Abstract
Objectives. We aimed to investigate population-level changes in smoking initiation during California's Tobacco Control (CTC) Program from 1990 to 2005, a period during which tobacco industry marketing practices also changed.
Methods. We used a discrete time survival analysis of data from the California Tobacco Survey to model changes in age of first smoking experimentation across birth cohorts.
Results. Smoking initiation patterns were stable across cohorts aged 9 years or older at the start of the CTC program. For children entering preadolescence since 1990, initiation declined with each more recent cohort. By 2005, the observed decline in experimentation was 80% for male participants and 92% for female participants at age 12 to 14 years; by age 15 to 17 years, 10% of Californian adolescents had experimented in 2005 compared with 45% in preprogram cohorts. However, rates of new experimentation after age 17 years did not change, except for a recent increase in late experimentation (after age 20 years) among young adult men.
Conclusion. Our models suggest that the CTC program greatly reduced adolescent smoking initiation among younger adolescents. Late experimentation may have recently increased among young adult men in California, coincident with an increase in tobacco industry marketing aimed at young adults.
Since the 1960s, adolescent smoking rates have changed in response to the competing influences of tobacco industry marketing campaigns1–3 and public health tobacco control programs.4,5 Reducing adolescent smoking has been a primary goal of the California Tobacco Control (CTC) Program,6 the longest-running large tobacco control program in the world. As an evaluation component, this program sponsors a population survey of tobacco use every 3 years. Previous survey estimates indicated that the CTC Program was associated with a lower age-specific prevalence of smoking from age 12 years, which was probably a consequence of reduced experimentation.5 However, it is not clear whether these age-specific changes led to an overall reduction in lifetime smoking initiation within a birth cohort, especially given the apparent effectiveness of recent tobacco advertising targeting young adults. We investigated changes in the trajectories of smoking experimentation across the age window of 10 to 24 years, in which almost all first experimentation has been documented to occur.7
Previously, an age-period-cohort model8 identified that smoking experimentation for California was stable for cohorts born before 1979. Subsequent cohorts, those aged 12 years or younger when the California program started in 1990, had lower experimentation levels on average over the adolescent years. However, the model in that analysis used additive effects for age, period, and cohort, with the consequence that, for example, changes in experimentation rates at a given time were averaged across cohorts and ages. Period and cohort effects, which can identify time changes, applied equally across all ages. Thus, that analysis was unable to identify changes in smoking uptake at specific ages within the age window of 10 to 24 years. This may be of concern, because other interventions that reduced smoking in early adolescence were shown to not be associated with reduced smoking in later years.9,10 In addition, there is specific concern that tobacco industry marketing campaigns have changed to target young adults, and this may have increased rates of smoking initiation in young adulthood.11
Recent contributions to the methodologic literature12–17 have addressed shortcomings of age-period-cohort models in identifying age- and period-specific effects.18,19 Incorporating suggestions from this literature, we modeled age-specific changes in the trajectory of smoking initiation among young Californians from 1990 to 2005. We hypothesized that age-specific changes have occurred in the pattern of smoking uptake among recent birth cohorts.
METHODS
The California Tobacco Surveys (CTSs) are random-digit-dialed telephone surveys that have been conducted by Westat, Inc, every 3 years since 1990.20,21 Complete documentation is available online (http://libraries.ucsd.edu/ssds/tobacco.htm). An initial survey asks 1 adult respondent to enumerate household members aged 12 years and older. All adolescents and a stratified random sample of adults are then scheduled to complete an extended 25-minute survey (in 1999 a random sample of adolescents was used). Survey weights accounted for selection probabilities and were poststratified to US Census population totals for California by sex, race/ethnicity, and education to account for under-coverage and nonresponse. Replicate weights were provided to compute jackknife estimates of variance that accounted for the random sampling and the weighting adjustment. Here, we weighted estimates by using SAS version 9.01 PROC SURVEYFREQ or SURVEYLOGISTIC (SAS Institute Inc, Cary, NC) and used a macro to compute confidence intervals and P values with the use of the replicate weights.
Participants
We included in the 6 cross-sectional CTSs (1990, 1993, 1996, 1999, 2002, and 2005) all participants aged 12 to 26 years who responded to the extended survey. Because age patterns and time trends in smoking initiation and prevalence differ considerably across racial/ethnic groups,22,23 we considered only the majority non-Hispanic white population (n = 34 342). The sample sizes grouped by the six 3-year birth cohorts we used in the analysis are shown in Table 1. We grouped age and birth cohorts so that the data within each table cell reflected the same survey year.12 The smallest sample size for any age-birth cohort cell was 884, and the average sample size was 2128.
TABLE 1.
Sample Sizes and Survey Years For Each Age Group and Birth Cohort: California Tobacco Survey, 1990–2005
| Sample Size and Survey Year |
|||||||
| Birth Cohort | Year Surveyed at Age 12–14 y | Age 12–14 Years, No. (Year) | Age 15–17 Years, No. (Year) | Age 18–20 Years, No. (Year) | Age 21–23 Years, No. (Year) | Age 24–26 Years, No. (Year) | Total Cohort, No. |
| 1976–1978 | 1990 | 2562 (1990) | 2660 (1993) | 1062 (1996) | 884 (1999) | 2123 (2002) | 9 291 |
| 1979–1981 | 1993 | 2835 (1993) | 3030 (1996) | 1007 (1999) | 2275 (2002) | 1267 (2005) | 10 414 |
| 1982–1984 | 1996 | 3183 (1996) | 3054 (1999) | 2912 (2002) | 1262 (2005) | … | 7 790 |
| 1985–1987 | 2009 | 2999 (1999) | 2882 (2002) | 1845 (2005) | … | … | 7 726 |
| 1988–1990 | 2002 | 2923 (2002) | 2250 (2005) | … | … | … | 5 173 |
| 1991–1993 | 2005 | 2172 (2005) | … | … | … | … | 2 172 |
Note. Survey period was every 3 years.
Measures
The categories of smoking uptake were susceptibility to smoking among never smokers,24 experimentation,21 established smoking,25,26 and ever-daily smoking.27 Adolescents aged 12 to 17 years were classified as experimenters or never-smokers by asking “Have you ever smoked a cigarette?” Experimenters were classified as established smokers if they responded affirmatively to “Have you smoked at least 100 cigarettes in your life?” Established smokers were classified as ever-daily smokers by asking “Have you ever smoked a cigarette every day for at least a month?” Adolescent never-smokers were further categorized as committed never-smokers if they answered “definitely not” to 2 questions measuring future intentions to smoke (“Do you think that you will try a cigarette soon?” and “Do you think you will be smoking one year from now?”) as well as 1 question measuring self-efficacy in refusing a cigarette (“If one of your best friends were to offer you a cigarette, would you smoke it?”). Any other answer categorized the respondent as a susceptible never smoker.
Adults aged 18–26 years who were not established smokers (i.e., adults who had never smoked 100 or more cigarettes in their lifetime) were asked, “What would you say is the total number of cigarettes that you have ever smoked?” A response of zero indicated a never-smoker and any other response an experimenter. To identify ever-daily smokers, established smokers were asked, “Have you ever smoked daily for six months or more?” Adult never-smokers were asked the following 2 questions: “Do you think you will smoke a cigarette soon?” and “Do you think you will smoke a cigarette in the next year?” A “definitely not” response to both questions categorized the respondent as a committed never-smoker and any other response indicated a susceptible never-smoker.
To compare categories of smoking initiation by age, we used the 2002 survey. For each age from 12 to 29 years, we computed the weighted cross-sectional prevalence for each category, and plotted prevalence by age by using PROC LOESS in SAS version 9.10. To present trajectories of uptake across ages by birth cohort, we computed the weighted prevalence of ever-experimentation by 3-year age groups and plotted it against age group for each birth cohort separately.
Statistical Models of Age of First Experimentation
We used a weighted logistic regression model with ever experimentation as the outcome and indicator variables for cumulative age, birth cohort, and age by cohort interactions as predictors. We parameterized this model by using the same period for age and cohort groups as the survey period, following Holford12 and using cumulative interaction terms as in Hanayama.17 The cumulative indicator variables that we used form a truncated power basis for first-degree regression splines.28
To understand the parameterization, consider a logistic regression model of ever experimentation that uses cumulative indicator variables of age. Each variable Aj indicates that a respondent is age j years or older, and logit(P(Y = 1)) = α12 A12 + α15 A15 + … + α24 A24· Because all respondents were aged at least 12 years, A12 is the intercept, and α12 is the log-odds of the proportion who have experimented by age 12 through 14 years. Next, variable A15 equals zero for those aged 12 through 14 years, and 1 for those older. Then α15 is the log-odds ratio of ever-experimentation by age 15 through 17 years relative to age 12 through 14 years. Any increase in ever-experimentation is necessarily because of new experimentation.
The full model incorporated cohort effects and age by cohort interactions. Let variable Bk indicate those born in year k or later. Then, an additive age-cohort model with 3-year birth cohorts and age groups is given by adding the 5 terms β1979 B1979 + β1982 B1982 + … + β1991 B1991 to the previous model. Each coefficient βj gives the relative log-odds of lifetime experimentation in the jth birth cohort relative to the next older cohort, with the 1976 through 1978 cohort as the reference. A fully parameterized model for the 20 age-cohort classes of Table 1 is achieved by adding 10 interaction parameters. For cohorts k = 1, … 4 and age groups 1 ≤ j ≤ 5 – k, (numbered as in Table 1), we construct the age by cohort product indicator variables AjBk and add the 10 corresponding terms to the model. Each interaction coefficient adjusts the odds of new experimentation between one age and the next for a given cohort, relative to the experience of the previous cohort. These interaction terms allow for tests of significant effects, which are specific to a given age group at a given time.
We used a backward model selection strategy from the fully parameterized model while retaining the intercept and age indicators. Starting from the next oldest cohort and youngest age group, cohort by age and cohort terms not significant at P > .25 were deleted, stepping through each older age group before considering a more recent cohort.
RESULTS
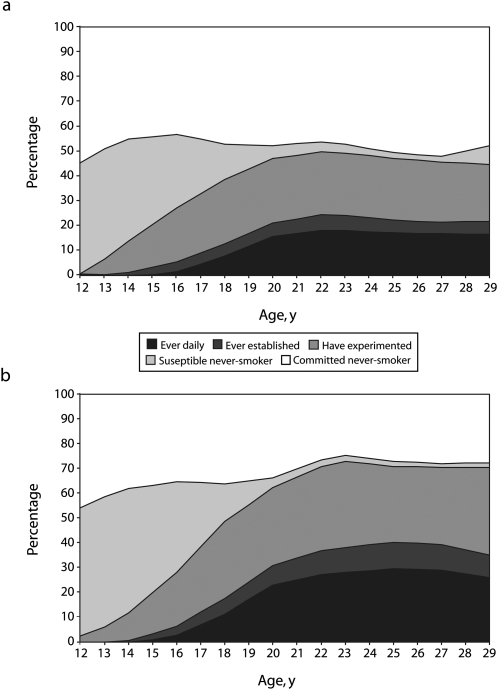
Figure 1 shows the cross-sectional distribution of the categories of smoking uptake by age and gender for 2002. The patterns of smoking uptake by age were qualitatively similar across survey years (data not shown). Almost no daily smoking and little established smoking occurred before age 16 years among these non-Hispanic white adolescents and young adults. Between age 16 and 20 years, ever-established smoking increased, closely followed by ever-daily smoking. After age 20 years, these proportions increased only slightly, to about age 22 to 23 years for women and age 24 to 25 years for men. Smoking experimentation was unusual before age 12 years, but increased rapidly from age 12 years into young adulthood. Little new experimentation occurred after age 22 years for women or age 23 years for men. By age 12 to 14 years, the proportion of susceptible never-smokers was already high and then remained comparatively stable. For both genders, it appeared that by age 23 years, most who were susceptible to smoking uptake had progressed at least to experimentation. Thus, we examined ever-experimentation (having ever smoked a whole cigarette) as the most sensitive measure of smoking uptake across adolescence into young adulthood.
FIGURE 1.
Prevalence of categories of smoking uptake by age for (a) females and (b) males: California Tobacco Survey, 2002.
Note. The smoking uptake categories are defined as follows: ever daily (have at some time smoked daily for ≥ 1 month), ever established (have smoked ≥ 100 lifetime cigarettes), ever experimented (have ever smoked a cigarette), susceptible never-smokers (have puffed on a cigarette or would not rule it out), and committed never-smokers (would “definitely not” try smoking).
Age–Birth Cohort Trends in Female Experimentation
The first 2 columns in Table 2 present each indicator variable and its coefficient from the final age-cohort-interaction logistic regression model for non-Hispanic white female adolescents and adults. Columns 3 and 4 translate these variables into between-group comparisons with their respective odds ratios (the exponential of the model coefficient). When the 1976 through 1978 cohort was first surveyed at age 12 to 14 years in 1990, 17.4% (95% confidence interval [CI] = 13.8%, 20.9%) had experimented with smoking, and this was the reference category (corresponding in Table 2 to the log odds of the intercept of −1.56). The fitted model and the raw data for these young Californian female adolescents and adults are presented in Figures 2a and 2b, respectively, as survival curves with age where the outcome is ever-experimentation.
TABLE 2.
Weighted Logistic Regression Model of Ever Experimenting Among California Non-Hispanic White Female Adolescents and Adults: California Tobacco Survey, 1990–2005
| Model Variables | Coefficient (95% CI) | Between-Group Comparisons From Model | OR (95% CI) |
| Intercept | −1.56 (−1.71, 1.41) | ||
| Cohort indicators (cumulative) | Comparing successive cohorts | ||
| Born 1976–1993 (Ref) | 1.00 | Cohort 1976–1978 | 1.00 |
| Born 1979–1993 | NS | Cohort 1979–1981 versus cohort 1976–1978 | NS |
| Born 1982–1993 | −0.34 (−0.51, −0.18) | Cohort 1982–1984 versus cohort 1979–1981 | 0.71 (0.60, 0.84) |
| Born 1985–1993 | −0.42 (−0.66, −0.19) | Cohort 1985–1987 versus cohort 1982–1984 | 0.66 (0.52, 0.83) |
| Born 1988–1993 | −0.70 (−1.07, −0.34) | Cohort 1988–1990 versus cohort 1985–1987 | 0.50 (0.34, 0.71) |
| Born 1991–1993 | −1.19 (−2.06, −0.32) | Cohort 1991–1993 versus cohort 1988–1990 | 0.30 (0.13, 0.73) |
| Age indicators (cumulative) | Comparing successive ages (main effects) | ||
| 12–26 y (Ref) | 1.00 | Age 12–14 y | |
| 15–26 y | 1.32 (1.15, 1.49) | Age 15–17 y versus age 12–14 y | 3.74 (3.16, 4.44)a |
| 18–26 y | 0.78 (0.61, 0.94) | Age 18–20 y versus age 15–17 y | 2.18 (1.84, 2.56) |
| 21–26 y | −0.05 (−0.31, 0.22) | Age 21–26 y versus age 18–20 y | 0.95 (0.73, 1.25) |
| 24–26 y | −0.08 (−0.39, 0.23) | Age 24–26 y versus age 21–24 y | 0.92 (0.67, 1.26) |
| Interaction term: Born 1985–1993 × age 15–26 y | −0.30 (−0.62, −0.03) | Age 15–17 versus age 12–14 y; born < 1985 versus born ≥ 1985 | 0.74 (0.53, 0.97) |
Note. CI = confidence interval; NS = not significant; OR = odds ratio. Terms not significant at P < .25 were dropped from the model by use of backward selection, starting from a fully parameterized model. Estimates are weighted to be representative of the total population.
Odds ratios for cohorts born before 1985. For cohorts born 1985 or later, multiply by interaction OR.
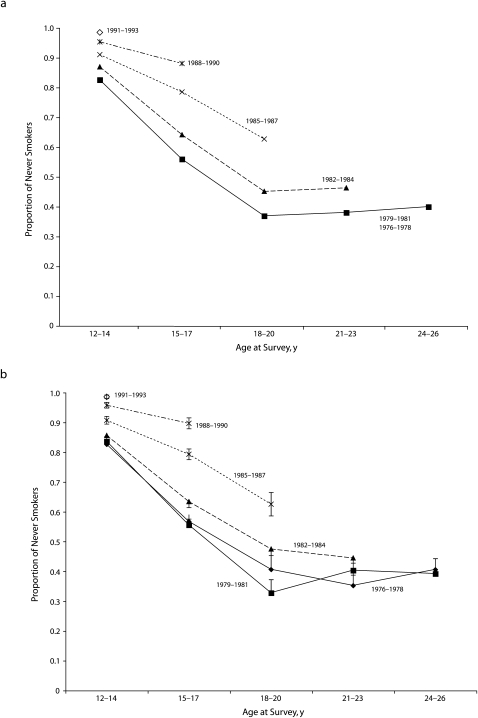
FIGURE 2.
Prevalence of never smokers among White female adolescents and adults, by 3-year birth cohorts and age groups from (a) the fitted model from logistic regression and (b) weighted percentages: California Tobacco Survey, 2002.
Note. All model terms are significant at P < .10. All estimates were weighted to be representative of the population using the published survey weights.
Ever-experimentation rates for the 1979 to 1981 birth cohort did not differ significantly from those for the 1976 to 1978 reference cohort. However, each successive cohort born after 1981 had a significantly lower rate of ever smoking than did each preceding birth cohort, as shown by the main effects for each cohort (in the absence of interaction terms) in Table 2. The 1982 to 1984 cohort ever-smoking rate was about 30% lower than that of the 1979 to 1981 cohort at all ages (odds ratio [OR] = 0.71; 95% CI = 0.6, 0.8). At age 12 to 14 years, the 1985 to 1987 cohort ever-smoking odds were 34% lower than were the 1982 to 1984 cohort, the 1988 to 1990 cohort rate was 50% lower than was the 1985 to 1987 cohort, and the 1991 to 1993 cohort was 70% lower than was the 1988 to 1990 cohort (OR = 0.30; 95% CI = 0.13, 0.73). For this most recent cohort, the ever-smoking rate among adolescent girls aged 12 to 14 years reached a low of 1.4% (95% CI = 2.7%, 0.2%).
Across all birth cohorts, the ever-smoking rate increased with age as seen by the main effects for age in Table 2. When these adolescents were aged 15 to 17 years, the odds of ever smoking was 3.7 times the odds at age 12 to 14 years (OR = 3.74; 95% CI = 3.16, 4.44). When aged 18 to 20 years, the odds of being an ever smoker were over twice as large as they were at age 15 to 17 years (OR = 2.18; 95% CI = 1.84, 2.56). The ever-smoking rate did not significantly increase further when these cohorts reached older ages such as 21 to 23 years or 23 to 26 years.
The interaction terms measured whether the transition rate across age groups had changed between birth cohorts. There was 1 significant interaction, and this modified the increase in ever smoking between age 12 to 14 years and 15 to 17 years for adolescents born 1985 or later. Adolescents in cohorts born after 1985 had a 26% smaller increase in the odds of becoming an ever smoker from age 12 to 14 years to age 15 to 17 years, compared with those born before 1985 (OR = 0.74; 95% CI = 0.53, 0.97). Together, the cohort main effects and the age-cohort interaction term combined so that the level of experimentation among those aged 15 to 17 years was much lower for the 1988 to 1990 cohort (10%; 95% CI = 6.7%, 14%) than it was for the 1976 to 1981 cohort (44%; 95% CI = 41%, 48%).
The major increase in ever smoking with age was apparent through to age 18 to 20 years in plots of both the raw and fitted data (Figure 2). Similarly, a lower rate of ever smoking at a given age was observed for each more recent birth cohort compared with each earlier birth cohort. The significant interaction term indicated that the line segment connecting age 12 to 14 years and age 15 to 17 years was less steep in slope for birth cohorts born after 1985 than it was for those born before 1985. The lack of a significant interaction term between age 15 to 17 years and aged 18 to 20 years means that the line segment connecting these ages was essentially parallel for all birth cohorts.
Age–Birth Cohort Trends in Male Experimentation
Similar data for male adolescents and adults are presented in Table 3 and Figure 3. For the reference birth cohort (1976–1978), the proportion of ever-smokers was 13.5% (95% CI = 10.6%, 16.5%) when aged 12 to 14 years. The proportion of ever smokers at each age was similar between the 1976 to 1978 and 1979 to 1981 birth cohorts. Considering the cohort main effects in Table 3, the 1982 to 1984 cohort did not differ significantly at age 12 to 14 years; however, the 1985 to 1987 birth cohort had a 40% lower odds of ever-smoking at this age than did the 1982 to 1984 cohort (OR = 0.60; 95% CI = 0.47, 0.76). Furthermore, the 1988 to 1990 cohort had a 57% lower ever-smoking odds at age 12 to 14 years than did the 1985 to 1987 cohort (OR = 0.43; 95% CI = 0.30, 0.59). Ever smoking at age 12 to 14 years was not significantly different in the 1991 to 1993 cohort than it was in the 1988 to 1990 cohort (estimated rate 2.8%; 95% CI = 5.2%, 0.4%).
TABLE 3.
Weighted Logistic Regression Model of Ever Experimenting Among California Non-Hispanic White Male Adolescents and Adults: California Tobacco Survey, 1990–2005
| Model Variables | Coefficient (95% CI) | Between-Group Comparisons From Model | OR (95% CI) |
| Intercept | −1.86 (−1.97, −1.74) | ||
| Cohort indicators (cumulative) | Comparing successive cohorts | ||
| Born 1976–1993 (Ref) | 1.00 | Cohort 1976–1978 | 1.00 |
| Born 1979–1993 | NS | Cohort 1979–1981 versus cohort 1976–1978 | NS |
| Born 1982–1993 | NS | Cohort 1982–1984 versus cohort 1979–1981 | NS |
| Born 1985–1993 | −0.51 (−0.75, −0.27) | Cohort 1985–1987 versus cohort 1982–1984 | 0.60 (0.47, 0.76) |
| Born 1988–1993 | −0.85 (−1.19, −0.52) | Cohort 1988–1990 versus cohort 1985–1987 | 0.43 (0.30, 0.59) |
| Born 1991–1993 | NS | Cohort 1991–1993 versus cohort 1988–1990 | |
| Age indicators (cumulative) | Comparing successive ages (main effects) | ||
| 12–26 y (Ref) | 1.00 | Age 12–14 y | 1.00 |
| 15–26 y | 1.60 (1.42, 1.78) | Age 15–17 y versus age 12–14 y | 4.95 (4.14, 5.93)a |
| 18–26 y | 0.89 (0.68, 1.09) | Age 18–20 y versus age 15–17 y | 2.44 (1.97, 2.97) |
| 21–26 y | 0.42 (0.11, 0.73) | Age 21–23 y versus age 18–20 y | 1.52 (1.12, 2.08)a |
| 24–26 y | −0.30 (−0.64, 0 04) | Age 24–26 y versus age 21–23 y | 0.74 (0.53, 1.04) |
| Interaction terms | Comparing successive ages (interaction) | ||
| Born 1982–1993 × age 15–26 y | −0.36 (−0.56, −0.16) | Age 15–17 y versus age 12–14 y; born < 1982 versus born ≥ 1982 | 0.70 (0.57, 0.85) |
| Born 1982–1993 × age 21–26 y | 0.37 (−0.17, 0.92) | Age 21–23 y versus age 18–20 y; born < 1982 versus born ≥ 1982 | 1.45 (0.84, 2.51) |
Note. CI = confidence interval; NS = not significant; OR = odds ratio. Terms not significant at P < .25 were dropped from the model using backward selection, starting from a fully parameterized model. Estimates are weighted to be representative of the population.
Odds ratio for cohorts born before 1982. For cohorts born 1982 or later, multiply by appropriate interaction OR.
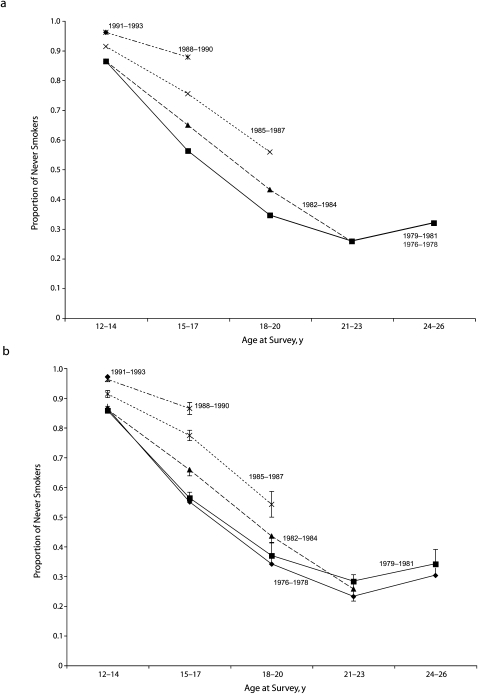
FIGURE 3.
Prevalence of never smokers among White male adolescents and adults, by 3-year birth cohorts and age groups from (a) the fitted model from logistic regression and (b) weighted percentages: California Tobacco Survey, 2002.
Note. All model terms are significant at P < .10. All estimates were weighted to be representative of the population using the published survey weights.
Considering the main effects for age, the odds of ever smoking at age 15 to 17 years was almost 5 times that at age 12 to 14 years (OR = 4.95; 95% CI = 4.14, 5.93) for male cohorts born before 1982. For every cohort, experimentation continued to increase with age, with those aged 18 to 20 years having an odds that was 2.42 times that for those aged 15 to 17 years, and with those aged 21 to 23 years having a rate significantly higher than that for those aged 18 to 20 years (OR = 1.52; 95% CI = 1.12, 2.08). Across cohorts, experimentation did not continue to increase at older ages.
This model had 2 significant age-by-cohort interaction terms. The first modified the increase in smoking between age 12 to 14 years and age 15 to 17 years and showed that those born in 1982 or later had a 30% lower transition rate at these ages than did those born before 1982 (OR = 0.70; 95% CI = 0.57, 0.85). As a result, the level of experimentation for the 1988 to 1990 male cohort at age 15 to 17 years (11%; 95% CI = 6.7%, 15%) was much lower than was that for the 1976 to 1981 cohorts (45%; 95% CI = 40%, 50%).
The second interaction term modified the increase in ever smoking between age 18 to 20 years and age 21 to 23 years, and showed the transition rate was 45% higher for the cohort born after 1982 than it was for those born before 1982 (OR = 1.45; 95% CI: 0.84, 2.51; P = .09).
As with female participants, for each cohort, plots of both the raw and fitted data showed the proportion of ever smokers increased rapidly with age and ever smoking at each fixed age decreased for more recent birth cohorts (Figure 3). The interaction term for the transition from age 12 to 14 years to age 15 to 17 years is apparent as a decrease in slope between these age groups for the youngest 2 birth cohorts compared with earlier birth cohorts. There was no interaction between age 15 to 17 years and age 18 to 20 years. The significant interaction for the transition between age 18 to 20 years and age 21 to 23 years was also seen: instead of the usual pattern (seen for earlier male cohorts and for female participants) in which ever-smoking stabilized across older age groups, the 1982 to 1984 male birth cohort had a marked increase in ever smokers in young adulthood.
DISCUSSION
Using suggestions from recent statistical modeling literature, we identified statistically significant changes in rates of adolescent smoking uptake that were associated with the California Tobacco Control Program. For California cohorts born in 1981 or before, the trajectory of smoking initiation from age 12 through 23 years was stable for both male and female participants, which is consistent with previous reports for the 1974 to 1978 birth cohorts.8 Thus, smoking initiation patterns were stable among those who were aged 9 years or older by the start of the California campaign in 1990. However, initiation trajectories changed dramatically starting with children in the 1982 to 1984 birth cohort, who were aged 6 to 8 years at the start of the California Program. Experimentation rates at age 12 to 14 years declined markedly and significantly with this and each subsequent birth cohort, although after the 1988 to 1990 cohort initiation rates held steady in more recent male cohorts in this youngest age group. By 2005, the observed decline in experimentation at age 12 to 14 years was 92% for female adolescents and 80% for male adolescents, and this decline appeared to be associated with the start of the California Program.
This significant decline in uptake trajectories continued at older ages. The rate of new experimentation from age 12 to 14 years to age 15 to 17 years decreased markedly across birth cohorts, starting from the 1982 to 1984 (male participants) and 1985 to 1987 (female participants) cohorts. By 2005, only 10% of adolescents aged 15 to 17 years had experimented (1988–1990 cohort), compared with about 45% of same-age adolescents in the 1976 to 1981 cohorts.
The odds of new smoking experimentation from age 15 to 17 years to age 18 to 20 years were stable for adolescents across the birth cohorts studied, which suggests that the California program did not have its major impact on experimentation in older adolescence or among young adults. However, other work has shown a major impact of the California program on smoking cessation among young adults.29
Among male participants only, there was the suggestion (P = .09) of an increase in experimentation after age 20 years, specific to the most recent birth cohort to reach age 21 to 23 years. This increase brought adult ever-smoking rates for this cohort up to those of previous male cohorts.
The increase in late experimentation among young adult men starting around 2002 suggests that an opposing social influence may have affected this cohort and overwhelmed the initial lower rates of experimentation at younger ages. Tobacco industry marketing practices are 1 such social influence. In 1998, the Master Settlement Agreement between State Attorney's General and the Tobacco Industry prohibited cartoon character advertising, including the Joe Camel campaign and other approaches shown to be effective with youth.30 However, tobacco industry expenditure on marketing rose dramatically after this agreement.31 Much of this effort appeared to target young adults,32 particularly tobacco brand-sponsored activities in bars and clubs, including distribution of free samples by “spokesmodels” and financial incentives for club owners and staff.33 This marketing appeared to be targeted more at young men than women. More than 30% of young adult men and 20% of young adult women aged 18 to 24 years in California attended at least 1 event sponsored by a tobacco company in 2002.34 It is possible that this increased marketing to young adults was responsible for the increase in late experimentation seen in the most recent cohort of young adult Californian men.
A limitation of our study was that response rates in population surveys have decreased over the past 15 years. However, contemporaneous population surveys with markedly different response rates have obtained similar prevalence estimates, which suggests that any nonresponse bias (postulated to be from call screening and cell phone usage) is largely accounted for by weighting.35 Another potential limitation is the use of self-reported smoking status. Although for regular smoking this has been shown to be relatively unbiased,36,37 it is possible that prior experimentation is increasingly subject to recall bias with increasing denormalization of smoking in society.38 We observed a small decline in reported ever experimentation among young adults, which suggests that, although the phenomenon exists, it is not causing a major bias.
The California Tobacco Control Program was associated with a marked reduction in smoking experimentation, particularly among adolescents. Each new birth cohort that entered preadolescence during the California campaign had decreased, or at least steady, new experimentation rates in early adolescence compared with earlier cohorts at the same age, resulting in a marked cumulative decline across the 12-year period. However, we found no evidence of a similar California campaign effect on experimentation after age 17 years. Rather, the data suggested that a recent tobacco industry marketing campaign11 may have increased late experimentation rates among young adult men, effectively overcoming the California Tobacco Control Program's effect on early experimentation rates in these recent male cohorts. However, experimentation is only the first step toward smoking dependence, and others have argued that progression to established smoking and eventual consumption levels may be influenced by the age of initiation.39,40 To properly address these hypotheses, recent birth cohorts should be followed to older ages.
Acknowledgments
The 1990, 1993, 1996, 1999, 2002, and 2005 California Tobacco Surveys were supported by the California Department of Health Services, Tobacco Control Section, and are publicly available at http://libraries.ucsd.edu/ssds/tobacco.htm. This work was supported by the Tobacco-Related Disease Research Program grant 15RT-0238 from the University of California.
Human Participant Protection
This research was approved by the institutional review board of the University of California, San Diego. Informed consent was obtained for the surveys in accordance with the guidelines of that board.
References
- 1.Pierce JP, Lee L, Gilpin EA. Smoking initiation by adolescent girls, 1944 through 1988. An association with targeted advertising [see comments]. JAMA 1994;271(8):608–611 [PubMed] [Google Scholar]
- 2.Gilpin EA, Pierce JP. Trends in adolescent smoking initiation in the United States: is tobacco marketing an influence? Tob Control 1997;6(2):122–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierce JP, Gilmer TP, Lee L, Gilpin EA, de Beyer J, Messer K. Tobacco industry price-subsidizing promotions may overcome the downward pressure of higher prices on initiation of regular smoking. Health Econ 2005;14(10):1061–1071 [DOI] [PubMed] [Google Scholar]
- 4.Bauer UE, Johnson TM, Hopkins RS, Brooks RG. Changes in youth cigarette use and intentions following implementation of a tobacco control program: findings from the Florida Youth Tobacco Survey, 1998-2000. JAMA 2000;284(6):723–728 [DOI] [PubMed] [Google Scholar]
- 5.Pierce JP, White MM, Gilpin EA. Adolescent smoking decline during California's tobacco control programme. Tob Control 2005;14(3):207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.California Department of Health Services Toward a Tobacco-Free California. A status report to the California legislature on the first fifteen months of California's Tobacco Control Program Sacramento, CA: CDHS; 1990 [Google Scholar]
- 7.Gilpin EA, Lee L, Evans N, Pierce JP. Smoking initiation rates in adults and minors: United States, 1944-1988. Am J Epidemiol 1994;140(6):535–543 [DOI] [PubMed] [Google Scholar]
- 8.Chen XG, Li GH, Unger JB, Liu XW, Johnson CA. Secular trends in adolescent never smoking from 1990 to 1999 in California: an age-period-cohort analysis. Am J Public Health 2003;93(12):2099–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glynn TJ. Essential elements of school-based smoking prevention programs. J School Health 1989;59(5):181–188 [DOI] [PubMed] [Google Scholar]
- 10.Glied S. Is smoking delayed smoking averted? Am J Public Health 2003;93(3):412–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling PGS. Why and how the tobacco industry sells cigarettes to young adults: evidence from industry documents. Am J Public Health 2002;92(6):908–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holford TR. Approaches to fitting age-period-cohort models with unequal intervals. Stat Med 2006;25(6):977–993 [DOI] [PubMed] [Google Scholar]
- 13.Keiding N, Knuiman MW. Survival analysis in natural history studies of disease. Stat Med 1990;9(10):1221–1222 [DOI] [PubMed] [Google Scholar]
- 14.Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: Age-period and age-cohort models. Stat Med 1987;6(4):449–467 [DOI] [PubMed] [Google Scholar]
- 15.Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: Age-period-cohort models. Stat Med 1987;6(4):469–481 [DOI] [PubMed] [Google Scholar]
- 16.Heuer C. Modeling of time trends and interactions in vital rates using restricted regression splines. Biometrics 1997;53(1):161–177 [PubMed] [Google Scholar]
- 17.Hanayama N. An extended age period cohort model for analysing (age, period)-tabulated data. Stat Med 2007;26(18):3459–475 [DOI] [PubMed] [Google Scholar]
- 18.Rothman KJ, Sanders G, eds. Modern Epidemiology Philadelphia, PA: Lippencott-Raven Publishers; 1998 [Google Scholar]
- 19.Carstensen B. Age-period-cohort models for the Lexis diagram. Stat Med 2007;26(15):3018–3045 [DOI] [PubMed] [Google Scholar]
- 20.Bal DG, Kizer KW, Felten PG, Mozar HN, Niemeyer D. Reducing tobacco consumption in California. Development of a statewide anti-tobacco use campaign. JAMA 1990;264(12):1570–1574 [PubMed] [Google Scholar]
- 21.Pierce JP, Gilpin EA, Emery SL, et al. Has the California tobacco control program reduced smoking? JAMA 1998;280(10):893–899 [DOI] [PubMed] [Google Scholar]
- 22.Trinidad DR, Gilpin EA, Messer K, White MM, Pierce JP. Trends in smoking among Hispanic women in California: Relationship to English language use. Am J Prev Med 2006;31(3):257–260 [DOI] [PubMed] [Google Scholar]
- 23.Trinidad DR, Messer K, Gilpin EA, Al-Delaimy WK, White MM, Pierce JP. The California Tobacco Control Program's effect on adult smokers: (3) Similar effects for African Americans across states. Tob Control 2007;16(2):96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce JP, Choi WS, Gilpin EA, Farkas AJ, Merritt RK. Validation of susceptibility as a predictor of which adolescents take up smoking in the United States. Health Psychol 1996;15:355–361 [DOI] [PubMed] [Google Scholar]
- 25.Haenszel W, Shimkin MB, Miller HP. Tobacco smoking patterns in the US. Public Health Monogr 1956;45:1–111 [PubMed] [Google Scholar]
- 26.Pierce JP, Fiore MC, Novotny TE, Hatziandreu EJ, Davis RM. Trends in cigarette smoking in the United States. Educational differences are increasing. JAMA 1989;261(1):56–60 [PubMed] [Google Scholar]
- 27.Giovino GA, Schooley MW, Zhu BP, et al. Surveillance for selected tobacco-use behaviors—United States, 1900-1994. MMWR CDC Surveill Summ 1994;43(3):1–43 [PubMed] [Google Scholar]
- 28.Suits DB, Mason A, Chan L. Spline functions fitted by standard regression methods. Rev Econ Stat 1978;60(1):132–139 [Google Scholar]
- 29.Messer K, Pierce JP, Zhu SH, et al. The California Tobacco Control Program's effect on adult smokers: (1) Smoking cessation. Tob Control 2007;16(2):85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilpin EA, Distefan JM, Pierce JP. Population receptivity to tobacco advertising/promotions and exposure to anti-tobacco media: effect of Master Settlement Agreement in California: 1992-2002. Health Promot Pract 2004;5(3 Suppl):91S–98S [DOI] [PubMed] [Google Scholar]
- 31.Pierce JP, Gilpin EA. How did the Master Settlement Agreement change tobacco industry expenditures for cigarette advertising and promotions? Health Promot Pract 2004;5(3 Suppl):84S–90S [DOI] [PubMed] [Google Scholar]
- 32.Gilpin EA, White VM, Pierce JP. What fraction of young adults are at risk for future smoking, and who are they? Nicotine Tob Res. 2005;7(5):747–759 [DOI] [PubMed] [Google Scholar]
- 33.Sepe E, Ling PM, Glantz SA. Smooth moves: bar and nightclub tobacco promotions that target young adults. Am J Public Health 2002;92(3):414–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Delaimy W, White MM, Gilmer T, Zhu SH, Pierce JP. The California Tobacco Control Program: Can We Maintain the Progress? Vol 1 La Jolla, CA: University of California, San Diego; 2008 [Google Scholar]
- 35.Biener L, Garrett CA, Gilpin EA, Roman AM, Currivan DB. Consequences of declining survey response rates for smoking prevalence estimates. Am J Prev Med 2004;27(3):254–257 [DOI] [PubMed] [Google Scholar]
- 36.Gilpin EA, Pierce JP, Cavin SW, et al. Estimates of population smoking prevalence: self- vs proxy reports of smoking status. Am J Public Health 1994;84(10):1576–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierce JP, Dwyer T, DiGiusto, et al. Cotinine validation of self-reported smoking in commercially run community surveys. J Chronic Dis 1987;40(7):689–695 [DOI] [PubMed] [Google Scholar]
- 38.Warner KE. Possible increases in the underreporting of cigarette consumption. J Am Stat Assoc. 1978;73(362):314–318 [Google Scholar]
- 39.Taioli E, Wynder EL. Effect of the age at which smoking begins on frequency of smoking in adulthood. N Engl J Med 1991;325(13):968–969 [DOI] [PubMed] [Google Scholar]
- 40.Breslau N. Daily cigarette consumption in early adulthood: age of smoking initiation and duration of smoking. Drug Alcohol Depend 1993;33(3):287–291 [DOI] [PubMed] [Google Scholar]