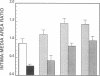
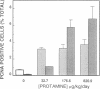
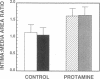
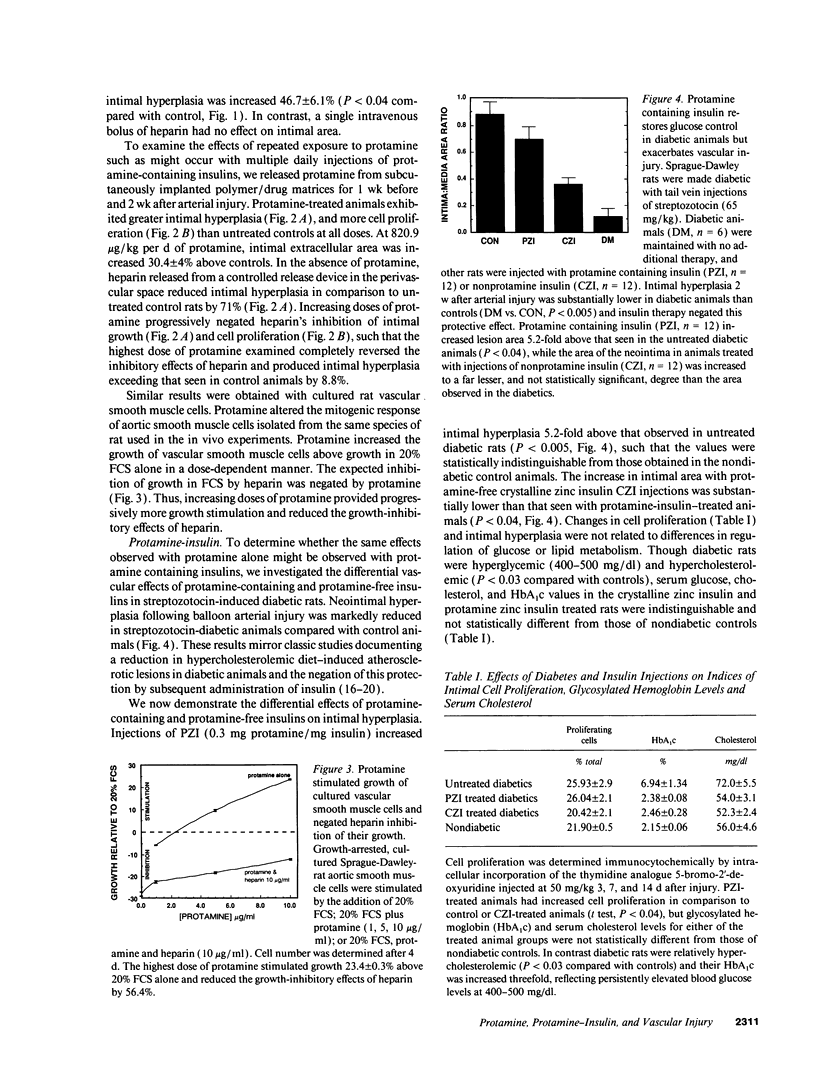
Abstract
Endothelial cells and smooth muscle cells produce heparinlike compounds that are growth inhibitory for vascular smooth muscle cells, and it has been suggested that these compounds play a regulatory role that is perturbed with vascular injury. Indeed, exogenous heparin preparations effectively suppress smooth muscle cell proliferation following injury imposed on vascular endothelium. We now report that protamine, an agent that binds heparin and negates its anticoagulant properties, has potent stimulatory effects on vascular smooth muscle cell proliferation. The administration of protamine, alone or as part of commonly used insulin preparations, stimulated the proliferation of cultured smooth muscle cells, exacerbated vascular smooth muscle cell proliferative lesions in laboratory rats, and interfered with the growth-inhibitory effects of heparin in culture and in vivo. These results confirm the importance of endogenous heparinlike compounds in arterial homeostasis and may require reconsideration of protamine use following vascular reparative procedures and in diabetics.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade S. P., Fan T. P., Lewis G. P. Quantitative in-vivo studies on angiogenesis in a rat sponge model. Br J Exp Pathol. 1987 Dec;68(6):755–766. [PMC free article] [PubMed] [Google Scholar]
- Au Y. P., Kenagy R. D., Clowes A. W. Heparin selectively inhibits the transcription of tissue-type plasminogen activator in primate arterial smooth muscle cells during mitogenesis. J Biol Chem. 1992 Feb 15;267(5):3438–3444. [PubMed] [Google Scholar]
- Bar-Ner M., Eldor A., Wasserman L., Matzner Y., Cohen I. R., Fuks Z., Vlodavsky I. Inhibition of heparanase-mediated degradation of extracellular matrix heparan sulfate by non-anticoagulant heparin species. Blood. 1987 Aug;70(2):551–557. [PubMed] [Google Scholar]
- Bar-Ner M., Kramer M. D., Schirrmacher V., Ishai-Michaeli R., Fuks Z., Vlodavsky I. Sequential degradation of heparan sulfate in the subendothelial extracellular matrix by highly metastatic lymphoma cells. Int J Cancer. 1985 Apr 15;35(4):483–491. doi: 10.1002/ijc.2910350411. [DOI] [PubMed] [Google Scholar]
- COOK D. L., MILLS L. M., GREEN D. M. The mechanism of alloxan protection in experimental atherosclerosis. J Exp Med. 1954 Feb;99(2):119–124. doi: 10.1084/jem.99.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Choay J., Lormeau J. C., Petitou M., Sache E., Karnovsky M. J. Structural determinants of the capacity of heparin to inhibit the proliferation of vascular smooth muscle cells. II. Evidence for a pentasaccharide sequence that contains a 3-O-sulfate group. J Cell Biol. 1986 May;102(5):1979–1984. doi: 10.1083/jcb.102.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Cochran D. L., Karnovsky M. J. Effect of heparin on vascular smooth muscle cells. I. Cell metabolism. J Cell Physiol. 1985 Jul;124(1):21–28. doi: 10.1002/jcp.1041240105. [DOI] [PubMed] [Google Scholar]
- Choay J., Petitou M., Lormeau J. C., Sinaÿ P., Casu B., Gatti G. Structure-activity relationship in heparin: a synthetic pentasaccharide with high affinity for antithrombin III and eliciting high anti-factor Xa activity. Biochem Biophys Res Commun. 1983 Oct 31;116(2):492–499. doi: 10.1016/0006-291x(83)90550-8. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Cook J. J., Niewiarowski S., Yan Z., Schaffer L., Lu W., Stewart G. J., Mosser D. M., Myers J. A., Maione T. E. Platelet factor 4 efficiently reverses heparin anticoagulation in the rat without adverse effects of heparin-protamine complexes. Circulation. 1992 Mar;85(3):1102–1109. doi: 10.1161/01.cir.85.3.1102. [DOI] [PubMed] [Google Scholar]
- DUFF G. L., BRECHIN D. J., FINKELSTEIN W. E. The effect of alloxan diabetes on experimental cholesterol atherosclerosis in the rabbit. IV. The effect of insulin therapy on the inhibition of atherosclerosis in the alloxan-diabetic rabbit. J Exp Med. 1954 Oct 1;100(4):371–380. doi: 10.1084/jem.100.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUFF G. L., PAYNE T. P. B. The effect of alloxan diabetes on experimental cholesterol atherosclerosis in the rabbit. III. The mechanism of the inhibition of experimental cholesterol atherosclerosis in alloxan-diabetic rabbits. J Exp Med. 1950 Oct 1;92(4):299–317. doi: 10.1084/jem.92.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchel M. C., Courty J., Mereau A., Barritault D. Modulation of mitogenic activity and cellular binding of basic fibroblast growth factor by basic proteins. J Cell Biochem. 1989 Apr;39(4):411–420. doi: 10.1002/jcb.240390407. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman E. R., Adams D. H., Karnovsky M. J. Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. Proc Natl Acad Sci U S A. 1990 May;87(10):3773–3777. doi: 10.1073/pnas.87.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritze L. M., Reilly C. F., Rosenberg R. D. An antiproliferative heparan sulfate species produced by postconfluent smooth muscle cells. J Cell Biol. 1985 Apr;100(4):1041–1049. doi: 10.1083/jcb.100.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan K. M., Hollman J. L. Recurrence of stenoses after coronary angioplasty. Heart Lung. 1986 Nov;15(6):585–587. [PubMed] [Google Scholar]
- Guyton J. R., Rosenberg R. D., Clowes A. W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ Res. 1980 May;46(5):625–634. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- Holmes D. R., Jr, Vlietstra R. E., Smith H. C., Vetrovec G. W., Kent K. M., Cowley M. J., Faxon D. P., Gruentzig A. R., Kelsey S. F., Detre K. M. Restenosis after percutaneous transluminal coronary angioplasty (PTCA): a report from the PTCA Registry of the National Heart, Lung, and Blood Institute. Am J Cardiol. 1984 Jun 15;53(12):77C–81C. doi: 10.1016/0002-9149(84)90752-5. [DOI] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Kennedy B., Deuel T. F. Platelet-derived growth factor. Specific binding to target cells. J Biol Chem. 1982 Jul 25;257(14):8130–8136. [PubMed] [Google Scholar]
- Huang J. S., Nishimura J., Huang S. S., Deuel T. F. Protamine inhibits platelet derived growth factor receptor activity but not epidermal growth factor activity. J Cell Biochem. 1984;26(4):205–220. doi: 10.1002/jcb.240260402. [DOI] [PubMed] [Google Scholar]
- Kurtz A. B., Gray R. S., Markanday S., Nabarro J. D. Circulating IgG antibody to protamine in patients treated with protamine-insulins. Diabetologia. 1983 Oct;25(4):322–324. doi: 10.1007/BF00253194. [DOI] [PubMed] [Google Scholar]
- Langer R., Brown L., Edelman E. Controlled release and magnetically modulated release systems for macromolecules. Methods Enzymol. 1985;112:399–422. doi: 10.1016/s0076-6879(85)12032-x. [DOI] [PubMed] [Google Scholar]
- Lowary L. R., Smith F. A., Coyne E., Dunham N. W. Comparative neutralization of lung- and mucosal-derived heparin by protamine sulfate using in vitro and in vivo methods. J Pharm Sci. 1971 Apr;60(4):638–640. doi: 10.1002/jps.2600600436. [DOI] [PubMed] [Google Scholar]
- Massonnet-Castel S., Pelissier E., Bara L., Terrier E., Abry B., Guibourt P., Swanson J., Jaulmes B., Carpentier A., Samama M. Partial reversal of low molecular weight heparin (PK 10169) anti-Xa activity by protamine sulfate: in vitro and in vivo study during cardiac surgery with extracorporeal circulation. Haemostasis. 1986;16(2):139–146. doi: 10.1159/000215283. [DOI] [PubMed] [Google Scholar]
- McGILL G. C., Jr, HOLMAN R. L. The influence of alloxan diabetes on cholesterol atheromatosis in the rabbit. Proc Soc Exp Biol Med. 1949 Oct;72(1):72–75. doi: 10.3181/00379727-72-17335. [DOI] [PubMed] [Google Scholar]
- McKay D. J., Renaux B. S., Dixon G. H. Rainbow trout protamines. Amino acid sequences of six distinct proteins from a single testis. Eur J Biochem. 1986 Jul 15;158(2):361–366. doi: 10.1111/j.1432-1033.1986.tb09759.x. [DOI] [PubMed] [Google Scholar]
- Montalescot G., Lowenstein E., Ogletree M. L., Greene E. M., Robinson D. R., Hartl K., Zapol W. M. Thromboxane receptor blockade prevents pulmonary hypertension induced by heparin-protamine reactions in awake sheep. Circulation. 1990 Nov;82(5):1765–1777. doi: 10.1161/01.cir.82.5.1765. [DOI] [PubMed] [Google Scholar]
- Montalescot G., Zapol W. M., Carvalho A., Robinson D. R., Torres A., Lowenstein E. Neutralization of low molecular weight heparin by polybrene prevents thromboxane release and severe pulmonary hypertension in awake sheep. Circulation. 1990 Nov;82(5):1754–1764. doi: 10.1161/01.cir.82.5.1754. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. Protamine sulfate inhibits mitogenic activities of the extracellular matrix and fibroblast growth factor, but potentiates that of epidermal growth factor. J Cell Physiol. 1987 Aug;132(2):287–294. doi: 10.1002/jcp.1041320213. [DOI] [PubMed] [Google Scholar]
- Racanelli A., Fareed J., Walenga J. M., Coyne E. Biochemical and pharmacologic studies on the protamine interactions with heparin, its fractions and fragments. Semin Thromb Hemost. 1985 Apr;11(2):176–189. doi: 10.1055/s-2007-1004373. [DOI] [PubMed] [Google Scholar]
- Schapira M., Christman B. W. Neutralization of heparin by protamine. Time for a change? Circulation. 1990 Nov;82(5):1877–1879. doi: 10.1161/01.cir.82.5.1877. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Bolender R. P., Wight T. N., Clowes A. W. Heparin modulates the composition of the extracellular matrix domain surrounding arterial smooth muscle cells. Am J Pathol. 1990 Aug;137(2):313–330. [PMC free article] [PubMed] [Google Scholar]
- Stewart W. J., McSweeney S. M., Kellett M. A., Faxon D. P., Ryan T. J. Increased risk of severe protamine reactions in NPH insulin-dependent diabetics undergoing cardiac catheterization. Circulation. 1984 Nov;70(5):788–792. doi: 10.1161/01.cir.70.5.788. [DOI] [PubMed] [Google Scholar]
- Strasser A., Whittingham S., Vaux D. L., Bath M. L., Adams J. M., Cory S., Harris A. W. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S., Folkman J. Protamine is an inhibitor of angiogenesis. Nature. 1982 May 27;297(5864):307–312. doi: 10.1038/297307a0. [DOI] [PubMed] [Google Scholar]
- Weiler J. M., Freiman P., Sharath M. D., Metzger W. J., Smith J. M., Richerson H. B., Ballas Z. K., Halverson P. C., Shulan D. J., Matsuo S. Serious adverse reactions to protamine sulfate: are alternatives needed? J Allergy Clin Immunol. 1985 Feb;75(2):297–303. doi: 10.1016/0091-6749(85)90061-2. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Potter-Perigo S., Aulinskas T. Proteoglycans and vascular cell proliferation. Am Rev Respir Dis. 1989 Oct;140(4):1132–1135. doi: 10.1164/ajrccm/140.4.1132. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]