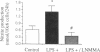Abstract
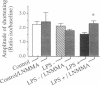
The mechanism by which soluble mediators of immune cell origin depress myocardial contractility, either globally as in systemic sepsis, or regionally in areas of inflammatory myocardial infiltrates, remains unclear. When freshly isolated ventricular myocytes from adult rat hearts were preincubated for at least 24 h in medium conditioned by endotoxin (LPS)-activated rat alveolar macrophages, their subsequent inotropic response to the beta-adrenergic agonist isoproterenol was reduced from 225 +/- 19% to 155 +/- 10% of the baseline amplitude of shortening (mean +/- SEM, P < 0.05). Neither baseline contractile function nor the contractile response to high extracellular calcium were affected. To determine whether an endogenous nitric-oxide (NO)-signaling pathway within ventricular myocytes was responsible for their decreased responsiveness to isoproterenol, the L-arginine analogue L-NMMA was added to the preincubation medium. While L-NMMA did not affect baseline contractile function or the response of control myocytes to isoproterenol, it completely restored the positive inotropic response to isoproterenol in myocytes preincubated in LPS-activated macrophage medium. Release of NO by ventricular myocytes following exposure to activated macrophage medium was detected as an increase in cGMP content in a reporter-cell (RFL-6) bioassay and also as increased nitrite content in myocyte-conditioned medium. Thus, the depressed contractile response of adult rat ventricular myocytes to beta-adrenergic agonists by a 24-h exposure to soluble inflammatory mediators is mediated at least in party by induction of an autocrine NO signaling pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Balligand J. L., Kelly R. A., Marsden P. A., Smith T. W., Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borzak S., Murphy S., Marsh J. D. Mechanisms of rate staircase in rat ventricular cells. Am J Physiol. 1991 Mar;260(3 Pt 2):H884–H892. doi: 10.1152/ajpheart.1991.260.3.H884. [DOI] [PubMed] [Google Scholar]
- Brain J. D., Frank N. R. Recovery of free cells from rat lungs by repeated washings. J Appl Physiol. 1968 Jul;25(1):63–69. doi: 10.1152/jappl.1968.25.1.63. [DOI] [PubMed] [Google Scholar]
- Chung M. K., Gulick T. S., Rotondo R. E., Schreiner G. F., Lange L. G. Mechanism of cytokine inhibition of beta-adrenergic agonist stimulation of cyclic AMP in rat cardiac myocytes. Impairment of signal transduction. Circ Res. 1990 Sep;67(3):753–763. doi: 10.1161/01.res.67.3.753. [DOI] [PubMed] [Google Scholar]
- Claycomb W. C., Palazzo M. C. Culture of the terminally differentiated adult cardiac muscle cell: a light and scanning electron microscope study. Dev Biol. 1980 Dec;80(2):466–482. doi: 10.1016/0012-1606(80)90419-4. [DOI] [PubMed] [Google Scholar]
- Eid H., Larson D. M., Springhorn J. P., Attawia M. A., Nayak R. C., Smith T. W., Kelly R. A. Role of epicardial mesothelial cells in the modification of phenotype and function of adult rat ventricular myocytes in primary coculture. Circ Res. 1992 Jul;71(1):40–50. doi: 10.1161/01.res.71.1.40. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Finkel M. S., Oddis C. V., Jacob T. D., Watkins S. C., Hattler B. G., Simmons R. L. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992 Jul 17;257(5068):387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- Geng Y., Hansson G. K., Holme E. Interferon-gamma and tumor necrosis factor synergize to induce nitric oxide production and inhibit mitochondrial respiration in vascular smooth muscle cells. Circ Res. 1992 Nov;71(5):1268–1276. doi: 10.1161/01.res.71.5.1268. [DOI] [PubMed] [Google Scholar]
- Gross S. S., Jaffe E. A., Levi R., Kilbourn R. G. Cytokine-activated endothelial cells express an isotype of nitric oxide synthase which is tetrahydrobiopterin-dependent, calmodulin-independent and inhibited by arginine analogs with a rank-order of potency characteristic of activated macrophages. Biochem Biophys Res Commun. 1991 Aug 15;178(3):823–829. doi: 10.1016/0006-291x(91)90965-a. [DOI] [PubMed] [Google Scholar]
- Gross S. S., Stuehr D. J., Aisaka K., Jaffe E. A., Levi R., Griffith O. W. Macrophage and endothelial cell nitric oxide synthesis: cell-type selective inhibition by NG-aminoarginine, NG-nitroarginine and NG-methylarginine. Biochem Biophys Res Commun. 1990 Jul 16;170(1):96–103. doi: 10.1016/0006-291x(90)91245-n. [DOI] [PubMed] [Google Scholar]
- Gulick T., Chung M. K., Pieper S. J., Schreiner G. F., Lange L. G. Immune cytokine inhibition of beta-adrenergic agonist stimulated cyclic AMP generation in cardiac myocytes. Biochem Biophys Res Commun. 1988 Jan 15;150(1):1–9. doi: 10.1016/0006-291x(88)90478-0. [DOI] [PubMed] [Google Scholar]
- Gulick T., Pieper S. J., Murphy M. A., Lange L. G., Schreiner G. F. A new method for assessment of cultured cardiac myocyte contractility detects immune factor-mediated inhibition of beta-adrenergic responses. Circulation. 1991 Jul;84(1):313–321. doi: 10.1161/01.cir.84.1.313. [DOI] [PubMed] [Google Scholar]
- Ishii K., Sheng H., Warner T. D., Förstermann U., Murad F. A simple and sensitive bioassay method for detection of EDRF with RFL-6 rat lung fibroblasts. Am J Physiol. 1991 Aug;261(2 Pt 2):H598–H603. doi: 10.1152/ajpheart.1991.261.2.H598. [DOI] [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Kelly R. A., Eid H., Krämer B. K., O'Neill M., Liang B. T., Reers M., Smith T. W. Endothelin enhances the contractile responsiveness of adult rat ventricular myocytes to calcium by a pertussis toxin-sensitive pathway. J Clin Invest. 1990 Oct;86(4):1164–1171. doi: 10.1172/JCI114822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Moncada S. Nitric oxide as a signal in blood vessels. Trends Biochem Sci. 1992 Oct;17(10):399–402. doi: 10.1016/0968-0004(92)90008-w. [DOI] [PubMed] [Google Scholar]
- Lamas S., Michel T., Brenner B. M., Marsden P. A. Nitric oxide synthesis in endothelial cells: evidence for a pathway inducible by TNF-alpha. Am J Physiol. 1991 Oct;261(4 Pt 1):C634–C641. doi: 10.1152/ajpcell.1991.261.4.C634. [DOI] [PubMed] [Google Scholar]
- Lowenstein C. J., Snyder S. H. Nitric oxide, a novel biologic messenger. Cell. 1992 Sep 4;70(5):705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- Marsden P. A., Ballermann B. J. Tumor necrosis factor alpha activates soluble guanylate cyclase in bovine glomerular mesangial cells via an L-arginine-dependent mechanism. J Exp Med. 1990 Dec 1;172(6):1843–1852. doi: 10.1084/jem.172.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natanson C., Eichenholz P. W., Danner R. L., Eichacker P. Q., Hoffman W. D., Kuo G. C., Banks S. M., MacVittie T. J., Parrillo J. E. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med. 1989 Mar 1;169(3):823–832. doi: 10.1084/jem.169.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M., Carley W. W., Gerritsen M. E., Ellingsen O., Kelly R. A., Smith T. W. Isolation and characterization of human and rat cardiac microvascular endothelial cells. Am J Physiol. 1993 Feb;264(2 Pt 2):H639–H652. doi: 10.1152/ajpheart.1993.264.2.H639. [DOI] [PubMed] [Google Scholar]
- Rakusan K., Flanagan M. F., Geva T., Southern J., Van Praagh R. Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation. 1992 Jul;86(1):38–46. doi: 10.1161/01.cir.86.1.38. [DOI] [PubMed] [Google Scholar]
- Reilly J. M., Cunnion R. E., Burch-Whitman C., Parker M. M., Shelhamer J. H., Parrillo J. E. A circulating myocardial depressant substance is associated with cardiac dysfunction and peripheral hypoperfusion (lactic acidemia) in patients with septic shock. Chest. 1989 May;95(5):1072–1080. doi: 10.1378/chest.95.5.1072. [DOI] [PubMed] [Google Scholar]
- Schulz R., Nava E., Moncada S. Induction and potential biological relevance of a Ca(2+)-independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992 Mar;105(3):575–580. doi: 10.1111/j.1476-5381.1992.tb09021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Burden T., Schini V. B., Elizondo E., Junquero D. C., Vanhoutte P. M. Platelet-derived growth factor suppresses and fibroblast growth factor enhances cytokine-induced production of nitric oxide by cultured smooth muscle cells. Effects on cell proliferation. Circ Res. 1992 Nov;71(5):1088–1100. doi: 10.1161/01.res.71.5.1088. [DOI] [PubMed] [Google Scholar]
- Springhorn J. P., Ellingsen O., Berger H. J., Kelly R. A., Smith T. W. Transcriptional regulation in cardiac muscle. Coordinate expression of Id with a neonatal phenotype during development and following a hypertrophic stimulus in adult rat ventricular myocytes in vitro. J Biol Chem. 1992 Jul 15;267(20):14360–14365. [PubMed] [Google Scholar]
- Volz A., Piper H. M., Siegmund B., Schwartz P. Longevity of adult ventricular rat heart muscle cells in serum-free primary culture. J Mol Cell Cardiol. 1991 Feb;23(2):161–173. doi: 10.1016/0022-2828(91)90103-s. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Zhang J., Snyder S. H. Nitric oxide stimulates auto-ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9382–9385. doi: 10.1073/pnas.89.20.9382. [DOI] [PMC free article] [PubMed] [Google Scholar]