Abstract
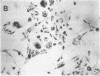
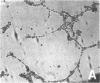
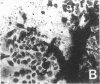
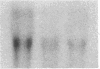
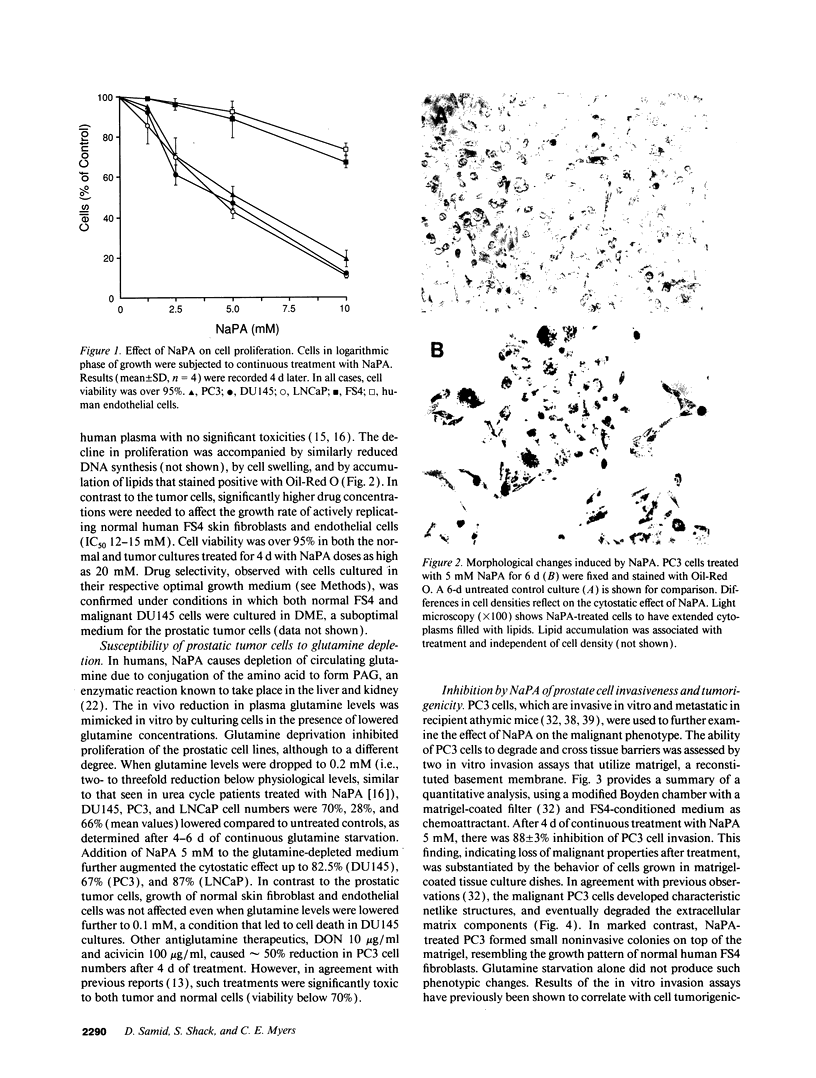
Differentiation therapy may provide an alternative for treatment of cancers that do not respond to cytotoxic chemotherapy or hormonal manipulations. This hypothesis led us to evaluate the effect of a nontoxic differentiation inducer, sodium phenylacetate (NaPA), on hormone-refractory prostate cancer, the second most common cause of cancer deaths in men. NaPA treatment of androgen-independent PC3 and DU145 prostate cell lines, like that of hormone-responsive LNCaP cultures, resulted in dose-dependent inhibition of cell proliferation. Similar treatments were not significantly inhibitory to replicating normal endothelial cells and skin fibroblasts. In addition to the selective cytostatic effect, NaPA induced reversion of the prostatic cells to a nonmalignant phenotype, evidenced by their reduced invasiveness and loss of tumorigenicity in athymic mice. Phenotypic reversion was accompanied by alterations in gene expression, including selective reduction in tumor growth factor-beta 2 mRNA levels and increased amounts of class I major histocompatibility complex HLA transcripts. Furthermore, there was a decrease in tumor-associated proteolysis mediated by urokinase plasminogen activator, a molecular marker of disease progression in humans. When tumor cells were treated with NaPA together with suramin, a drug with demonstrable activity in patients, there was complete abrogation of cell growth under conditions in which each treatment alone produced only a partial effect. The in vitro antineoplastic activity was observed with drug concentrations that have been achieved in humans with no significant toxicities, suggesting that PA, used alone or in combination with other antitumor agents, warrants evaluation in the treatment of advanced prostatic cancer.
Full text
PDF

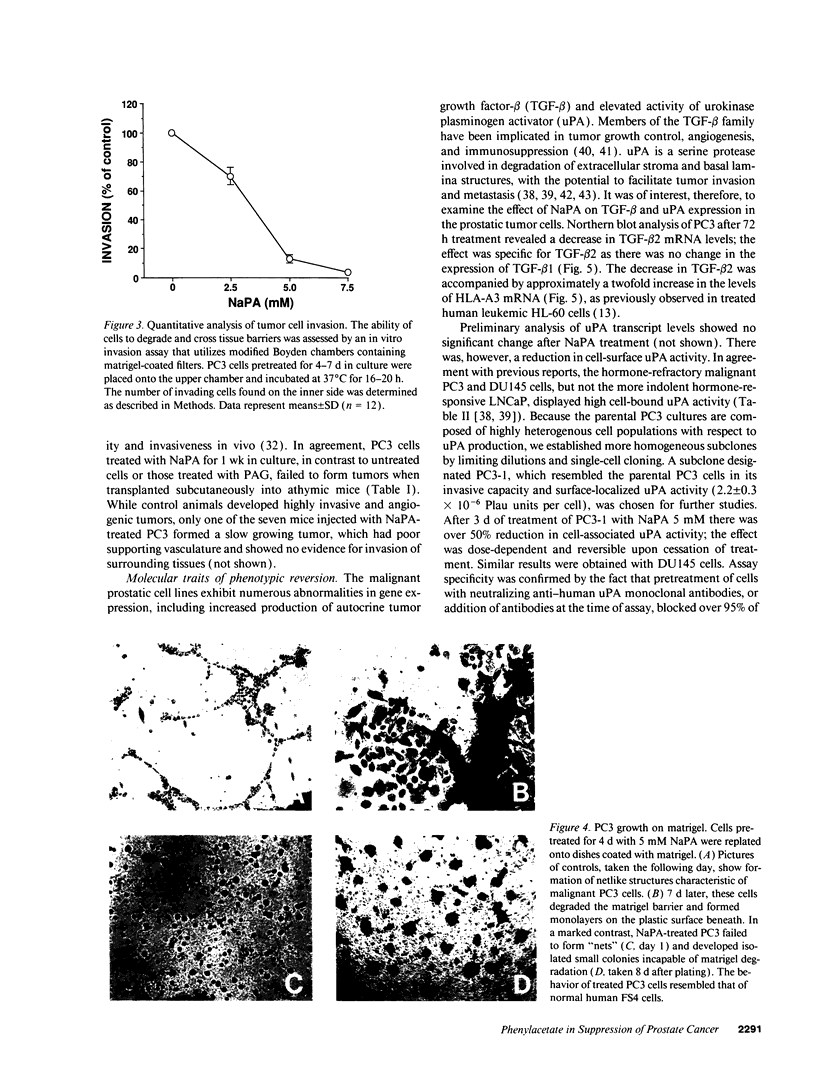
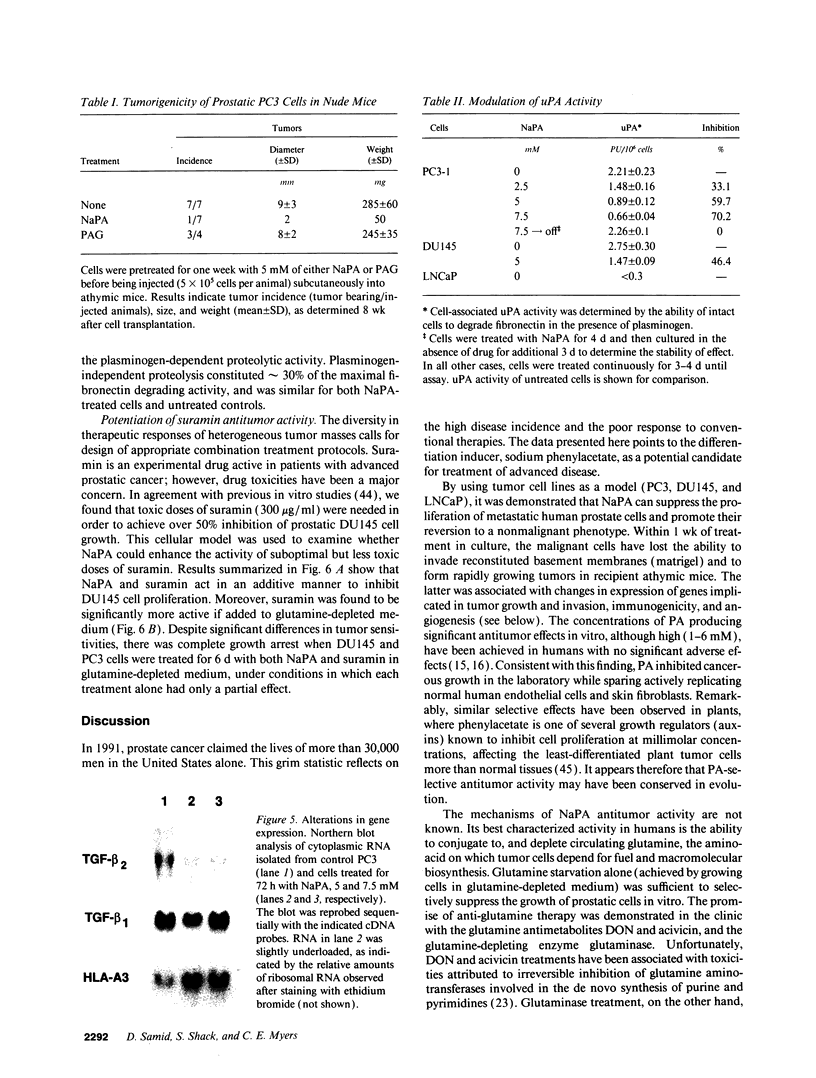
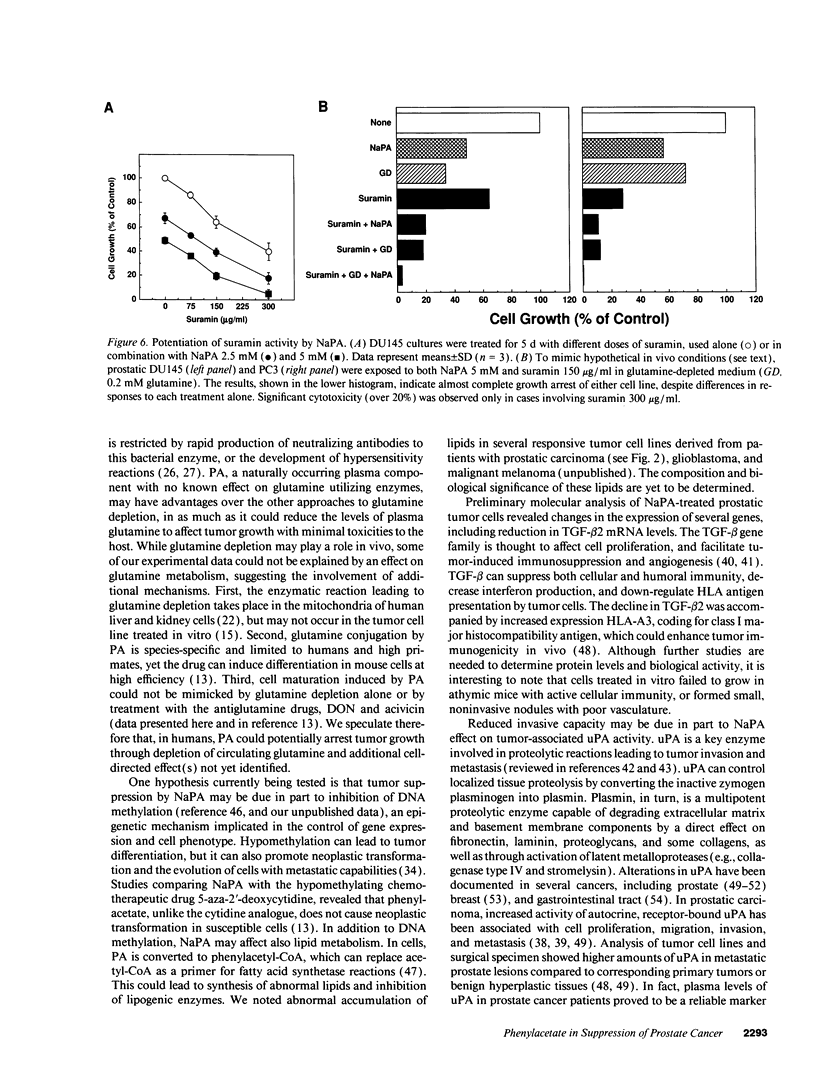


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Iwamoto Y., Kleinman H. K., Martin G. R., Aaronson S. A., Kozlowski J. M., McEwan R. N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987 Jun 15;47(12):3239–3245. [PubMed] [Google Scholar]
- Blasi F., Verde P. Urokinase-dependent cell surface proteolysis and cancer. Semin Cancer Biol. 1990 Apr;1(2):117–126. [PubMed] [Google Scholar]
- Bloch A. Induced cell differentiation in cancer therapy. Cancer Treat Rep. 1984 Jan;68(1):199–205. [PubMed] [Google Scholar]
- Brusilow S. W., Danney M., Waber L. J., Batshaw M., Burton B., Levitsky L., Roth K., McKeethren C., Ward J. Treatment of episodic hyperammonemia in children with inborn errors of urea synthesis. N Engl J Med. 1984 Jun 21;310(25):1630–1634. doi: 10.1056/NEJM198406213102503. [DOI] [PubMed] [Google Scholar]
- Camiolo S. M., Markus G., Englander L. S., Siuta M. R., Hobika G. H., Kohga S. Plasminogen activator content and secretion in explants of neoplastic and benign human prostate tissues. Cancer Res. 1984 Jan;44(1):311–318. [PubMed] [Google Scholar]
- Chiarodo A. National Cancer Institute roundtable on prostate cancer: future research directions. Cancer Res. 1991 May 1;51(9):2498–2505. [PubMed] [Google Scholar]
- Cowan E. P., Jordan B. R., Coligan J. E. Molecular cloning and DNA sequence analysis of genes encoding cytotoxic T lymphocyte-defined HLA-A3 subtypes: the E1 subtype. J Immunol. 1985 Oct;135(4):2835–2841. [PubMed] [Google Scholar]
- Dover G. J., Brusilow S., Samid D. Increased fetal hemoglobin in patients receiving sodium 4-phenylbutyrate. N Engl J Med. 1992 Aug 20;327(8):569–570. doi: 10.1056/NEJM199208203270818. [DOI] [PubMed] [Google Scholar]
- Duffy M. J., Reilly D., O'Sullivan C., O'Higgins N., Fennelly J. J., Andreasen P. Urokinase-plasminogen activator, a new and independent prognostic marker in breast cancer. Cancer Res. 1990 Nov 1;50(21):6827–6829. [PubMed] [Google Scholar]
- Eisenberger M. A. Chemotherapy for endocrine resistant cancer of the prostate. Prog Clin Biol Res. 1990;359:155–180. [PubMed] [Google Scholar]
- Gaylis F. D., Keer H. N., Wilson M. J., Kwaan H. C., Sinha A. A., Kozlowski J. M. Plasminogen activators in human prostate cancer cell lines and tumors: correlation with the aggressive phenotype. J Urol. 1989 Jul;142(1):193–198. doi: 10.1016/s0022-5347(17)38709-8. [DOI] [PubMed] [Google Scholar]
- Gittes R. F. Carcinoma of the prostate. N Engl J Med. 1991 Jan 24;324(4):236–245. doi: 10.1056/NEJM199101243240406. [DOI] [PubMed] [Google Scholar]
- Golomb H. M., Ratain M. J., Mick R., Daly K. The treatment of hairy cell leukemia: an update. Leukemia. 1992;6 (Suppl 2):24–27. [PubMed] [Google Scholar]
- Grant D. S., Lelkes P. I., Fukuda K., Kleinman H. K. Intracellular mechanisms involved in basement membrane induced blood vessel differentiation in vitro. In Vitro Cell Dev Biol. 1991 Apr;27A(4):327–336. doi: 10.1007/BF02630910. [DOI] [PubMed] [Google Scholar]
- Gross J. L., Behrens D. L., Mullins D. E., Kornblith P. L., Dexter D. L. Plasminogen activator and inhibitor activity in human glioma cells and modulation by sodium butyrate. Cancer Res. 1988 Jan 15;48(2):291–296. [PubMed] [Google Scholar]
- Hanks S. K., Armour R., Baldwin J. H., Maldonado F., Spiess J., Holley R. W. Amino acid sequence of the BSC-1 cell growth inhibitor (polyergin) deduced from the nucleotide sequence of the cDNA. Proc Natl Acad Sci U S A. 1988 Jan;85(1):79–82. doi: 10.1073/pnas.85.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner G. H., Miller B. E. Tumor heterogeneity: biological implications and therapeutic consequences. Cancer Metastasis Rev. 1983;2(1):5–23. doi: 10.1007/BF00046903. [DOI] [PubMed] [Google Scholar]
- Hienert G., Kirchheimer J. C., Christ G., Pflüger H., Binder B. R. Plasma urokinase-type plasminogen activator correlates to bone scintigraphy in prostatic carcinoma. Eur Urol. 1988;15(3-4):256–258. doi: 10.1159/000473447. [DOI] [PubMed] [Google Scholar]
- Hienert G., Kirchheimer J. C., Pflüger H., Binder B. R. Urokinase-type plasminogen activator as a marker for the formation of distant metastases in prostatic carcinomas. J Urol. 1988 Dec;140(6):1466–1469. doi: 10.1016/s0022-5347(17)42074-x. [DOI] [PubMed] [Google Scholar]
- Holcenberg J. S. Enzyme therapy of cancer, future studies. Cancer Treat Rep. 1981;65 (Suppl 4):61–65. [PubMed] [Google Scholar]
- Hoosein N. M., Boyd D. D., Hollas W. J., Mazar A., Henkin J., Chung L. W. Involvement of urokinase and its receptor in the invasiveness of human prostatic carcinoma cell lines. Cancer Commun. 1991 Aug;3(8):255–264. doi: 10.3727/095535491820873146. [DOI] [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Chu T. M., Wajsman Z. L., Friedman M., Papsidero L., Kim U., Chai L. S., Kakati S., Arya S. K. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- James M. O., Smith R. L., Williams R. T., Reidenberg M. The conjugation of phenylacetic acid in man, sub-human primates and some non-primate species. Proc R Soc Lond B Biol Sci. 1972 Jul 25;182(1066):25–35. doi: 10.1098/rspb.1972.0064. [DOI] [PubMed] [Google Scholar]
- Kaighn M. E., Lechner J. F., Narayan K. S., Jones L. W. Prostate carcinoma: tissue culture cell lines. Natl Cancer Inst Monogr. 1978 Dec;(49):17–21. [PubMed] [Google Scholar]
- Keating M. T., Escobedo J. A., Williams L. T. Ligand activation causes a phosphorylation-dependent change in platelet-derived growth factor receptor conformation. J Biol Chem. 1988 Sep 15;263(26):12805–12808. [PubMed] [Google Scholar]
- Kirchheimer J. C., Pflüger H., Ritschl P., Hienert G., Binder B. R. Plasminogen activator activity in bone metastases of prostatic carcinomas as compared to primary tumors. Invasion Metastasis. 1985;5(6):344–355. [PubMed] [Google Scholar]
- Kishore G., Sugumaran M., Vaidyanathan C. S. Metabolism of DL-(+/-)-phenylalanine by Aspergillus niger. J Bacteriol. 1976 Oct;128(1):182–191. doi: 10.1128/jb.128.1.182-191.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rocca R. V., Danesi R., Cooper M. R., Jamis-Dow C. A., Ewing M. W., Linehan W. M., Myers C. E. Effect of suramin on human prostate cancer cells in vitro. J Urol. 1991 Feb;145(2):393–398. doi: 10.1016/s0022-5347(17)38351-9. [DOI] [PubMed] [Google Scholar]
- Maxwell M., Galanopoulos T., Neville-Golden J., Antoniades H. N. Effect of the expression of transforming growth factor-beta 2 in primary human glioblastomas on immunosuppression and loss of immune surveillance. J Neurosurg. 1992 May;76(5):799–804. doi: 10.3171/jns.1992.76.5.0799. [DOI] [PubMed] [Google Scholar]
- Medina M. A., Sánchez-Jiménez F., Márquez F. J., Pérez-Rodríguez J., Quesada A. R., Núez de Castro I. Glutamine and glucose as energy substrates for Ehrlich ascites tumour cells. Biochem Int. 1988 Feb;16(2):339–347. [PubMed] [Google Scholar]
- Momparler R. L., Rivard G. E., Gyger M. Clinical trial on 5-aza-2'-deoxycytidine in patients with acute leukemia. Pharmacol Ther. 1985;30(3):277–286. doi: 10.1016/0163-7258(85)90052-x. [DOI] [PubMed] [Google Scholar]
- Muindi J. R., Frankel S. R., Huselton C., DeGrazia F., Garland W. A., Young C. W., Warrell R. P., Jr Clinical pharmacology of oral all-trans retinoic acid in patients with acute promyelocytic leukemia. Cancer Res. 1992 Apr 15;52(8):2138–2142. [PubMed] [Google Scholar]
- Myers C., Cooper M., Stein C., LaRocca R., Walther M. M., Weiss G., Choyke P., Dawson N., Steinberg S., Uhrich M. M. Suramin: a novel growth factor antagonist with activity in hormone-refractory metastatic prostate cancer. J Clin Oncol. 1992 Jun;10(6):881–889. doi: 10.1200/JCO.1992.10.6.881. [DOI] [PubMed] [Google Scholar]
- Nuti M. P., De Bertoldi M., Lepidi A. A. Influence of phenylacetic acid on poly- -hydroxybutyrate (PHB) polymerization and cell elongation in Azotobacter chroococcum Beij. Can J Microbiol. 1972 Aug;18(8):1257–1261. doi: 10.1139/m72-194. [DOI] [PubMed] [Google Scholar]
- Qian S. W., Kondaiah P., Casscells W., Roberts A. B., Sporn M. B. A second messenger RNA species of transforming growth factor beta 1 in infarcted rat heart. Cell Regul. 1991 Mar;2(3):241–249. doi: 10.1091/mbc.2.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rago R., Mitchen J., Cheng A. L., Oberley T., Wilding G. Disruption of cellular energy balance by suramin in intact human prostatic carcinoma cells, a likely antiproliferative mechanism. Cancer Res. 1991 Dec 15;51(24):6629–6635. [PubMed] [Google Scholar]
- Redondo M., Concha A., Oldiviela R., Cueto A., Gonzalez A., Garrido F., Ruiz-Cabello F. Expression of HLA class I and II antigens in bronchogenic carcinomas: its relationship to cellular DNA content and clinical-pathological parameters. Cancer Res. 1991 Sep 15;51(18):4948–4954. [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J., Holcenberg J. S., Dolowy W. C. Antineoplastic activity of highly purified bacterial glutaminases. Nature. 1970 Sep 12;227(5263):1136–1137. doi: 10.1038/2271136a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld H., Roberts J. Enhancement of antitumor activity of glutamine antagonists 6-diazo-5-oxo-L-norleucine and acivicin in cell culture by glutaminase-asparaginase. Cancer Res. 1981 Apr;41(4):1324–1328. [PubMed] [Google Scholar]
- Samid D., Flessate D. M., Friedman R. M. Interferon-induced revertants of ras-transformed cells: resistance to transformation by specific oncogenes and retransformation by 5-azacytidine. Mol Cell Biol. 1987 Jun;7(6):2196–2200. doi: 10.1128/mcb.7.6.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samid D., Shack S., Sherman L. T. Phenylacetate: a novel nontoxic inducer of tumor cell differentiation. Cancer Res. 1992 Apr 1;52(7):1988–1992. [PubMed] [Google Scholar]
- Samid D., Yeh A., Prasanna P. Induction of erythroid differentiation and fetal hemoglobin production in human leukemic cells treated with phenylacetate. Blood. 1992 Sep 15;80(6):1576–1581. [PubMed] [Google Scholar]
- Scheidt S. Angina: evolution of the role of nitrates. Am Heart J. 1990 Sep;120(3):757–772. doi: 10.1016/0002-8703(90)90049-4. [DOI] [PubMed] [Google Scholar]
- Simell O., Sipilä I., Rajantie J., Valle D. L., Brusilow S. W. Waste nitrogen excretion via amino acid acylation: benzoate and phenylacetate in lysinuric protein intolerance. Pediatr Res. 1986 Nov;20(11):1117–1121. doi: 10.1203/00006450-198611000-00011. [DOI] [PubMed] [Google Scholar]
- Smith S., Stern A. The effect of aromatic CoA esters on fatty acid synthetase: biosynthesis of omega-phenyl fatty acids. Arch Biochem Biophys. 1983 Apr 1;222(1):259–265. doi: 10.1016/0003-9861(83)90523-4. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Mickey D. D., Wunderli H., Mickey G. H., Paulson D. F. Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer. 1978 Mar 15;21(3):274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- Testa J. E., Quigley J. P. The role of urokinase-type plasminogen activator in aggressive tumor cell behavior. Cancer Metastasis Rev. 1990 Dec;9(4):353–367. doi: 10.1007/BF00049524. [DOI] [PubMed] [Google Scholar]
- Weber G. Biochemical strategy of cancer cells and the design of chemotherapy: G. H. A. Clowes Memorial Lecture. Cancer Res. 1983 Aug;43(8):3466–3492. [PubMed] [Google Scholar]
- de Bruin P. A., Verspaget H. W., Griffioen G., Verheijen J. H., Dooijewaard G., Lamers C. B. Plasminogen activators in endoscopic biopsies as indicators of gastrointestinal cancer: comparison with resection specimens. Br J Cancer. 1989 Sep;60(3):397–400. doi: 10.1038/bjc.1989.293. [DOI] [PMC free article] [PubMed] [Google Scholar]