Summary
Marijuana impairs learning and memory through actions of its psychoactive constituent, delta-9-tetrahydrocannabinol (Δ9-THC), in the hippocampus, through activation of cannabinoid CB1 receptors (CB1R). CB1Rs are found on glutamate and GABA neuron axon terminals in the hippocampus where they control neurotransmitter release. Previous studies suggest that Δ9-THC is a partial agonist of CB1Rs on glutamate axon terminals in the hippocampus, whereas its effects on GABA terminals have not been described. Using whole-cell electrophysiology in brain slices from C57BL6/J mice, we examined Δ9-THC effects on synaptic GABA IPSCs, and postsynaptic GABA currents elicited by laser-induced photo-uncaging (photolysis) of α-carboxy-2-nitrobenzyl (CNB) caged GABA. Despite robust inhibition of synaptic IPSCs in wildtype mice by the full synthetic agonist WIN55,212-2, using a Tween-80 and DMSO vehicle, Δ9-THC had no effects on IPSCs in this, or in a low concentration of another vehicle, randomly-methylated β-cyclodextrin (RAMEB, 0.023%). However, IPSCs were inhibited by Δ9-THC in 0.1% RAMEB, but not in neurons from CB1R knockout mice. Whereas Δ9-THC did not affect photolysis-evoked GABA currents, these responses were prolonged by a GABA uptake inhibitor. Concentration-response curves revealed that the maximal effects of Δ9-THC and WIN55,212-2 were similar, indicating that Δ9-THC is a full agonists at CB1Rs on GABA axon terminals. These results suggest that Δ9-THC inhibits GABA release but does not directly alter GABAA receptors or GABA uptake in the hippocampus. Furthermore, full agonist effects of Δ9-THC on IPSCs likely result from a much higher expression of CB1Rs on GABA versus glutamate axon terminals in the hippocampus.
Keywords: marijuana, brain slice, learning, memory, synaptic plasticity, cannabinoid
Introduction
Marijuana (Cannabis sativa) is a psychoactive plant that is widely used throughout the world. The marijuana plant contains approximately 70 cannabinoid compounds (Burns & Ineck, 2006). However, Δ9-THC is thought to be the main psychoactive cannabinoid responsible for mood alterations, feelings of euphoria, and cognitive impairments that are hallmarks of its effects in humans (Gaoni & Mechoulam, 1964). Δ9-THC exerts its effects on cellular processes by activating cannabinoid CB1 and CB2 receptors that are members of the G-protein coupled receptor family (Pertwee, 1997). However, compared to CB2Rs, CB1Rs are highly expressed in brain tissue, and mediate most of the central actions of marijuana in humans (Huestis et al., 2001). The discovery of the cannabinoid receptors (Matsuda et al., 1990; Munro et al., 1993), and identification of endogenous ligands (Devane et al., 1992; Mechoulam et al., 1995; Stella et al., 1997) demonstrated the existence of a brain endocannabinoid system.
An important cognitive effect of marijuana in humans is the impairment of memory through the disruption of information encoding and recall of newly acquired information (Abel, 1971a; Abel, 1971b; Ranganathan & D'Souza, 2006; Wilson et al., 1994). In addition, working memory is necessary for normal performance in many cognitive tasks in humans and animals, and it relies upon intact hippocampal function (Hampson & Deadwyler, 1998; Ranganathan & D'Souza, 2006). In animals, spatial working memory is profoundly impaired by Δ9-THC given systemically, injected directly into the hippocampus (Lichtman et al., 1995), or by exposure to marijuana smoke (Niyuhire et al., 2007), and this appears to be mediated exclusively by CB1Rs in the hippocampus (Varvel & Lichtman, 2002; Wise et al., 2009). In addition to disruption of spatial working memory, non-spatial operant learning and memory is also disrupted by Δ9-THC (Hampson & Deadwyler, 1998; Heyser et al., 1993). In support of the central role of the hippocampus in mediating the cognitive effects of marijuana, this structure contains a dense population of CB1Rs that mediate the effects of exogenous and endogenous cannabinoids (Herkenham et al., 1990; Katona et al., 1999), and a recent study has provided strong evidence for the involvement of CB1Rs on GABA axon terminals in the effects of Δ9-THC on spatial memory in mice (Puighermanal et al., 2009).
At the cellular level, cannabinoids presynaptically inhibit the release of both GABA and glutamate in the hippocampus and throughout the brain (Gerdeman & Lovinger, 2001; Hoffman et al., 2003; Hoffman & Lupica, 2000; Hoffman & Lupica, 2001; Levenes et al., 1998; Robbe et al., 2001; Wilson & Nicoll, 2002). Furthermore, it is likely that this is the primary means through which cannabinoids alter hippocampal neuronal network activity (Hajos et al., 2000; Robbe et al., 2006). Another mechanism that may be involved in the disruption of memory by cannabinoids is the inhibition of forms of synaptic plasticity, such as long-term potentiation and depression (LTP, LTD), that are proposed cellular correlates of learning and memory (Bliss & Collingridge, 1993; Lynch, 2004). Acutely, synthetic cannabinoids, endocannabinoids, and Δ9-THC block LTP in the CA1 region of the hippocampus in vitro (Misner & Sullivan, 1999; Nowicky et al., 1987; Stella et al., 1997), and long-term exposure to Δ9-THC in vivo can block LTP in vitro during withdrawal, despite the absence of detectable tissue levels of Δ9-THC at the time of LTP induction (Hoffman et al., 2007; Fan et al., 2010).
Although ample evidence implicates the hippocampus as a site for the actions of Δ9-THC in the disruption of memory, surprisingly little is known of its physiological actions on specific intact neural pathways in the CNS. Although a some studies have described the effects of Δ9-THC in adult hippocampal brain slices (Foy et al., 1982; Nowicky et al., 1987), these were conducted prior to identification of CB1Rs, and the development of antagonists and CB1R knockout animals. More recent functional studies with Δ9-THC in vitro have utilized immature hippocampal neurons maintained in culture to demonstrate that this phytocannabinoid can act as an agonist, partial agonist or antagonist at CB1Rs coupled to the inhibition of glutamate release (Kelley & Thayer, 2004; Roloff & Thayer, 2009; Shen & Thayer, 1999; Straiker & Mackie, 2005). However, these studies have not examined the effects of Δ9-THC in mature hippocampal circuits, nor have they directly examined its effects on GABAergic neurotransmission, despite the much higher density of CB1Rs on these axon terminals compared to glutamate terminals (Kawamura et al., 2006; Marsicano & Lutz, 1999). In addition to the paucity of information of Δ9-THC effects on GABA release in the hippocampus, it has also been proposed to inhibit the uptake of GABA and other neurotransmitters (Banerjee et al., 1975; Coull et al., 1997; Maneuf et al., 1996). Furthermore, our limited understanding of the effects of Δ9-THC in specific CNS circuits might be due to its high lipophilicity and poor solubility in aqueous media (Banerjee et al., 1975; Jarho et al., 1998).
In an attempt to identify the specific sites at which Δ9-THC acts to alter hippocampal function, we have examined its effects on GABA release in mature hippocampal slices obtained from CB1+/+ and CB1-/- mice, and compared these actions to those of a full synthetic agonist. Additionally, since Δ9-THC proved to be highly insoluble for in vitro use, we describe procedures permitting is solubilization.
Methods
Animals
Animal protocols were approved by the Animal Care and Use Committee of the NIDA Intramural Research Program, and were conducted in strict accordance with NIH guidelines to minimize the number of animals used in these studies. Wildtype (WT, CB1+/+) and CB1 receptor knockout (KO, CB1-/-) littermate C57BL6\J mice (4-10 weeks) were obtained from the NIDA Intramural Research Program transgenic facility colony. These animals were descendants of 3 heterozygous (CB1+/-) breeding pairs, donated by Dr. Andreas Zimmer and the National Institute of Mental Health (Bethesda, MD, USA). Genotyping was performed by Charles River Laboratories (Raleigh, NC, USA). Several of the observations made in mice were confirmed using a smaller number of wildtype male Sprague-Dawley rats (4-6 weeks of age), obtained from Charles River Laboratories (Raleigh, NC, USA).
Brain slice preparation
Hippocampal brain slices were prepared as previously described (Hoffman & Lupica, 2000). Briefly, animals were killed by cervical dislocation followed by decapitation. The brains were rapidly removed and immersed in cold (4°C), oxygenated high-sucrose, low-Ca2+-containing artificial cerebrospinal fluid (aCSF) of the following composition (mM): NaCl, 87; KCl, 2.5; MgCl2, 7; CaCl2, 0.5; NaH2PO4, 1.25; glucose, 25; sucrose 75; NaHCO3, 25. Coronal slices were then cut at 280 μm thickness using a vibrating tissue slicer (VT1000S, Leica Instruments, Germany). Hemi-sectioned brain slices containing the hippocampus were then incubated in a solution composed of 50% high-sucrose and 50% normal aCSF of the following composition (mM): NaCl, 126; KCl, 3.0; MgCl2, 1.5; CaCl2, 2.4; NaH2PO4, 1.2; glucose, 11.0; NaHCO3, 26, saturated with 95% O2 and 5% CO2, at room temperature for ≥ 90 minutes before recordings. Individual brain slices were placed into a low-volume (∼300 μL) recording chamber integrated into the fixed stage of a differential interference (DIC) contrast microscope (Olympus America, Center Valley, PA, USA), and submerged in normal aCSF of a fixed volume (∼15 mL) that was recirculated at 2 mL/min using a peristaltic pump. This solution was continuously bubbled with 95% O2 and 5% CO2, and maintained at 30-32°C using a solution heater (TC-324B, Warner Instruments, Hamden, CT).
Electrophysiology
Whole-cell electrophysiological recordings were performed using an Axopatch 200B amplifier (Axon Instruments, Foster CA) and electrodes pulled from borosilicate glass (1.5 mm O.D., 0.86 mm I.D., Sutter Instruments, Burlingame, CA). Electrodes were filled with a solution containing (mM): D-gluconic acid, 125.0; HEPES, 10.0; EGTA, 1.0; CaCl2, 0.1; KCl, 10.0; Mg2+-ATP, 1.0; Na+-GTP, 0.2, or CsCH3SO3, 100; CsCl, 60; EGTA 0.2; HEPES, 10; MgCl2, 2.0; Mg2+-ATP, 1.0; Na+-GTP, 0.3. All intracellular solutions also contained the quaternary lidocaine derivative, QX-314 (Sigma, St. Louis, MO; 1 mg/ml), to block action potentials only in the recorded cells. The internal solutions were adjusted to pH 7.2-7.4 using CsOH. Series resistance was monitored with a -10 mV voltage step (200 ms), initiated every 30 sec. Series resistance measurements and the synaptic and photolysis-evoked current amplitudes were all plotted versus time on the same graph to determine whether the observed changes in these currents were associated with altered cellular access resistance. Only cells maintaining stable series resistance (< 10% change over the duration of the recording) were included in analyses. Data were directly acquired to a personal computer using an A/D board (Instrutech ITC-18, Bellmore, NY) and Windows-based software (WinWCP, courtesy of Dr. John Dempster, University of Strathclyde, Glasgow, UK; http://spider.science.strath.ac.uk/sipbs/software_ses.htm).
GABAergic currents were measured in hippocampal CA1 pyramidal neurons identified under visual control using differential-interference contrast (DIC) videomicroscopy and infrared illumination. To measure evoked IPSCs, CA1 pyramidal neurons were voltage clamped at −20 mV when using the gluconic acid intracellular solution (outward currents), and at -70 mV when the CSCH3SO3 intracellular solution (inward currents) was used. IPSCs were evoked using a custom-built bipolar formvar-insulated nickel-chromium stimulating electrode placed near stratum pyramidale. Synaptic IPSCs and photolysis-evoked GABAA-mediated Cl- currents were pharmacologically isolated using the glutamate receptor antagonists D-(-)-2-amino-5-phosphonopentanoic acid (APV, 40 μM) to block NMDA receptors, and 6,7-dinitroquinoxaline-2,3-dione (DNQX, 10 μM) to block AMPA/kainate receptors. Synaptic IPSCs were evoked once per minute and alternated with photolysis-evoked postsynaptic GABA currents throughout the duration of the recordings. Photolysis was performed using a solid state, pulsed Nd:YAG laser (Minilite I, Continuum, Santa Clara, CA, USA). The laser output beam was channeled to a 40× water immersion microscope objective using a 400 μm diameter fiber optic light guide. This arrangement yielded a circular illumination area, approximately 25 μm in diameter. This spot was focused upon the pyramidal neuron soma to uncage α-carboxy-2-nitrobenzyl (CNB)-caged GABA (Invitrogen, Carlsbad, CA, USA). Once whole-cell access was obtained, the objective was focused upon the pyramidal neuron and the laser output was adjusted to yield a postsynaptic response that was similar in amplitude to a 50% of maximum electrically-evoked synaptic response. The settings of the laser and the electrical stimulator were then left undisturbed throughout the remainder of the experiment.
Drugs
WIN55,212-2 and AM251 were purchased from Tocris-Cookson (Ballwin, MO, USA). DNQX, APV, picrotoxin and randomly-methylated β-cyclodextrin (RAMEB) were purchased from Sigma (St. Louis, MO). CNB-caged GABA was purchased from Invitrogen (Carlsbad, CA, USA). Δ9-THC (200 mg/ml in EtOH) was obtained from the National Institute on Drug Abuse drug supply system (Bethesda, MD). The Δ9-THC resin was suspended in an equivalent volume of DMSO. The Δ9-THC solution was then diluted to 10 mM (3 mg/ml) in 22.5% RAMEB in 50% EtOH, and a stock solution prepared at 1 mM in 10% or 2.3% RAMEB. The final bath concentration of RAMEB was 0.1% in all experiments, unless indicated. For experiments described in Figure 1B, Δ9-THC was also prepared in Tween-80 (10%), DMSO (20%), and saline (70%). WIN55,212-2 was initially prepared as 10 mM stock solution in DMSO, and then diluted in Tween-80 and normal aCSF. Final concentrations of Tween-80 and DMSO in the tissue bath were 0.01% and 0.02%, respectively, and have been found have no effects on the hippocampal synaptic transmission at these concentrations.
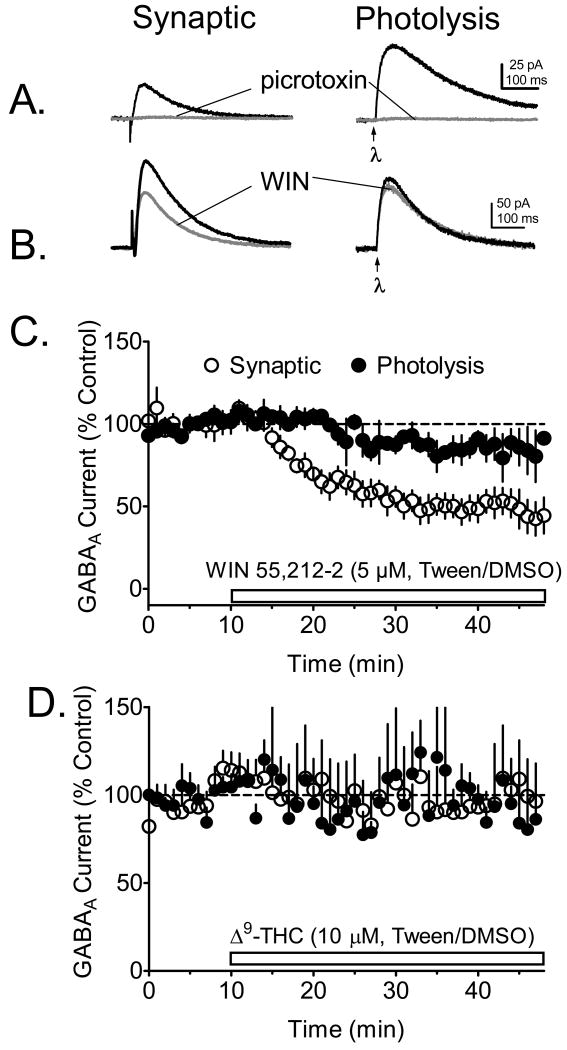
Figure 1.
Effects of cannabinoid agonists on GABAA receptor-mediated currents evoked synaptically, or through uv laser photolysis of CNB-caged GABA (50 μM) in WT mouse hippocampal CA1 pyramidal neurons. All recordings were performed during blockade of AMPA/kainate receptors with DNQX (10 μM) and NMDA receptors with APV (40 μM). A. Representative signal averages of outward GABAA receptor currents activated through either electrical stimulation (synaptic) or local photolysis of caged GABA by uv light (λ, applied at arrow) focused through the microscope objective. Note that both the synaptic and the photolysis GABA responses were completely blocked by the GABAA channel blocker picrotoxin (100 μM). B. Effects of the cannabinoid agonist WIN55,212-2 (dissolved in Tween-80/DMSO) on the mean time course of GABAA currents elicited through electrical stimulation or CNB-GABA photolysis in the same CA1 pyramidal neurons (n = 6 neurons). C. Mean time course of the effect Δ9-THC (dissolved in Tween-80/DMSO) on photolysis- and synaptically-evoked GABAA currents (n = 5 pyramidal neurons). Note the absence of an effect of Δ9-THC on IPSCs or photolysis-evoked currents in these experiments. A gluconic acid-based intracellular solution was used in these experiments.
Data analysis
All data are presented either as the mean ± SEM, or the mean ± C.I. (95% confidence interval). The n represents the number of neurons tested, with no more than 2 neurons obtained from a single animal. Peak amplitudes of IPSCs during drug application were normalized to the pre-drug (control) baseline period. All statistical analyses and curve fits were performed using Prism (v5.02, GraphPad Scientific, San Diego, CA). In all instances where time courses of drug effects were measured, a repeated measures analysis of variance (RM-ANOVA) was used, with appropriate post hoc analyses. The Δ9-THC concentration-response curve was fit using the sigmoidal non-linear regression function:
where Top and Bottom represent the plateau values of the Y-axis (% inhibition of the IPSC). IPSC decay time constants were fit using a single phase exponential decay function:
Where Y0 is the Y value when X (time) = zero, K is the rate constant, and tau (time constant of decay) = 1/K.
Results
Vehicle-dependent effects of Δ9-THC on GABA currents
Synaptic currents (IPSCs) and those activated by laser photolysis of caged GABA (CNB-GABA) were recorded in the same CA1 pyramidal neurons during application of the ionotropic glutamate receptor antagonists DNQX (10 μM), and APV (40 μM), in hippocampal slices from WT C57BL6/J mice. In general, the CNB-GABA photolysis currents exhibited kinetic properties that were similar to those of the synaptic IPSCs (Fig. 1A). In addition, both the synaptic IPSCs and the photolysis-evoked GABA currents were completely abolished by the GABAA blocker picrotoxin (100 μM, Fig. 1A). The cannabinoid receptor agonist WIN55,212-2 (5 μM), solubilized in a Tween-80/DMSO vehicle (Hoffman & Lupica, 2000), caused robust inhibition of the synaptic IPSC in wildtype mice, whereas the photolysis-evoked GABAA current was only slightly affected (Fig. 1B, 1C). We have shown previously that this effect of WIN55,212-2 on synaptic IPSCs in hippocampal CA1 pyramidal neurons is mediated by presynaptic CB1Rs and is absent in CB1-/- mice (Hoffman et al., 2005). In contrast to the effects of WIN55,212-2 on IPSCs, Δ9-THC (10 μM) had no effect on these responses or on photolysis-evoked GABA currents when it was solubilized in the same Tween-80/DMSO vehicle (Fig. 1D).
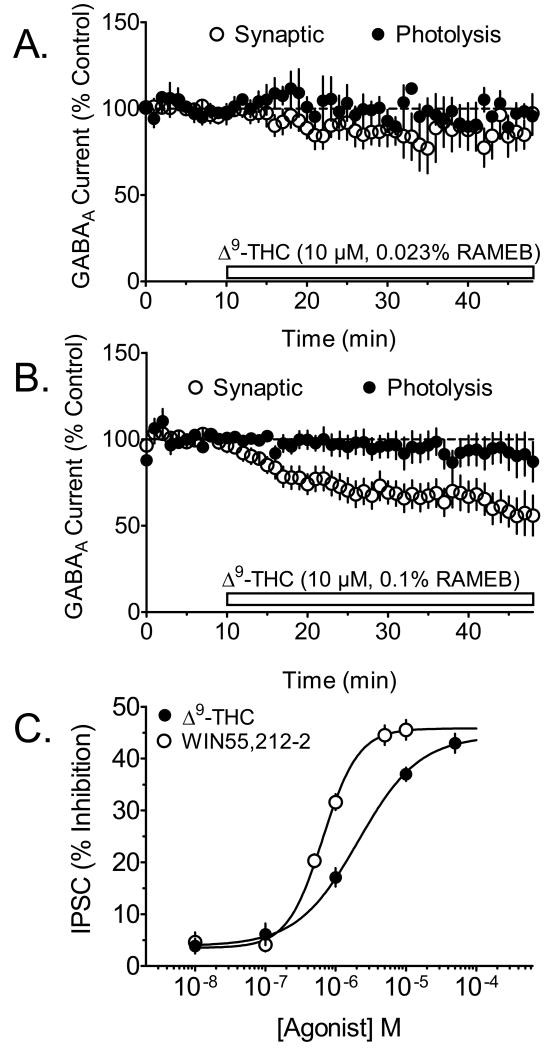
Since Δ9-THC is highly insoluble in aqueous solutions, we hypothesized that unlike WIN55,212-2, the Tween-80/DMSO vehicle may not permit adequate suspension of the phytocannabinoid. Recent chemical studies have shown that the solubility of Δ9-THC can be greatly increased by suspension in randomly-methylated-β-cyclodextrin (RAMEB) (Hazekamp & Verpoorte, 2006). Therefore, the effects of Δ9-THC dissolved in this vehicle were determined on IPSCs and photolysis-GABAergic currents. Δ9-THC (10 μM) dispersed in 0.023% RAMEB did not significantly affect IPSCs or photolysis-evoked GABA currents recorded in CA1 pyramidal neurons (Fig. 2A). However, when the same concentration of Δ9-THC was suspended in a higher concentration of RAMEB (0.1%) synaptic IPSCs were significantly inhibited (Fig. 2B). Also, this concentration of RAMEB alone had no effect on these currents (103 ± 16.9% of control at 50 min of application, n = 6 neurons). The inhibition of GABAergic IPSCs by Δ9-THC differed from that of WIN55,212-2 in that it was much slower to develop (compare Fig. 1C to Fig. 2B). Thus, a mean single exponential time constant for inhibition of IPSCs for Δ9-THC was 25.5 min, whereas that for by WIN55,212-2 (5 μM) was 9.8 min. This delay in the pharmacological effect of Δ9-THC likely resulted from the slower partition of the Δ9-THC-RAMEB complex in the brain slice, as compared to that for WIN55,212-2 in Tween-80/DMSO. Since the 0.1% RAMEB effectively permitted the inhibition of evoked synaptic IPSCs by Δ9-THC, but did not affect these responses by itself, this concentration of the vehicle was used for the remainder of the Δ9-THC experiments.
Figure 2.
Effects of different concentrations of RAMEB on Δ9-THC inhibition of IPSCs, and concentration response comparison with WIN55,212-2. A. Mean time course of the effect of Δ9-THC (10 μM) dissolved in 0.023% RAMEB solution on synaptic- and photolysis-evoked GABAA-mediated currents recorded in the same CA1 pyramidal neurons (n = 9 neurons). Note the absence of a significant effect of Δ9-THC on GABA currents in this concentration of the vehicle. B. Mean time course of the effect of Δ9-THC (10 μM) on photolysis- and synaptically-evoked GABAA currents (n = 11 neurons), using a higher concentration of the RAMEB vehicle (0.1%). Note the significant inhibition of the synaptically-evoked IPSCs by Δ9-THC using the 0.1% RAMEB solution, and the absence of effects on the photolysis-evoked currents. C. Concentration-dependent effect of Δ9-THC, suspended in 0.1% RAMEB, and WIN55,212-2 in Tween-80-DMSO, on synaptically evoked IPSCs. The EC50 for Δ9-THC was 1.22 μM (95% C.I. = 0.86 μM to 1.73 μM). Note that the maximal effects of Δ9-THC and WIN55,212-2 on IPSCs are comparable (p > 0.05, t-test). The number of neurons used for each point on the concentration-response curve was 5-7. The data shown in all subsequent figures used Δ9-THC in the 0.1 % concentration of RAMEB.
Full agonist properties of Δ9-THC at inhibitory axon terminals in the hippocampus
The inhibition of synaptic GABAergic IPSCs by Δ9-THC was dependent upon the concentration of this agonist (Fig. 2C). Thus, 50 μM Δ9-THC produced a mean maximal inhibition of the IPSCs of 42.9 ± 1.8%, and exhibited an EC50 value of 1.22 μM (95% C.I. = 0.86 μM to 1.73 μM). For comparison with the effects of Δ9-THC, a concentration-response relationship was also determined with the full CB1R agonist, WIN55,212-2. The maximal inhibition of IPSCs by WIN55,212-2 (10 μM, Fig 2C) suspended in DMSO/Tween 80, was, 45.5 ± 1.9%, which is comparable to the maximal effect of this drug in rat brain slices in our laboratory (46.6 ± 5.3%, Hoffman & Lupica, 2000). Since this value did differ significantly from the maximal inhibition of IPSCs produced by 50 μM Δ9-THC described above (p > 0.05, t-test), we conclude that WIN55,212-2 and Δ9-THC acted as full agonists in the inhibition of GABA release in the hippocampus.
Δ9-THC inhibits GABA neurotransmission via CB1R activation
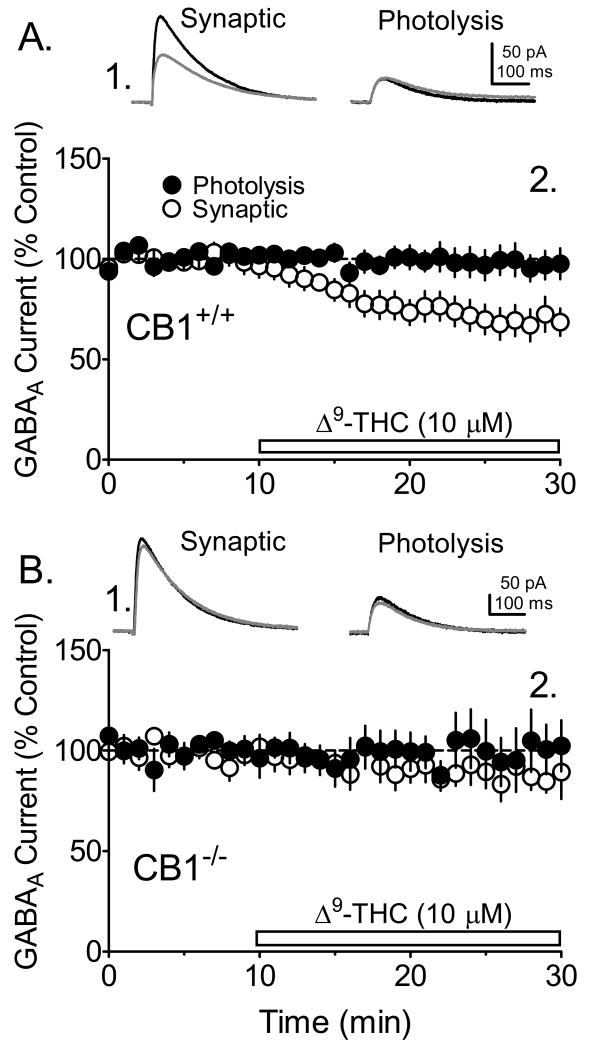
To determine whether the inhibition of GABAergic IPSCs by Δ9-THC occurred through activation of CB1Rs, we examined its effects in hippocampal brain slices obtained from CB1-/- mice that were littermates of the WT mice described in the experiments above. No effects of Δ9-THC on evoked IPSC amplitudes were observed in neurons from CB1-/- mice (Fig. 3B), and, as described above in WT hippocampal slices, Δ9-THC had no effect upon photolysis-evoked GABAA current amplitudes in CA1 pyramidal neuron recordings obtained from the CB1-/- mice (Fig. 3B).
Figure 3.
Effects of Δ9-THC on photolysis-evoked and synaptic GABAA currents in WT (CB1+/+) and KO(CB1-/-) mice. The GABAA receptor-mediated currents were evoked by alternating electrical stimulation of the hippocampal slice (synaptic) with uv laser photolysis of caged GABA in the same pyramidal neurons. A1. Signal averages of synaptic and photolysis-evoked GABA currents obtained prior to (black lines), and during Δ9-THC (10 μM) application (gray lines) in a CA1 pyramidal neuron from a CB1+/+ mouse. A2. The mean time course of the effect of Δ9-THC on photolysis-evoked and synaptic GABAA currents (n = 11 CA1 pyramidal neurons). Note the significant effect of Δ9-THC on synaptic IPSCs and the absence of an effect on photolysis-evoked currents. B1. Representative signal averages demonstrating the lack of Δ9-THC (gray lines) effect on synaptic and photolysis-evoked responses in CA1 pyramidal neuron obtained from CB1-/- mice (n = 6). B2. Mean time course demonstrating the lack of Δ9-THC effect on the GABAA currents. A gluconic acid-based intracellular solution was used in these experiments.
The effect of Δ9-THC on GABA neurotransmission is presynaptic
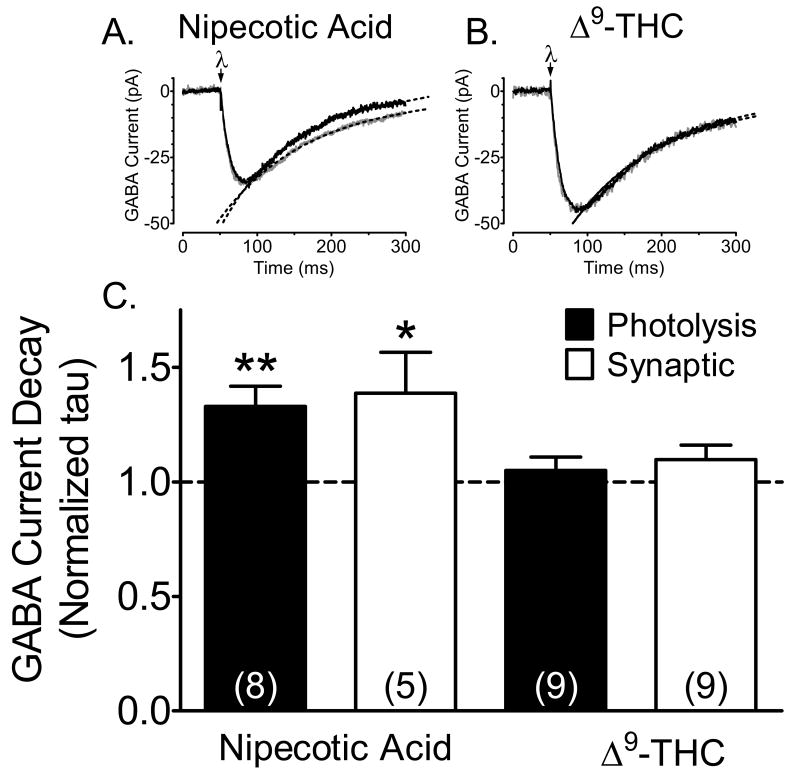
Although our observation that Δ9-THC inhibited synaptic IPSC amplitudes without altering photolysis-evoked GABA currents provided good evidence for a presynaptic action of this drug, others have suggested that Δ9-THC affects GABAergic neurotransmission by inhibiting GABA uptake and prolonging the duration of action of this neurotransmitter at the synapse (Banerjee et al., 1975; Coull et al., 1997; Maneuf et al., 1996). To address this we examined the effects of Δ9-THC, at a concentration that was maximal (10 μM) for inhibition of IPSCs via CB1R activation, on the decay kinetics of synaptic and photolysis-evoked GABA currents. In addition, the effects of Δ9-THC were compared with the GABA uptake inhibitor, nipecotic acid (10 μM). The decay phase of averaged IPSCs and photolysis-evoked currents were fitted with single exponential curves using an iterative best fit algorithm, and decay time constants (tau) were determined from this fit. The change in tau was then determined in each neuron by calculating a ratio of tau in normal aCSF to that measured 30 min after Δ9-THC or nipecotic acid was applied to the hippocampal brain slices (Fig. 4C). Whereas nipecotic acid significantly prolonged the duration of both synaptic and photolysis-evoked GABAA receptor-mediated currents (Fig. 4C), Δ9-THC (10 μM) had no effect on tau values of either the synaptic or photolysis-evoked currents (Fig. 4). This suggests that Δ9-THC did not directly alter GABA uptake, nor GABAa receptor/Cl- channel function on CA1 pyramidal neurons.
Figure 4.
Δ9-THC acts presynaptically to reduce GABAergic neurotransmission in the hippocampus. A. Effect of the competitive GABA uptake inhibitor nipecotic acid (10 μM, gray line) on averaged (n = 6-9 individual sweeps) inward currents evoked by CNB-GABA photolysis (uv laser flash applied at λ) in a CA1 pyramidal neuron. The control sweep is shown in black. Also shown (dashed lines) are single exponential curves fitted to the decay phase of the currents that were used to calculate the decay time constants (tau). Note that nipecotic acid significantly increased the tau value in this cell (control tau =100.8 ms, 95% C.I. = 99.0-102.7 ms; nipecotic acid tau =120.9 ms, 95% C.I. = 118.3-123.5 ms). Currents shown here and in B are scaled to the same amplitude. B. Lack of an effect of Δ9-THC (10 μM, gray line) on photolysis-evoked GABA current decay in a CA1 pyramidal neuron (control tau = 116.8 ms, 95% C.I. = 115.0-118.7 ms; Δ9-THC tau = 116.9 ms, 95% C.I. = 114.4-119.5 ms). C. Summary of the effect of nipecotic acid and Δ9-THC on decay tau values for GABA currents evoked by synaptic stimulation and photolysis of CNB caged GABA. Values represent ratios of the tau value acquired after drug application to that obtained during the control period. Note that Δ9-THC did not significantly affect synaptic or photolysis-evoked GABA current decay, whereas nipecotic acid prolonged the tau values for each of these currents. *, p < 0.01; **, p < 0.001, paired t-test. These data indicate that GABA uptake was unaffected by Δ9-THC under the present recording conditions.
Discussion
The central and peripheral effects of marijuana and its primary psychoactive constituent Δ9-THC have been the subject of intense study for many years. Furthermore, marijuana is a widely used illicit drug whose potency has increased in recent years due to selective hybridization to increased Δ9-THC levels (McLaren et al., 2008). Despite its widespread use and increased potency, few modern studies have examined the pharmacological and physiological actions of Δ9-THC on intact mature neuronal pathways. Additionally, although most studies show that cannabinoid agonists act at presynaptic CB1Rs to inhibit neurotransmitter release in the CNS, several groups report other actions of Δ9-THC, including inhibition of neurotransmitter uptake (Banerjee et al., 1975; Coull et al., 1997; Maneuf et al., 1996). The relative lack of knowledge regarding the effects of Δ9-THC and interest in the specific sites at which this widely used drug acts to disrupt memory processes (Hoffman et al., 2003; Hoffman et al., 2007), prompted us to examine these issues more directly using transgenic mice and optical uncaging methods to permit unequivocal distinction between pre- and postsynaptic effects of Δ9-THC in the hippocampus. Additionally, a recent study has demonstrated that selective deletion of the CB1R gene on GABA, but not glutamate neurons in the hippocampus can disrupt spatial learning in mice (Puighermanal et al., 2009), suggesting that this may be the primary site of Δ9-THC action in this brain structure.
Our initial experiments in hippocampal brain slices demonstrated that whereas the synthetic cannabinoid agonist WIN55,212-2 inhibited GABAergic IPSCs, no effect of Δ9-THC was observed when it was suspended in the same DMSO/Tween-80 vehicle (Fig. 1D). This vehicle is commonly used in this (Hoffman & Lupica, 2000), and other laboratories to suspend hydrophobic cannabinoid agonists that exhibit poor solubility in aqueous solutions (Banerjee et al., 1975). Cyclodextrins are naturally occurring water-soluble oligosaccharides that form inclusion complexes with hydrophobic molecules, greatly increasing their solubility (Fahr & Liu, 2007), and the cyclodextrin RAMEB greatly increases the solubility of Δ9-THC in water (Hazekamp & Verpoorte, 2006). Although these compounds increase lipophilic drug solubility, they can also alter baseline synaptic transmission in the hippocampus, secondary to extraction of cholesterol from cellular membranes (Frank et al., 2008). Interestingly, RAMEB-mediated cholesterol depletion selectively reduces glutamatergic and not GABAergic synaptic transmission (Frank et al., 2008; Hoffman et al., 2010). We also found that baseline GABergic synaptic transmission was unaltered by RAMEB, and that Δ9-THC significantly inhibited synaptic GABAA receptor-mediated IPSCs when it was suspended in this vehicle. The fact that RAMEB was necessary to solubilize Δ9-THC suggests that, even in comparison with other hydrophobic cannabinoid agonists like WIN55,212-2, the chemical properties of this molecule render it highly insoluble in standard vehicles used for studies in brain slices. This insolubility, and the relatively slow onset of Δ9-THC effects compared to compounds such as WIN55,212-2 should be taken into consideration when examining the pharmacological effect of this drug in vitro.
In contrast to its effects on IPSCs in pyramidal neurons from CB1+/+ mice, Δ9-THC did not affect these synaptic currents in brain slices obtained from CB1-/- mice. This, together with the lack of Δ9-THC effects on postsynaptic currents activated by the photolysis of caged GABA in the same neurons, strongly suggests that Δ9-THC acted at CB1Rs located on inhibitory axon terminals to affect GABA neurotransmission. This is consistent with the effects of synthetic cannabinoids on IPSCs in pyramidal neurons (Hoffman & Lupica, 2000; Katona et al., 1999), and with the dense expression of CB1Rs on GABAergic axon terminals in the CA1 region of the hippocampus (Marsicano & Lutz, 1999; Tsou et al., 1998; Tsou et al., 1999).
Previous studies of Δ9-THC's effects in hippocampal brain slices have demonstrated relatively complex actions on pyramidal neuron population responses. Thus, at a very low concentration (10 pM) Δ9-THC increased population spike amplitude, whereas it inhibited these responses at concentrations as high as 10 nM (Foy et al., 1982; Nowicky et al., 1987). However, in the latter study, population EPSPs (field EPSPs) were uniformly inhibited by Δ9-THC across this same range of concentrations (Nowicky et al., 1987). In the present study, we found that significant effects of Δ9-THC on GABAergic IPSCs were not observed at concentrations below ∼100 nM (Fig 2C). At present the reason for this discrepancy is unknown. However, one subsequent study reported an EC50 value of 20 nM for Δ9-THC's inhibition of glutamate synaptic responses in cultured hippocampal neurons (Shen & Thayer, 1999), whereas another reported no inhibition of these responses in cultured hippocampal cells (Straiker & Mackie, 2005). This high degree of variability in the pharmacological effects of Δ9-THC may relate to its insolubility in aqueous buffers used during in vitro physiological experiments, and the relatively high EC50 in the present study (∼1.2 μM) may reflect the comparatively poor degree of agonist penetration in the hippocampal brain slice, compared to more accessible receptor sites in cell culture systems.
Based on its relatively limited effects on glutamate release from axon terminals in hippocampal cultures (Shen & Thayer, 1999, and see Sim et al., 1996), Δ9-THC has been proposed to act as a partial agonist in the hippocampus. In addition, it has also been shown that that Δ9-THC was without agonist activity at hippocampal glutamate terminals, but could antagonize the effects of full CB1R agonists at this site (Straiker & Mackie, 2005). This observation prompted the hypothesis that some of the psychotropic effects of Δ9-THC may be mediated, not by the activation of CB1Rs, but rather by antagonism of endogenously released cannabinoids (Straiker & Mackie, 2005). Classical receptor theory predicts that low efficacy ligands like Δ9-THC will behave as pure antagonists or partial agonists in systems where the stimulation of a large proportion of receptors is required to see a physiological response, or if there is a limited spare receptor reserve (Hoyer & Boddeke, 1993; Morissette et al., 2007). In contrast, the same ligand can exhibit full efficacy when there is a large receptor reserve, and a smaller fraction of receptor occupancy is required to see a complete physiological response. Since CB1Rs are much more densely expressed on GABAergic versus glutamatergic axon terminals in the hippocampus (Kawamura et al., 2006), one would predict that Δ9-THC should exhibit higher efficacy in inhibiting GABA release than that observed for the inhibition of glutamate release. Commensurate with this hypothesis, Δ9-THC's maximal inhibition of GABAergic IPSCs was similar to the full agonist WIN55,212-2 in the present study (and see Hoffman & Lupica, 2000), and the maximal effects of Δ9-THC on EPSCs was smaller than WIN55,212-2 using the same brain slices under identical recording conditions, when the independent effects of RAMEB on EPSCs were considered (Hoffman et al., 2010).
This differential pharmacological profile of Δ9-THC at glutamate and GABAergic axon terminals has important implications for interpreting its effects on hippocampal circuitry, and for a more complete understanding of its ability to disrupt hippocampal function. Since Δ9-THC would preferentially activate CB1Rs on inhibitory axon terminals to inhibit GABA release, and have smaller effects on glutamate release, it is likely that the deleterious effects of marijuana on learning and memory are primarily mediated through GABAergic mechanisms. This may occur as a result of the disruption of hippocampal network activity that is driven by synchronized GABA neuron activity, which is known to be disrupted by cannabinoids (Hajos et al., 2000; Robbe et al., 2006). Further support for hippocampal GABAergic networks in mediating the effects of Δ9-THC is also found in a recent study showing that disruption of hippocampal-dependent behavior by Δ9-THC was absent in transgenic animals containing a targeted deletion of the CB1R gene in GABAergic, but not glutamatergic neurons (Puighermanal et al., 2009). This suggests that alterations in hippocampal GABAergic transmission are primarily responsible for the memory disrupting effects of Δ9-THC, and the present results demonstrate that this is likely through full agonist actions of this drug at these abundant CB1Rs.
Several studies have demonstrated that Δ9-THC can inhibit the clearance of neurotransmitters, including GABA, from the extracellular space in the CNS (Banerjee et al., 1975; Coull et al., 1997; Maneuf et al., 1996). However, we found that despite clear effects of the GABA uptake inhibitor nipecotic acid on the rates of decay of synaptic and photolysis-evoked GABAA currents, Δ9-THC had no effect on these measures at concentrations that strongly inhibited IPSCs via CB1Rs. This suggests that whereas the synaptic release of GABA is inhibited by Δ9-THC acting at CB1Rs, it is unlikely that the influence of GABA will be prolonged at this synapse by the inhibition of GABA uptake at these synapses.
Conclusion
Marijuana can profoundly impair human memory through interference with the acquisition of new information and its recall (Abel, 1971a; Abel, 1971b; Ranganathan & D'Souza, 2006; Wilson et al., 1994), and working memory is profoundly impaired in animals exposed to marijuana smoke, or by hippocampal injection of Δ9-THC (Lichtman et al., 1995; Niyuhire et al., 2007). More recently it has been shown that intrahippocampal injection of a CB1R antagonist can block the memory disruptive effects of systemic Δ9-THC, strongly supporting the involvement of CB1Rs and the hippocampus in this effect of the drug (Wise et al., 2009). The present study demonstrates that Δ9-THC inhibits GABA release via presynaptic CB1Rs in the absence of postsynaptic effects and that, unlike its actions at glutamate axon terminals in the hippocampus, it appears to act as a full agonist at GABA terminals. Thus, the modulation of the inhibitory hippocampal circuitry should be considered as a primary site for actions of Δ9-THC in this brain structure.
Acknowledgments
We would like to acknowledge the National Institutes of Health and the National Institute on Drug Abuse Intramural Research Program for support of this work.
This work supported by the National Institutes of Health and the National Institute on Drug Abuse Intramural Research Program.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- APV
D-(-)-2-amino-5-phosphonopentanoic acid
- CB1+/+
wildtype C57BL6/J mice
- CB1-/-
C57BL6/J mice lacking the gene for the CB1 receptor
- CB1R
cannabinoid type 1 receptor
- CNB
of α-carboxy-2-nitrobenzyl
- Δ9-THC
delta-9-tetrahydrocannabinol
- DIC
differential interference contrast microscopy
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- Nd
YAG, neodymium-doped yttrium aluminum garnet
- LTD
long-term depression
- LTP
long-term potentiation
- RAMEB
randomly methylated β-cyclodextrin
- WIN55212-2
(R)-(+)-[2,3-Dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. Marihuana and memory: acquisition or retrieval? Science. 1971a;173:1038–1040. doi: 10.1126/science.173.4001.1038. [DOI] [PubMed] [Google Scholar]
- Abel EL. Retrieval of information after use of marihuana. Nature. 1971b;231:58. doi: 10.1038/231058a0. [DOI] [PubMed] [Google Scholar]
- Banerjee SP, Snyder SH, Mechoulam R. Cannabinoids: influence on neurotransmitter uptake in rat brain synaptosomes. J Pharmacol Exp Ther. 1975;194:74–81. [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Burns TL, Ineck JR. Cannabinoid Analgesia as a Potential New Therapeutic Option in the Treatment of Chronic Pain. Ann Pharmacother. 2006;40:251–260. doi: 10.1345/aph.1G217. [DOI] [PubMed] [Google Scholar]
- Coull MA, Johnston AT, Pertwee RG, Davies SN. Action of Delta-9-tetrahydrocannabinol on GABAA receptor- mediated responses in a grease-gap recording preparation of the rat hippocampal slice. Neuropharmacol. 1997;36:1387–1392. doi: 10.1016/s0028-3908(97)00110-x. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Fahr A, Liu X. Drug delivery strategies for poorly water-soluble drugs. Expert Opinion on Drug Delivery. 2007;4:403–416. doi: 10.1517/17425247.4.4.403. [DOI] [PubMed] [Google Scholar]
- Fan N, Yang H, Zhang J, Chen C. Reduced expression of glutamate receptors and phosphorylation of CREB are responsible for in vivoDelta9-THC exposure-impaired hippocampal synaptic plasticity. J Neurochem. 2010;112:691–702. doi: 10.1111/j.1471-4159.2009.06489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Teyler TJ, Vardaris RM. delta 9-THC and 17-beta-estradiol in hippocampus. Brain Res Bull. 1982;8:341–345. doi: 10.1016/0361-9230(82)90070-3. [DOI] [PubMed] [Google Scholar]
- Frank C, Rufini S, Tancredi V, Forcina R, Grossi D, D'Arcangelo G. Cholesterol depletion inhibits synaptic transmission and synaptic plasticity in rat hippocampus. Experimental Neurology. 2008;212:407–414. doi: 10.1016/j.expneurol.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Amer Chem Soc. 1964;86:1646. [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, Mackie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Role of cannabinoid receptors in memory storage. Neurobiol Dis. 1998;5:474–482. doi: 10.1006/nbdi.1998.0223. [DOI] [PubMed] [Google Scholar]
- Hazekamp A, Verpoorte R. Structure elucidation of the tetrahydrocannabinol complex with randomly methylated [beta]-cyclodextrin. European Journal of Pharmaceutical Sciences. 2006;29:340–347. doi: 10.1016/j.ejps.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, De Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther. 1993;264:294–307. [PubMed] [Google Scholar]
- Hoffman AF, Laaris N, Kawamura M, Masino SA, Lupica CR. Control of Cannabinoid CB1 Receptor Function on Glutamate Axon Terminals by Endogenous Adenosine Acting at A1 Receptors. Journal of Neuroscience. 2010;30:545–555. doi: 10.1523/JNEUROSCI.4920-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J Neurophysiol. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Yang R, Lichtman AH, Lupica CR. Opposing actions of chronic Δ9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn Mem. 2007;14:63–74. doi: 10.1101/lm.439007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Riegel AC, Lupica CR. Functional localization of cannabinoid receptors and endogenous cannabinoid production in distinct neuron populations of the hippocampus. Eur J Neurosci. 2003;18:524–534. doi: 10.1046/j.1460-9568.2003.02773.x. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Macgill AM, Smith D, Oz M, Lupica CR. Species and strain differences in the expression of a novel glutamate-modulating cannabinoid receptor in the rodent hippocampus. European Journal of Neuroscience. 2005;22:2387–2391. doi: 10.1111/j.1460-9568.2005.04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Boddeke HW. Partial agonists, full agonists, antagonists: dilemmas of definition. Trends Pharmacol Sci. 1993;14:270–275. doi: 10.1016/0165-6147(93)90129-8. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Jarho P, Pate DW, Brenneisen R, Jarvinen T. Hydroxypropyl-beta-cyclodextrin and its combination with hydroxypropyl-methylcellulose increases aqueous solubility of delta9-tetrahydrocannabinol. Life Sci. 1998;63:L381–L384. doi: 10.1016/s0024-3205(98)00528-1. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BG, Thayer SA. Delta 9-tetrahydrocannabinol antagonizes endocannabinoid modulation of synaptic transmission between hippocampal neurons in culture. Neuropharmacol. 2004;46:709–715. doi: 10.1016/j.neuropharm.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Levenes C, Daniel H, Soubrie P, Crepel F. Cannabinoids decrease excitatory synaptic transmission and impair long- term depression in rat cerebellar Purkinje cells. J Physiol (Lond) 1998;510:867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 1995;119:282–290. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Maneuf YP, Nash JE, Crossman AR, Brotchie JM. Activation of the cannabinoid receptor by [Delta]9-tetrahydrocannabinol reduces [gamma]-aminobutyric acid uptake in the globus pallidus. European Journal of Pharmacology. 1996;308:161–164. doi: 10.1016/0014-2999(96)00326-3. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McLaren J, Swift W, Dillon P, Allsop S. Cannabis potency and contamination: a review of the literature. Addiction. 2008;103:1100–1109. doi: 10.1111/j.1360-0443.2008.02230.x. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Misner DL, Sullivan JM. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J Neurosci. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette G, Houle S, Gera L, Stewart JM, Marceau F. Antagonist, partial agonist and antiproliferative actions of B-9870 (CU201) as a function of the expression and density of the bradykinin B1 and B2 receptors. Br J Pharmacol. 2007;150:369–379. doi: 10.1038/sj.bjp.0706982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, bu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Niyuhire F, Varvel SA, Martin BR, Lichtman AH. Exposure to Marijuana Smoke Impairs Memory Retrieval in Mice. Journal of Pharmacology And Experimental Therapeutics. 2007;322:1067–1075. doi: 10.1124/jpet.107.119594. [DOI] [PubMed] [Google Scholar]
- Nowicky AV, Teyler TJ, Vardaris RM. The modulation of long-term potentiation by delta-9- tetrahydrocannabinol in the rat hippocampus, in vitro. Brain Res Bull. 1987;19:663–672. doi: 10.1016/0361-9230(87)90052-9. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D'Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Roloff AM, Thayer SA. Modulation of Excitatory Synaptic Transmission by Delta(9)-Tetrahydrocannabinol Switches from Agonist to Antagonist Depending on Firing Rate. Molecular Pharmacology. 2009;75:892–900. doi: 10.1124/mol.108.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Thayer SA. Delta 9-Tetrahydrocannabinol Acts as a Partial Agonist to Modulate Glutamatergic Synaptic Transmission between Rat Hippocampal Neurons in Culture. Molecular Pharmacology. 1999;55:8–13. doi: 10.1124/mol.55.1.8. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16:8057–8066. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tsou K, Mackie K, MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Wilson WH, Ellinwood EH, Mathew RJ, Johnson K. Effects of marijuana on performance of a computerized cognitive-neuromotor test battery. Psychiatry Res. 1994;51:115–125. doi: 10.1016/0165-1781(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Wise LE, Thorpe AJ, Lichtman AH. Hippocampal CB1 Receptors Mediate the Memory Impairing Effects of [Delta]9-Tetrahydrocannabinol. Neuropsychopharmacology. 2009;34:2072–2080. doi: 10.1038/npp.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]