Abstract
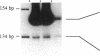
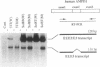
Approximately 2% of Caucasians and African-Americans are homozygous for a nonsense mutation in exon 2 of the AMPD1 (AMP deaminase) gene. These individuals have a high grade deficiency of AMPD activity in their skeletal muscle. More than 100 patients with AMPD1 deficiency have been reported to have symptoms of a metabolic myopathy, but it is apparent many individuals with this inherited defect are asymptomatic given the prevalence of this mutant. Results of the present study provide a potential molecular explanation for "correction" of this genetic defect. Alternative splicing eliminates exon 2 in 0.6-2% of AMPD1 mRNA transcripts in adult skeletal muscle. Expression studies document that AMPD1 mRNA, which has exon 2 deleted, encodes a functional AMPD peptide. A much higher percentage of alternatively spliced transcripts are found during differentiation of human myocytes in vitro. Transfection studies with human minigene constructs demonstrate that alternative splicing of the primary transcript of human AMPD1 is controlled by tissue-specific and stage-specific signals. Alternative splicing of exon 2 in individuals who have inherited this defect provides a mechanism for phenotypic rescue and variations in splicing patterns may contribute to the variability in clinical symptoms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowsky W., Gershon A. A., Shenkman L., Hirschhorn R. Adenosine deaminase deficiency without immunodeficiency: clinical and metabolic studies. Pediatr Res. 1980 Jul;14(7):885–889. doi: 10.1203/00006450-198007000-00009. [DOI] [PubMed] [Google Scholar]
- Cobianchi F., Wilson S. H. Enzymes for modifying and labeling DNA and RNA. Methods Enzymol. 1987;152:94–110. doi: 10.1016/0076-6879(87)52013-4. [DOI] [PubMed] [Google Scholar]
- Daddona P. E., Mitchell B. S., Meuwissen H. J., Davidson B. L., Wilson J. M., Koller C. A. Adenosine deaminase deficiency with normal immune function. An acidic enzyme mutation. J Clin Invest. 1983 Aug;72(2):483–492. doi: 10.1172/JCI110996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B. L., Tarlé S. A., Palella T. D., Kelley W. N. Molecular basis of hypoxanthine-guanine phosphoribosyltransferase deficiency in ten subjects determined by direct sequencing of amplified transcripts. J Clin Invest. 1989 Jul;84(1):342–346. doi: 10.1172/JCI114160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein W. N., Armbrustmacher V. W., Griffin J. L. Myoadenylate deaminase deficiency: a new disease of muscle. Science. 1978 May 5;200(4341):545–548. doi: 10.1126/science.644316. [DOI] [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen J., Rice D. R., Bradley W. G., Munsat T. L., DiMauro S., Hogan E. L. Familial myoadenylate deaminase deficiency and exertional myalgia. Neurology. 1982 Aug;32(8):857–863. doi: 10.1212/wnl.32.8.857. [DOI] [PubMed] [Google Scholar]
- Mineo I., Clarke P. R., Sabina R. L., Holmes E. W. A novel pathway for alternative splicing: identification of an RNA intermediate that generates an alternative 5' splice donor site not present in the primary transcript of AMPD1. Mol Cell Biol. 1990 Oct;10(10):5271–5278. doi: 10.1128/mcb.10.10.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo I., Holmes E. W. Exon recognition and nucleocytoplasmic partitioning determine AMPD1 alternative transcript production. Mol Cell Biol. 1991 Oct;11(10):5356–5363. doi: 10.1128/mcb.11.10.5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarras D., Pinset C., Chelly J., Kahn A., Gros F. Expression of MyoD1 coincides with terminal differentiation in determined but inducible muscle cells. EMBO J. 1989 Aug;8(8):2203–2207. doi: 10.1002/j.1460-2075.1989.tb08343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki T., Gross M., Morisaki H., Pongratz D., Zöllner N., Holmes E. W. Molecular basis of AMP deaminase deficiency in skeletal muscle. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6457–6461. doi: 10.1073/pnas.89.14.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki T., Sabina R. L., Holmes E. W. Adenylate deaminase. A multigene family in humans and rats. J Biol Chem. 1990 Jul 15;265(20):11482–11486. [PubMed] [Google Scholar]
- Ogden R. C., Adams D. A. Electrophoresis in agarose and acrylamide gels. Methods Enzymol. 1987;152:61–87. doi: 10.1016/0076-6879(87)52011-0. [DOI] [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabina R. L., Fishbein W. N., Pezeshkpour G., Clarke P. R., Holmes E. W. Molecular analysis of the myoadenylate deaminase deficiencies. Neurology. 1992 Jan;42(1):170–179. doi: 10.1212/wnl.42.1.170. [DOI] [PubMed] [Google Scholar]
- Sabina R. L., Morisaki T., Clarke P., Eddy R., Shows T. B., Morton C. C., Holmes E. W. Characterization of the human and rat myoadenylate deaminase genes. J Biol Chem. 1990 Jun 5;265(16):9423–9433. [PubMed] [Google Scholar]
- Sabina R. L., Ogasawara N., Holmes E. W. Expression of three stage-specific transcripts of AMP deaminase during myogenesis. Mol Cell Biol. 1989 May;9(5):2244–2246. doi: 10.1128/mcb.9.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalstieg F. C., Mills G. C., Tsuda H., Goldman A. S. Severe combined immunodeficiency in a child with a healthy adenosine deaminase deficient mother. Pediatr Res. 1983 Dec;17(12):935–940. doi: 10.1203/00006450-198312000-00002. [DOI] [PubMed] [Google Scholar]
- Shumate J. B., Katnik R., Ruiz M., Kaiser K., Frieden C., Brooke M. H., Carroll J. E. Myoadenylate deaminase deficiency. Muscle Nerve. 1979 May-Jun;2(3):213–216. doi: 10.1002/mus.880020309. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Stout J. T., Palella T. D., Davidson B. L., Kelley W. N., Caskey C. T. A molecular survey of hypoxanthine-guanine phosphoribosyltransferase deficiency in man. J Clin Invest. 1986 Jan;77(1):188–195. doi: 10.1172/JCI112275. [DOI] [PMC free article] [PubMed] [Google Scholar]