Over the last approximately 30 years, the neuroscience community has made terrific strides in its understanding of the small region in the temporal lobe named for its peculiar almond shape, the amygdala. This area now provides among the best examples of how neural circuits control specific behaviors. In terms of our depth of understanding of its afferent and efferent connections, the role of incoming signals in modulating emotion-related behavior, and the functional and anatomical results of its projection patterns, the detailed understanding of the amygdala is unsurpassed. Examination of these functions has allowed great progress in dissecting the neural circuitry of emotion regulation. It is involved in many processes, including appetitive behavior (such as affiliation, sex, and drug abuse), but its role as an integral part of the fear circuitry may be the most fully described [1–3]. Recent work in two manuscripts in this issue of Biological Psychiatry, add to our understanding of the breadth of amygdale function, and in particular, how chronic stress may affect amygdala processing, and conversely how amygdala-mediated defensive behaviors may help protect against stress.
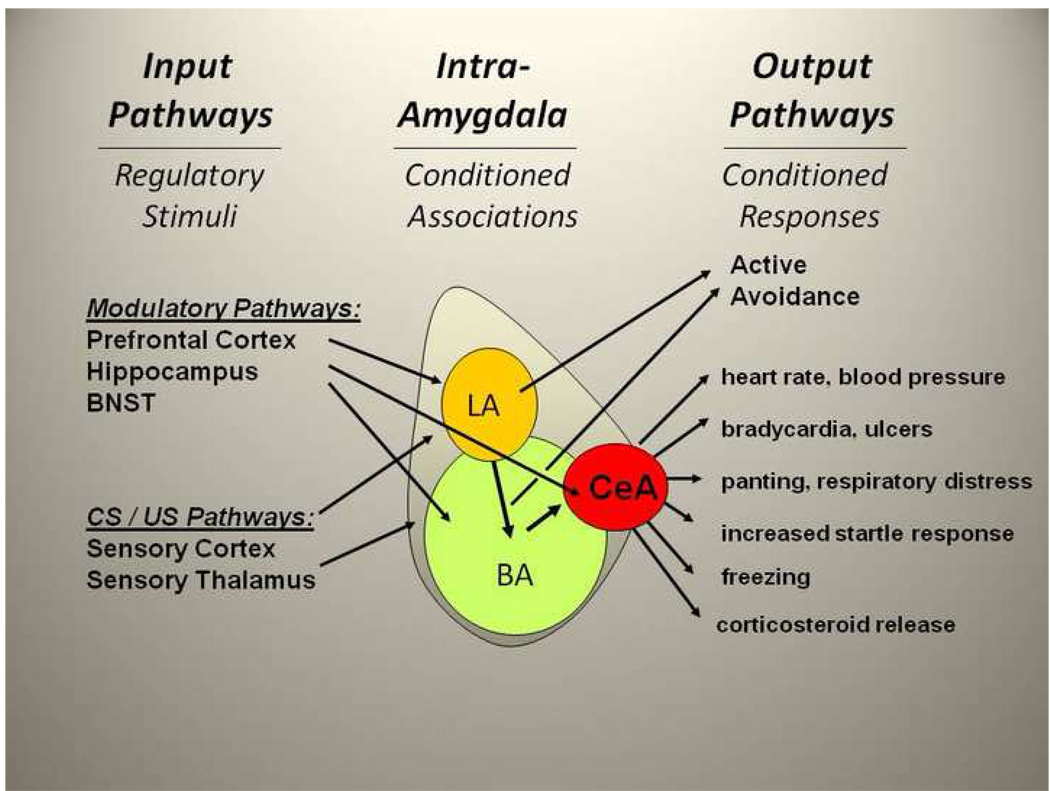
The amygdala is comprised of at least 13 different subnuclei, the most clearly defined of which are the central (CeA), the basal (BA) and lateral (LA) nuclei (see Figure). The CeA regulates many aspects of the fear response, including regulation of the release of cortisol through the paraventricular nucleus of the hypothalamus, increase in startle response via the midbrain, and modulation of the autonomic nervous system through the lateral hypothalamus [2]. Lesions of the CeA eliminate fear conditioned responses, such as fear-potentiated startle and freezing [1] in rodents. Thus the CeA can be thought of as the primary output or effector region. The LA and BA are involved in the learning or associative processing within the amygdala. In particular, the LA receives projections from auditory and visual areas, and is thought to be a principal locus for associations between previously neutral conditioned stimuli (CS) and aversive, e.g. shock or trauma, unconditioned stimuli (US), resulting in the acquisition of conditioned fear. The BA receives some direct CS and US pathways, but is also a target area for further processing of information from the LA prior to sending CS-US information to the CeA.
Figure 1. Amygdala Circuitry and the Fear Response.
Input, intra-amygdala, and output projections are shown schematically. Input pathways: these include connections with areas that mediate conditioned (CS) and unconditioned stimulus (US) pathways such as sensory cortical and thalamic areas, as well as areas that modulate stress-dependent effects on amygdala activation (e.g. bed nucleus of the stria terminalis (BNST) and prelimbic prefrontal cortex). Other areas may be involved in inhibiting amygdala activity and extinction of fear responses (e.g. infralimbic prefrontal cortex and hippocampus). Intra-Amygdala pathways: these include the projections from lateral amygdala (LA) and from LA and basolateral amygdala (BA) to the central amygdala (CeA). The LA and BA regions are involved in associative CS-US pairings as well as outputs to the CeA and other extra-amygdala areas which control avoidance and other behaviors. Output pathways: include projections to brainstem, hypothalamic, and cortical areas mediating fear and other emotional responses.
Studies have also found that the amygdala modulates the fear response in humans. Fearful stimuli including fearful faces, fear inducing images, and fear conditioned cues, have been found to activate amygdala in several brain imaging studies using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) [3–5]. In a recent review of 55 imaging studies of the functional neuroanatomy of emotion, 25 studies found amygdala activation to fearful stimuli while 4 studies found activation to positive stimuli [5]. To complement the imaging work, it has been demonstrated that temporal lobectomy patients with resulting amygdala loss have impaired fear-conditioned startle [6]. Together, these findings indicate that the amygdala plays an extensive role in regulating the fear response in humans as well as animals.
Posttraumatic stress disorder (PTSD) appears to combine aspects of both severe stress responsiveness and either enhanced conditioned fear or an inability to extinguish, or inhibit, conditioned fear. Notably, many neuroimaging studies have demonstrated that patients with PTSD have greater amygdala activation compared to controls [7]. PET studies using combat scripts [8] and images [9, 10] as well as single proton emission tomography (SPECT) studies comparing combat sounds to white noise [11] have all found greater levels of amygdala activation in subjects with PTSD. Similarly, recent fMRI studies have found that even the presentation of trauma-relevant words increase amygdale activation in PTSD cohorts [12]. Notably, this increased fear response extends beyond trauma-specific imagery, with fearful faces activating the amygdala in subjects with PTSD more than in controls [13, 14]. These and other clinical data examining the neural substrates of PTSD suggest that it is a disorder of enhanced stress responsiveness combined with dysregulation of fear and its inhibition.
Many rodent models of PTSD combine either chronic or acute stress with fear conditioning [15–17]. However, there is not yet a consensus on the relative validity and specificity of some of the different model systems. An ongoing and critically important question for the field is how stress, both acute and chronic, regulates fear conditioning. Notably, chronic stress, and anxiety-related behaviors from chronic, unpredictable stress are thought to be more related to functioning within the bed nucleus of the stria terminalis (BNST) than the amygdala [18]. The BNST shares many projection targets with the nuclei of the amygdala, and has reciprocal connections with the amygdala. It is out of the scope of this commentary to review BNST function and projections, but see recent review [19]. Although not examined within the current studies, the role of the BNST in the stress-related phenotypes is surely an area of active interest for future examination.
The new work by Rosenkranz and colleagues, “Chronic stress and amygdala neuronal dysfunction” directly addresses how chronic stress may interact with amygdala function and amygdala-related behaviors. They first demonstrate that chronic stress increases fear, measured with conditioned freezing – a robust, and easy to quantify readout of amygdala-mediated fear. Using electrophysiological examination of acute amygdala slice preparation, they then demonstrate that this same chronic stress procedure enhances neural excitability within LA circuits. After demonstrating the association between chronic stress amygdala activity, they examine possible molecular mechanisms that may underlie this effect, finding that chronic stress reduces a specific potassium (K+) channel-dependent regulation of action potential firing. Since K+ channels are normally inhibitory, and serve to hyperpolarize the neuronal membrane following an action potential, a decrease in K+ channel inhibition effectively facilitates LA excitability. Together these interesting findings suggest that chronic stress increases emotional responding including fear and anxiety responses, in part through the local effects on amygdala neuron excitability mediated by K+ channel function. These local effects within the amygdala are likely to lead to an over-active fear and anxiety related circuit and to decrease the ability of other areas involved in fear inhibition, e.g. hippocampus and medial prefrontal cortex, to dampen amygdala output.
Another question of great importance is how separate amygdala regions may differentially mediate separate fear-related behavioral outputs. For example Pavlovian conditioned fear responses support a range of defensive behaviors, such as freezing, fear-potentiated startle, aggression, and avoidance. Active avoidance, while sometimes detrimental (in that avoidance can impede extinction of fear), may also be protective. These questions are examined in the study by Lazaro-Munoz and colleagues, “Sidman Instrumental Avoidance Initially Depends on Lateral and Basal Amygdala-Mediated Pavlovian Processes.” They note that the LA and BA are critical for the acquisition of instrumental avoidance learning, but the CeA is not. After a number of repetitions, well-trained active avoidance responses become LA- and BA-independent, while continuing to be CeA-independent. Lesions of CeA abolished freezing and rescued avoidance behaviors. This suggests that an intact CeA actually constrains avoidance behaviors, possibly by inducing Pavlovian responses, such as freezing, that compete with the performance of active avoidance. Together, their findings reinforce prior observations that fear activates multiple possible behavioral outcomes. They propose that active avoidance in particular may lead to less long-term negative stress effects, and thus in some cases serve as an active and productive coping style, by minimizing re-exposure to fear- and stress-inducing stimuli, compared to reactive and passive defensive behaviors, such as freezing. Although it is premature to know how parallel this is in humans, one wonders if the psychological sense of being ‘frozen’ with fear and anxiety is a similarly passive and chronic stress-inducing process as physical freezing is in rodents. If so, engaging alternative coping approaches, such as active avoidance and other active coping strategies, likely through cortical and other interacting areas with the amygdala, are likely to lead to decreased stress activation and improved psychological function.
In summary, there have now been decades of work examining mechanisms of amygdala function and how these functions, coupled with known output pathways may mediate emotion related behavior. This area of neuroscience has advanced rapidly and carries with it significant translational insight because the mammalian amygdala and many of its connections are highly conserved across species. Thus, the new studies outlined here, which combine neural circuitry, neurophysiology, molecular biology and behavior, are particularly compelling. We are in exciting times, and it is hoped that continued functional dissection of amygdala-relevant pathways, across preclinical and clinical studies, will facilitate increasingly detailed clarification of how neural circuits create and modulate behavior. Through such work, novel and robust prevention and treatment strategies may be closer at hand to help those with debilitating fear- and stress-related psychopathology.
Acknowledgements
This work was primarily supported by National Institutes of Mental Health (MH071537) and the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Within the last two years, Dr. Ressler has received awards and/or funding support from Burroughs Wellcome Foundation, NARSAD, NIMH, NIDA, and is a cofounder of Extinction Pharmaceuticals for NMDA-based therapeutics.
References
- 1.LeDoux J. Brain mechanisms of emotion and emotional learning. Current Opinions in Neurobiology. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- 2.Davis M. The role of the amygdala in conditioned fear. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. New York: Wiley-Liss; 1992. pp. 255–305. [Google Scholar]
- 3.Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1(1):70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- 4.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human Amygdala Activation during Conditioned Fear Acquisition and Extinction: a Mixed-Trial fMRI Study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 5.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 6.Funayama ES, Grillon C, Davis M, Phelps EA. A double dissociation in the affective modulation of startle in humans: effects of unilateral temporal lobectomy. J Cogn Neurosci. 2001;13(6):721–729. doi: 10.1162/08989290152541395. [DOI] [PubMed] [Google Scholar]
- 7.Liberzon I, Sripada CS. Progress in Brain Research. Elsevier; 2007. The functional neuroanatomy of PTSD: a critical review; pp. 151–169. [DOI] [PubMed] [Google Scholar]
- 8.Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script driven imagery. Archives of General Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 9.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. American Journal of Psychiatry. 1999;156(4):575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 10.Bremner J, Staib L, Kaloupek D D, Southwick S, Soufer R R, Charney D. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological psychiatry. 1999;45(7):806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 12.Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, Silbersweig D, Stern E. Differential Time Courses and Specificity of Amygdala Activity in Posttraumatic Stress Disorder Subjects and Normal Control Subjects. Biological Psychiatry. 2005;57(5):464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 14.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 15.Brewin CR. What is it that a neurobiological model of PTSD must explain? Prog Brain Res. 2008;167:217–228. doi: 10.1016/S0079-6123(07)67015-0. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto S, Morinobu S S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009;26(12):1110–1117. doi: 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]
- 17.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56(1):19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann N Y Acad Sci. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- 19.Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]