further characterization of its interaction with Cx43 and its functional importance. The antibodies can be used to detect endogenous CIP85 in multiple mammalian cell lines by immunoprecipitation and by immunofluorescence microscopy. Use of these antibodies will help to further characterize the function of CIP85, its role in the trafficking of Cx43, and its influence on the regulation of Cx43 gap junction communication. This will lead to a better understanding of how connexins are endocytosed from the plasma membrane and their degradation by lysosomes.
Methods
Bacterial expression and purification of His-tagged CIP85 protein
Full-length CIP85 (GenBank accession no. AY382616) was subcloned into the pTrcHisA vector (Invitrogen, Carlsbad, CA), then transformed into BL21 Escherichia coli. His-CIP85 expression was induced with 0.1 mM IPTG for 1 h at 37°C. Bacteria were harvested by centrifugation, then lysed by sonication and washed once in PBS. Cell lysates were incubated with Ni+-Sepharose Fast Flow (GE Healthcare, Piscataway, NJ) for 3 h at 4°C to bind His-tagged proteins. Following washes in PBS, the bound proteins were eluted with 500 mM imidazole. His-Src was expressed in, and purified from, bacteria in a similar manner for use as a negative control.
Full-length CIP85, the SH3, TBC, RUN domain-containing regions, and carboxyl (C) and amino (N) terminal regions of CIP85 were subcloned into the pGEX-6P2 vector and expressed as glutathione S-transferase (GST) fusion proteins. The C and N terminal regions included the adjacent RUN and TBC domains, respectively. Proteins were expressed in E. coli following induction with 0.1 mM IPTG for 1 h at 37°C. Bacteria were harvested by centrifugation then lysed by sonication following a PBS wash. GST proteins were bound to glutathione agarose (Sigma, St. Louis, MO) by incubation with cell lysates for 2 h at 4°C. Following two PBS washes, proteins were eluted from the agarose with 20 mM glutathione in 50 mM Tris-HCL (pH 9.5). The GST-tagged C-terminal tail region of Cx43 (Cx43CT) was also expressed in, and purified from, bacteria in a similar manner for use as a negative control.
Immunization and production of CIP85 hybridomas
BALB/c mice were immunized with 115–145 μg of purified CIP85 protein in either Freund's complete or alum adjuvants. Booster immunizations were given at 3-week intervals in either Freund's incomplete or alum adjuvants. Test bleeds were assayed for positive reactions to CIP85 by indirect enzyme-linked immunoabsorbant assay (ELISA). Spleen cells from Freund's incomplete adjuvant immunized mice were fused to P3x63Ag8.653 mouse myeloma cells in the presence of polyethylene glycol (PEG) to produce monoclonal antibodies according to established techniques.(23,24) Hybridomas were then selected with hypoxanthine, aminopterin, and thymidine (HAT) supplemented medium and allowed to grow on macrophage plates in preparation for ELISA. Positive wells were subcloned two to three times and tested by indirect ELISA against His- and GST-tagged CIP85. Concentrated monoclonal antibody supernatants were later generated from selected hybridoma lines grown in BD serum-free cell medium using the CELLine flask system (BD Biosciences, San Jose, CA).
ELISA screening of monoclonal antibodies
Vinyl round-bottom 96-well plates (Corning, Lowell, MA) were coated with 1 μg of antigen diluted in PBS overnight at 4°C. Plates were washed three times with PBS, then blocked for 1 h with 5% non-fat milk in borate buffer (167 mM boric acid, 134 mM NaCl [pH 8.0]). Supernatants from hybridoma cultures were added and incubated for 1 h. Plates were washed three times with 0.5x borate buffer, then incubated with secondary goat anti-mouse IgG antibody conjugated to alkaline phosphatase (Southern Biotech, Birmingham, AL) for 1 h. Plates were washed three times with 0.5x borate buffer, then incubated with p-nitrophenyl phosphate (PnPP, Thermo Scientific, Waltham, MA) diluted to 1 mg/mL in diethanolamine substrate (DEA) buffer (Thermo Scientific) for 30 min. The OD at 405 nm was detected on a Victor3 plate reader (Perkin-Elmer, Waltham, MA).
Isotype determination
Isotyping was performed using the Mouse Mono-Ab-ID kit (Invitrogen) through the capture assay. The OD at 405 nm was detected on a Victor3 plate reader (Perkin-Elmer).
Cell culture conditions
Human cervical carcinoma cells not expressing (HeLa) or expressing (HeLa C1) endogenous Cx43 or exogenous Cx43 (HeLa 2-18), human embryonic kidney (HEK293), normal rat kidney (NRKe), mouse sarcoma with stably transfected L-cadherin (S180L), dog kidney epithelial (MDCK), and monkey kidney (COS-7) cells were cultured in high glucose Dulbecco's modified Eagle's medium (Invitrogen), supplemented with 10% fetal bovine serum, 20 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Immunoblotting and immunoprecipitation
Purified proteins were separated by 10% SDS-PAGE, transferred to PVDF membranes, and immunoblotted for CIP85 using monoclonal supernatants. Antibody binding was detected using ECL. For immunoprecipitation experiments, cells were lysed in NP-40 lysis buffer (0.2% NP-40, 150 mM NaCl, 20 mM Tris [pH 8.0], 1 mM DTT, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1 mM benzamidine, 10 mM NaF, 1 mM PMSF, and 160 μM Na3VO4), and lysates were clarified by centrifugation at 20,000 g for 20 min at 4°C. The BCA assay reagent kit (Bio-Rad, Hercules, CA) was used to determine protein concentrations. Clarified lysates were incubated with monoclonal CIP85 antibodies bound to protein G agarose (Thermo Scientific) for 1 h at 4°C. The agarose was washed three times with lysis buffer and then proteins were released from the agarose by boiling for 5 min in SDS-PAGE sample buffer. The proteins were analyzed by immnoblotting as described above. For blocking experiments MAbs were pre-incubated overnight at 4°C with the purified GST-tagged CIP85 protein domains that they were previously shown to recognize. Co-immunoprecipitation of Cx43 was determined by immunoblotting with a monoclonal Cx43 antibody, P2D12 (Fred Hutchinson Cancer Research Center, Seattle, WA).
Immunofluorescence microscopy
HeLa 2-18 and NRKe cells were transiently transfected for 24 h with pcDNA-Flag-CIP85 using Lipofectamine 2000 (Invitrogen). Cells were fixed in cold 80% methanol/20% acetone for 20 min at −20°C and washed three times with 0.1% Triton X-100 in PBS (PBSTx) for 5 min each. Then cells were blocked with 5% NGS in PBSTx for 30 min prior to incubation for 1 h with polyclonal rabbit CIP85 antibody, mouse anti-Flag M2 antibody (Sigma), rabbit anti-Cx43 antibody (Sigma), or the different CIP85 MAbs. After three 5 min washes with PBSTx, the cells were incubated with goat anti-mouse or goat anti-rabbit secondary antibodies conjugated to Alexa 488 or Alexa 594 (Molecular Probes, Eugene, OR). To identify nuclei, the cells were co-labeled with TO-PRO-3 iodide (Invitrogen). Subcellular localization of CIP85 was examined using a TCS SP5 AOBS laser scanning confocal microscope (Leica, Germany) with a 63x objective.
Results
Production and screening of MAbs
Mice were immunized with purified recombinant CIP85 that was expressed as a His-tagged fusion protein in bacteria. Test bleeds showed a positive immune reaction to CIP85 by ELISA assay. ELISA assays were also used to test hybridomas, generated from the immunized mice, for reactivity to different regions of the CIP85 protein. GST-Cx43CT and His-Src were used as negative controls to eliminate non-specific clones that were reactive against epitopes in GST and His, and possibly, Cx43CT or Src. Purified GST-tagged full-length CIP85, the individual TBC, SH3, or RUN domains, and the combined TBC/N-terminal and RUN/C-terminal regions of CIP85 were used to establish the domain specificity for each antibody (Fig. 1A). Hybridoma cell clones that demonstrated specific ELISA reactivity to the N-terminal/TBC region (G10 and D11) and the SH3 domain (E1 and F6) of CIP85 were generated.
FIG. 1.
The specificity of MAbs for selected CIP85 domains. Purified GST-tagged CIP85 protein and peptides, produced in and purified from bacteria, were used to test MAb specificity. (A) Schematic domain diagram of full-length CIP85, the N-terminal region with the TBC domain, the TBC, SH3, and RUN domains, and the C-terminal region with the RUN domain (not to scale). Molecular weights predicted from the amino acid sequences by MacVector software (Oxford Molecular, Madison, WI) are shown in parenthesis. (B) Purified Cx43CT (lane 1), CIP85 C-terminal region with the RUN domain (lane 2), CIP85 RUN (lane 3), SH3 (lane 4), and TBC (lane 5) domains, CIP85 N-terminal region with the TBC domain (lane 6), and full-length CIP85 (lane 7) were immunoblotted with GST antibody or the CIP85 E1, F6, G10, or D11 MAbs. The migration positions of purified protein/peptides are indicated at the right margin of each panel and the positions of molecular mass markers (kDa) are indicated at the left margin of each panel.
The same purified proteins or peptides were used to further test the specificity of the hybridoma clones to CIP85 reactivity by immunoblotting (Fig. 1B). MAbs E1 (Fig. 1B, second panel) and F6 (Fig. 1B, third panel) recognized only the isolated SH3 domain (lane 4) and full-length CIP85 (lane 7), which indicated their specificity to the SH3 domain. The E1 and F6 MAbs failed to recognize either the TBC or RUN domains or the N- or C-terminal CIP85 regions (lanes 2, 3, 5, and 6). Additionally, the E1 and F6 MAbs failed to recognize His-Src, which also contains a SH3 domain (data not shown). This suggests that the reactive epitope(s) of the E1 and F6 MAbs are specific to the CIP85 SH3 domain.
MAbs G10 (Fig. 1B, fourth panel) and D11 (Fig. 1B, fifth panel) recognized only the N-terminal/TBC region (lane 6) and full-length CIP85 (lane 7), which suggested the presence of a reactive epitope(s) in either the TBC domain or the N-terminal sequence. However, the observations that the G10 and D11 MAbs did not recognize the isolated TBC domain (lane 5) containing amino acids (aa) 114-326 indicated that they were specific to the N-terminal region of CIP85, encompassing aa 1-113.
The CIP85 MAbs also recognized faster migrating, lower molecular weight (mw) bands, which were evident in lanes containing the full-length CIP85 on the CIP85 G10 and D11 MAb blots (Fig. 1B, bottom two panels, lane 7) and the N-terminal region of CIP85 on the G10 and D11 MAb blots (Fig. 1B, bottom two panels, lane 6). The presence of these additional bands only in the lanes where the antibodies specifically recognized a full-length CIP85 protein suggests that the lower mw bands represent degradation products in which the reactive epitope(s) has been conserved. Thus, for example, the apparent lower mw bands in lanes 6 and 7 of the bottom two blots in Figure 1B contain the epitope for the G10 and D11 MAbs, which is located in the N-terminus of CIP85, but likely lack varying portions of the C-terminal region of CIP85.
Immunoprecipitation and immunoblotting detection of endogenous CIP85 proteins
MAbs were tested for reactivity against endogenously expressed CIP85 by immunoblotting (Fig. 2). The G10 MAb, which recognizes the N-terminal region of CIP85 detected full-length CIP85, or its homologues, endogenously expressed in human (HeLa, HEK, Fig. 2A, lanes 1 and 2), rat (NRKe, Fig. 2A, lane 3), dog (MDCK, Fig. 2A, lane 4), and mouse (S180L, Fig. 2A, lane 6) cells. The G10 MAb also detected endogenously expressed CIP85 in monkey (COS-7, Fig. 2A, lane 5) cells at a lower level. The D11 MAb as well as the SH3 domain-specific E1 and F6 MAbs also recognized CIP85 from these species.
FIG. 2.
Immunoprecipitation and immunoblotting of CIP85 expressed in mammalian cells. CIP85, endogenously or exogenously expressed in various mammalian cell lines was detected by immunoprecipitation and/or immunoblotting with CIP85 MAbs. (A) The G10 MAb was used to detect endogenous CIP85, or its homologues, by immunoblotting in lysates of human HeLa and HEK (lanes 1 and 2), rat NRKe (lane 3), dog MDCK (lane 4), monkey COS-7 (lane 5), and mouse S180L (lane 6) cells, as compared to purified GST-CIP85 protein (lane 7). (B) Endogenously expressed CIP85 was immunoprecipitated from rat NRKe cell lysates with MAb E1 preblocked with the SH3 domain (left panel, lane 1), unblocked MAb E1 (left panel, lane 2), MAb F6 preblocked with the SH3 domain (right panel, lane 1), and unblocked MAb F6 (right panel, lane 2). CIP85 in the immunoprecipitates and in lysates of nontransfected NRKe cells (right panel, lane 3) and cells transfected with Flag-tagged CIP85 (right panel, lane 4) were detected by immunoblotting with the N-terminal region specific G10 MAb. (C) Endogenously expressed CIP85 was immunoprecipitated from rat NRKe cell lysates with MAb G10 preblocked with the N-terminal region (lane 1), unblocked MAb G10 (lane 2), MAb D11 preblocked with the N-terminal region (lane 3), and unblocked MAb D11 (lane 4). CIP85 in the immunoprecipitates and in lysates of nontransfected NRKe cells (lane 5) and cells transfected with Flag-tagged CIP85 (lane 6) were detected by immunoblotting with the SH3 domain specific F6 MAb. The migration positions of CIP85 and the IgG heavy chain (HC, present in IP lanes only) are shown to the right of each panel and the positions of molecular mass markers (kDa) are shown to the left of all panels.
To establish if the MAbs were effective in immunoprecipitation experiments, endogenously expressed CIP85 was immunoprecipitated from NRKe cell lysates, resolved by SDS-PAGE, and detected by immunoblotting (Fig. 2B and C). All four MAbs were able to immunoprecipitate endogenous CIP85 from the NRKe cell lysates (Fig. 2B, left panel, lane 2 and right panel, lane 2; Fig. 2C, lanes 2 and 4). To establish the MAbs' specificity in immunoprecipitations, purified CIP85 domains were used to block the interaction between the various MAbs and endogenous CIP85 in cell lysates prior to the immunoprecipitation procedures. Pre-incubation of the E1 or F6 MAbs with the purified CIP85 SH3 domain peptide (Fig. 2B, left panel, lane 1 and right panel, lane 1) and the G10 or D11 MAbs with the purified CIP85 N-terminal region peptide (Fig. 2C, lanes 1 and 3), prior to the immunoprecipitation, resulted in a markedly decreased recognition of CIP85. Additionally, all four CIP85 MAbs were able to co-immunoprecipitate endogenous Cx43 from the various cell lysates that could be detected by immunoblotting using a mouse monoclonal Cx43 antibody (data not shown).
Detection of CIP85 by immunofluorescence laser scanning confocal microscopy
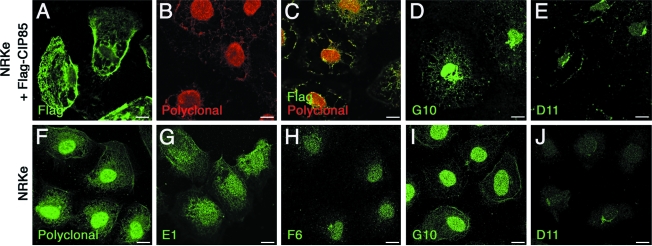
MAbs were also tested for their ability to recognize CIP85 in immunofluorescence microscopy. NRKe cells were transiently transfected with Flag-tagged CIP85 and visualized with Flag antibody, polyclonal rabbit CIP85 antibody, or the various MAbs (Fig. 3, upper panels). Co-staining using the Flag and polyclonal rabbit CIP85 antibodies is shown in Figure 3C. Staining of Flag-tagged CIP85 by the G10 and D11 MAbs was observed at the cell periphery, intracellular sites, and in the nucleus (Fig. 3D and E). Labeling of CIP85 endogenously expressed in NRKe cells using the polyclonal rabbit CIP85 antibody and the various CIP85 MAbs (Fig. 3F–J) was similar to that in Flag-CIP85 transfected cells by the Flag antibody, the rabbit CIP85 antibody, and the G10 and D11 MAbs (Fig. 3A–E).
FIG. 3.
Visualization of CIP85 in rat NRKe cells by immunofluorescence laser scanning confocal microscopy. Laser scanning confocal microscopy was employed to determine the subcellular localization of exogenous Flag-CIP85 or endogenously expressed CIP85 in NRKe cells. (Upper panels) Exogenously expressed Flag-CIP85 was localized by Flag antibody (A), polyclonal rabbit CIP85 antibody (B), both Flag and polyclonal rabbit CIP85 antibodies (C), G10 MAb (D), and D11 MAb (E). (Bottom panels) Endogenous CIP85 was localized by polyclonal rabbit CIP85 antibody (F), E1 MAb (G), F6 MAb (H), G10 MAb (I), and D11 MAb (J). Scale bar, 10 μm.
All four MAbs also recognized endogenous CIP85 in HeLa cells stably overexpressing Cx43 (Fig. 4, top panels). All MAbs were found to co-localize occasionally with Cx43 (Fig. 4, lower panels), which supported our previously published results.(11) Since CIP85 staining was observed at the cell periphery, intracellular sites, and in the nucleus (Figs. 3 and 4), we performed co-staining with the nuclear marker ToPRO-3 to determine the nuclear specificity of CIP85 labeling (Fig. 4, bottom panels). CIP85 staining was predominantly observed throughout the cytoplasm outside of the nucleus in images focused on the cell nucleus (Fig. 4C and D, bottom panels), which suggests that CIP85 is not localized in the nucleus.
FIG. 4.
Localization of endogenously expressed CIP85 in human HeLa 2-18 cells by immunofluorescence laser scanning confocal microscopy. Laser scanning confocal microscopy using the E1 (A), F6 (B), G10 (C), and D11 (D) MAbs determined the subcellular localization of endogenous CIP85 in human HeLa 2-18 cells. Cells were co-stained using ToPro3 nuclear stain and Cx43 polyclonal antibody (bottom panels A–D). White arrows indicate selected areas of co-localization between Cx43 and CIP85 (bottom panels B and C). Scale bar, 10 μm.
MAb isotyping
Isotyping revealed that all CIP85 MAbs contained kappa light chains combined with various heavy chains, including IgG1, IgG2a, and IgG2b (Table 1). The immunoblotting, immunoprecipitation, and immunofluorescence microscopy results are also summarized in Table 1.
Table 1.
Comparison of CIP85 MAb Specificities and Activities
| CIP85 MAb | Region specificity | Immunoblotting (multiple-species) | Immunoprecipitation | Immunofluorescence microscopy | Isotype |
|---|---|---|---|---|---|
| D11 | N-terminal (aa 1-113) | Yes | Yes | Yes | IgG2a, kappa |
| G10 | N-terminal (aa 1-113) | Yes | Yes | Yes | IgG2b, kappa |
| E1 | SH3 domain | Yes | Yes | Yes | IgG1, kappa |
| F6 | SH3 domain | Yes | Yes | Yes | IgG1, kappa |
Discussion
Intercellular gap junctional communication is vital for normal physiological functions.(1,2) Regulation of Cx43 levels in the cytoplasm and at the plasma membrane can have a significant effect on the level of gap junctional communication; thus it is important to understand the processes involved in the regulation of Cx43 levels and their influence on gap junctional intracellular communication.(4,21) The turnover of Cx43 is regulated by endocytic trafficking of Cx43 from the plasma membrane to lysosomes and proteosomes where it is degraded.(19–22) We have shown that CIP85, a Rab5GAP, is a Cx43-interacting partner that affects the Cx43 turnover, possibly through lysosomal degradation; however, the mechanism by which this occurs is still unclear.(11)
To enable further study of CIP85, we have successfully generated a panel of mouse MAbs specific to either the SH3 domain or N-terminal region encompassing aa 1-113 of CIP85. The MAbs consisted of IgG1, IgG2a, or IgG2b heavy chains together with kappa light chains. MAbs against additional identified domains of CIP85 (TBC, RUN, and the C-terminal region) were not detected in the serum of mice immunized with the full-length CIP85 protein. Purified peptides containing only these specific domains may be used as antigens in future immunizations to generate MAbs specific to these CIP85 regions. All of the CIP85 MAbs successfully generated in this present effort could be used to detect CIP85 in cell lysates by immunoblotting or immunoprecipitation, and they were useful in visualizing CIP85 by immunofluorescence laser scanning confocal microscopy. Importantly, all of the MAbs cross-reacted with CIP85 homologues in multiple mammalian species, including human, rat, dog, monkey, and mouse (Fig. 2A and Table 1). CIP85 homologues that are expressed in non-mammalian species may also be detectable by these MAbs.
After the collection of these data, hybridoma clones producing the CIP85 MAbs were successfully regrown in the CELLine flask system with serum-free medium. These flasks allow for the production of higher titer MAb supernatants and the removal of serum from the media, which should reduce the occurrence of non-specific protein detection.
We have previously demonstrated that the interaction between CIP85 and CX43 relies upon the SH3 domain of CIP85 binding to a proline-rich region of Cx43. We have also shown that the overexpression of CIP85 is associated with increased turnover of Cx43, which appears to be mediated in part by lysosomes.(11) This effect may be dependent upon the ability of CIP85 to function as a GAP for the Rab5 small GTPase, since Rab5 functions in the endocytic trafficking of proteins from the plasma membrane to endosomes and ultimately to lysosomes for degradation.(17,25) MAbs specific to the regions within and outside the SH3 domain of CIP85 will allow us to investigate the importance of the interaction between CIP85 and Cx43, the role of CIP85 in the endocytosis and increased turnover of Cx43 by lysosomes, and thus, the ability of CIP85 to indirectly regulate gap junctional communication.
Acknowledgments
We thank Dr. Vivian Su, Wendy Kurata, and members of the J. Berestecky laboratory for additional technical assistance; and P. Lampe for the Cx43 monoclonal antibody. This work was supported by grant # 5RO1CA052098-17 from the National Cancer Institute, National Institutes of Health (to AFL), a travel grant from the Graduate Student Organization of the University of Hawaii at Manoa (to KC), and a Cancer Research Center P30 core grant CURE award (to CK).
Disclosure Statement
No competing financial interests exist.
References
- 1.Vinken M. Vanhaecke T. Papeleu P. Snykers S. Henkens T. Rogiers V. Connexins and their channels in cell growth and cell death. Cell Signal. 2006;18:592–600. doi: 10.1016/j.cellsig.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Wei CJ. Xu X. Lo CW. Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol. 2004;20:811–838. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- 3.Goodenough DA. Goliger JA. Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- 4.Iacobas DA. Scemes E. Spray DC. Gene expression alterations in connexin null mice extend beyond the gap junction. Neurochem Int. 2004;45:243–250. doi: 10.1016/j.neuint.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Jin C. Lau AF. Characterization of a Novel SH3-containing Protein that May Interact with Connexin43. IOS Press; Amsterdam: 1998. [Google Scholar]
- 6.Jin C. Lau AF. Martyn KD. Identification of connexin-interacting proteins: application of the yeast two-hybrid screen. Methods. 2000;20:219–231. doi: 10.1006/meth.1999.0939. [DOI] [PubMed] [Google Scholar]
- 7.Yang H. Sasaki T. Minoshima S. Shimizu N. Identification of three novel proteins (SGSM1, 2, 3) which modulate small G protein (RAP and RAB)-mediated signaling pathway. Genomics. 2007;90:249–260. doi: 10.1016/j.ygeno.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Lupas A. Prediction and analysis of coiled-coil structures. Methods Enzymol. 1996;266:513–525. doi: 10.1016/s0076-6879(96)66032-7. [DOI] [PubMed] [Google Scholar]
- 9.Cohen GB. Ren R. Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995;80:237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 10.Yu H. Chen JK. Feng S. Dalgarno DC. Brauer AW. Schreiber SL. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994;76:933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]
- 11.Lan Z. Kurata WE. Martyn KD. Jin C. Lau AF. Novel rab GAP-like protein, CIP85, interacts with connexin43 and induces its degradation. Biochemistry. 2005;44:2385–2396. doi: 10.1021/bi048306w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callebaut I. de Gunzburg J. Goud B. Mornon JP. RUN domains: a new family of domains involved in Ras-like GTPase signaling. Trends Biochem Sci. 2001;26:79–83. doi: 10.1016/s0968-0004(00)01730-8. [DOI] [PubMed] [Google Scholar]
- 13.Itoh T. Satoh M. Kanno E. Fukuda M. Screening for target Rabs of TBC (Tre-2/Bub2/Cdc16) domain-containing proteins based on their Rab-binding activity. Genes Cells. 2006;11:1023–1037. doi: 10.1111/j.1365-2443.2006.00997.x. [DOI] [PubMed] [Google Scholar]
- 14.Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta. 2003;1603:47–82. doi: 10.1016/s0304-419x(02)00082-3. [DOI] [PubMed] [Google Scholar]
- 15.Neuwald AF. A shared domain between a spindle assembly checkpoint protein and Ypt/Rab-specific GTPase-activators. Trends Biochem Sci. 1997;22:243–244. doi: 10.1016/s0968-0004(97)01073-6. [DOI] [PubMed] [Google Scholar]
- 16.Pan X. Eathiraj S. Munson M. Lambright DG. TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature. 2006;442:303–306. doi: 10.1038/nature04847. [DOI] [PubMed] [Google Scholar]
- 17.Haas AK. Fuchs E. Kopajtich R. Barr FA. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat Cell Biol. 2005;7:887–893. doi: 10.1038/ncb1290. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs E. Haas AK. Spooner RA. Yoshimura S. Lord JM. Barr FA. Specific Rab GTPase-activating proteins define the Shiga toxin and epidermal growth factor uptake pathways. J Cell Biol. 2007;177:1133–1143. doi: 10.1083/jcb.200612068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laing JG. Tadros PN. Westphale EM. Beyer EC. Degradation of connexin43 gap junctions involves both the proteasome and the lysosome. Exp Cell Res. 1997;236:482–492. doi: 10.1006/excr.1997.3747. [DOI] [PubMed] [Google Scholar]
- 20.Qin H. Shao Q. Igdoura SA. Alaoui-Jamali MA. Laird DW. Lysosomal and proteasomal degradation play distinct roles in the life cycle of Cx43 in gap junctional intercellular communication-deficient and -competent breast tumor cells. J Biol Chem. 2003;278:30005–30014. doi: 10.1074/jbc.M300614200. [DOI] [PubMed] [Google Scholar]
- 21.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leithe E. Brech A. Rivedal E. Endocytic processing of connexin43 gap junctions: a morphological study. Biochem J. 2006;393:59–67. doi: 10.1042/BJ20050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gooding J. Monoclonal Antibodies: Principles, Practice, 2e. Academic Press; London: 1986. [Google Scholar]
- 24.Loo LW. Berestecky JM. Kanemitsu MY. Lau AF. pp60src-mediated phosphorylation of connexin 43, a gap junction protein. J Biol Chem. 1995;270:12751–12761. doi: 10.1074/jbc.270.21.12751. [DOI] [PubMed] [Google Scholar]
- 25.Stenmark H. Olkkonen VM. The Rab GTPase family [review] Genome Biol. 2001;2:3007. doi: 10.1186/gb-2001-2-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]