Abstract
Objectives. We used a simulation model to analyze whether the Healthy People 2010 goal of reducing smoking prevalence from the current 19.8% rate to 12% by 2010 could be accomplished by increasing quit attempts, increasing the use of treatments, or increasing the effectiveness of treatment.
Methods. We expanded on previous versions of the tobacco control simulation model SimSmoke to assess the effects of an increase in quit attempts, treatment use, and treatment effectiveness to reduce smoking prevalence. In the model, we considered increases in each of these parameters individually and in combination.
Results. Individually, 100% increases in quit attempts, treatment use, and treatment effectiveness reduced the projected 2020 prevalence to 13.9%, 16.7%, and 15.9%, respectively. With a combined 100% increase in all components, the goal of a 12% adult smoking prevalence could be reached by 2012.
Conclusions. If we are to come close to reaching Healthy People 2010 goals in the foreseeable future, we must not only induce quit attempts but also increase treatment use and effectiveness. Simulation models provide a useful tool for evaluating the potential to reach public health targets.
Since the 1964 Surgeon General's Report first warned of the hazards of smoking tobacco,1 enormous strides have been made in reducing adult smoking prevalence. At the peak of US tobacco use in 1965, the adult smoking prevalence was 42.4%2; now, only 19.8% of adults smoke.3 Nevertheless, an estimated 44.4 million American adults continue to smoke, incurring 443 000 premature deaths, with $97 billion in productivity losses and $96 billion in health care expenditures.4 In recognition of the problem, Healthy People 2010 set an ambitious goal of reducing smoking prevalence to 12% by 2010.5 With that goal now almost certainly unattainable,6 new approaches need to be explored.
Smoking prevalence can be reduced in 3 ways: (1) by preventing nonsmokers from initiating smoking, (2) by inducing current smokers to quit, and (3) by preventing those who have already quit from relapsing back to smoking. Because prevention strategies apply largely to persons in the 14- to 20-year-old age group,7 only a small percentage of the population is affected at any point in time and many years must pass before the strategies lead to large reductions in adult rates.8 By contrast, quitting strategies can be targeted at smokers of all ages and can lead to a more immediate drop in adult prevalence. Still, encouraging smokers to quit will only go so far in reducing prevalence unless something is done in tandem to help smokers maintain their abstinence.
Each year, fewer than 45% of smokers make a serious quit attempt and quit for even 24 hours.9 More than three fourths of smokers making a quit attempt each year do not use efficacious treatment,10 and only 3% to 5% of those untreated smokers remain abstinent for 12 months.11,12 Quit success increases 2- to 3-fold when proven treatments are used.13 Those at the lowest socioeconomic levels are the most vulnerable to smoking but the least successful at quitting when making a quit attempt.9,14–18 Thus, much is to be gained by improving treatment effectiveness along with increasing the number of smokers who attempt to quit and who use evidence-based treatment.
Simulation models are useful for understanding and predicting how changes in specific inputs (e.g., treatment use) lead to changes in outputs (e.g., quit rates) over time in complex social systems.19,20 Modeling helps to reveal relationships by organizing the channels of influence and by making assumptions about the relevant relationships more explicit. This process generally proves more robust than relying on intuition alone and is thereby useful for evaluating hypothetical future scenarios.
Numerous models of smoking behaviors have been developed to show the effect of tobacco control policies on smoking prevalence and health outcomes.8,21–28 These models use information from studies of past policies or smoking behaviors to predict future smoking rates. We were motivated by the need to better understand the changes that would be necessary to meet prevalence goals. Because changes in smoking initiation will have minimal impact on smoking prevalence within the next 15 years, we focused on the cessation process. We carried out a series of simulations that focused on the quitting process and were intended to examine hypothetical effects of changes in quitting behaviors on smoking prevalence. Specifically, we generated a model that predicted smoking prevalence by using 3 strategies: (1) by increasing the percentage of current smokers making a quit attempt, (2) by increasing the percentage of current smokers who use an evidence-based treatment, and (3) by improving treatment effectiveness. We explored the magnitude of the various strategies necessary to reach the Healthy People 2010 goal of a 12% smoking prevalence and the time frame in which it could be achieved.
METHODS
The model we used is based on the SimSmoke model. Discussed at length elsewhere,8,20,29–32 the model begins with the population divided by age and gender into the number of current smokers, never smokers, and former smokers. The model uses a discrete time, first-order Markov process33 with births and deaths to project a population from the base year forward. Similarly, smoking rates depend on initiation, cessation, and relapse. Individuals are classified as never smokers from birth until they initiate smoking. Current smokers may become former smokers through cessation and may return to smoking through relapse.
Quit rates were modified from our earlier models to account for the 3 primary elements of quitting.34 Based on data availability and the structure of the model, we considered quitting in the past year. A smoker first decides whether to make a quit attempt. Upon making a quit attempt, the smoker elects whether to use evidence-based treatment, the results of which depend on treatment effectiveness.
At a population level, the model can be expressed as:
where PQR is the population-level quit rate, QA is the proportion of current smokers who have made a quit attempt in the past year, TxUsei is the proportion of the quitting population who used an evidence-based treatment i (Σi = 1…4 TxUsei = 1), and TxEffi is the effectiveness of treatment i. Treatments fall into 4 mutually exclusive categories: (1) no evidence-based treatment, meaning no assistance from a health care provider, medication, or other evidence-based treatment (no evidence-based treatment may include the use of pamphlets, videos, other self-help materials; unproven quitting aids and remedies; or informal strategies such as cutting down on smoking before quitting); (2) pharmacotherapy only, including over-the-counter and prescription medications approved by the Food and Drug Administration (e.g., nicotine replacement products, bupropion/bupropion hydrochloride, nortriptyline, and clonidine); (3) behavioral therapy only, involving proactive telephone counseling or face-to-face group or individual counseling; and (4) any combination of both pharmacotherapy and behavioral therapy.
Input Measures
Smoking data were from the 2003 Tobacco Use Supplement of the Current Population Survey (TUS-CPS), a nationally representative household survey of the noninstitutionalized, civilian, US population aged 15 years and older.35 Data from 2003 were used because a special survey was conducted that included questions on cessation treatment along with tobacco use.
Persons who reported smoking at least 100 cigarettes in their lifetime and who were currently smoking some days or every day on the TUS-CPS survey were considered current smokers. Those who reported exceeding the 100-cigarette lifetime threshold but who were no longer smoking were categorized as ex-smokers. Those below the 100-cigarette lifetime threshold were treated as never smokers. Initiation rates were tracked through age 24 years and were measured at a particular age as the change in prevalence rate between those smoking at that age and those smoking at the previous age.
As part of the TUS-CPS, current smokers were asked, “Have you ever stopped smoking for one day or longer because you were trying to quit smoking?” followed by, “During the past 12 months, have you stopped smoking for one day or longer because you were trying to quit smoking?” Individuals who answered yes to both questions were designated as having made a quit attempt in the past year. In addition, individuals who were former smokers at the time the 2003 TUS-CPS was administered but who were smoking 1 year previously were designated as having made a quit attempt. Those who had made a quit attempt in the 12 months before the survey were asked about treatment use in their last quit attempt, which was used to measure treatment use.
The model developed PQRs for the following age groups: 18 to 24 years, five 10-year age groups from 25–34 years through 65–74 years, and 75 years and older. Relapse rates after the first year determined the percentage of quitters who returned to smoking. Relapse rates, which were not available from the TUS-CPS, were obtained from other sources.12,36–39
In the baseline scenario, treatment effectiveness was calculated for each treatment category in terms of its improvement over no evidence-based treatment. In accordance with the literature, we selected 4% as the quit rate per quit attempt.12,40–43 Each of the recommended and approved pharmacotherapies at least double quit rates compared with no evidence-based treatment with the exception of nicotine gum (odds ratio = 1.5).15,44–46 Compared with no evidence-based treatment, proactive telephone quit lines are approximately 1.6 times as effective,15,47 face-to-face individual counseling is 1.6 times as effective,48 and face-to-face group counseling is twice as effective.49 Adding behavioral therapy to pharmacotherapy yields quit rates 30% above rates achieved with pharmacotherapy alone,15 and adding pharmacotherapy to behavioral therapy increases these rates by 70%. Consistent with findings by others, quit rates can be enhanced 50% to 100% over rates for pharmacotherapy when combined with behavioral counseling.50–52 We estimated that compared with no evidence-based treatment, quit rates would increase 100% with pharmacotherapy, 60% with the different forms of behavioral therapy, and 200% when pharmacotherapy and behavioral therapy were used in combination.
The TUS-CPS asks about treatment use during the last quit attempt, but data show that smokers who make a quit attempt average more than 3.5 quit attempts per year. This rate is roughly consistent with a pattern whereby half of those who make at least one quit attempt go on to make at least a second quit attempt, and half of those make a third quit attempt, and so on (i.e., 1 + 0.5 * 2 + 0.25 * 3 + 0.0125 * 4 …). If the proportion of people who use each type of treatment remains constant across the multiple attempts, effectiveness rates double (i.e., 1 + 0.5 + 0.25 + 0.0125 … = 2), yielding quit rates of 8%, 12.8%, 16%, and 24% for no evidence-based treatment, behavioral therapy, pharmacotherapy, and combination behavioral and pharmacotherapy, respectively.
Model Calibration, Validation, and Projection
To calibrate the initial quit rates used in our model, we first computed the PQR for all ages and by age group using the 2003 TUS-CPS data on quit attempts and treatment use, as well as for treatment effectiveness estimates. We then compared these PQRs with a quit rate measure suggested by Burns et al.,53 which was measured with 2003 TUS-CPS data as the number of current ex-smokers who quit in the last year and who have quit for at least 3 months as a percentage of those who were smokers 1 year ago. This comparison was used to correct for age-related variations in the quit rate.
Our model takes into account the effects of actual policies implemented between 2003 and 2007, as described in Levy et al.54 We validated the model over the period from 2003 to 2007 by comparing the projected smoking prevalence over that period with the National Health Interview Survey data,2 which were also used in evaluating the progress toward specific Healthy People 2010 goals. Because our model was based on TUS-CPS data, we multiplied the value of smoking prevalence each year by the ratio of the average National Health Interview Survey 2003 to the average TUS-CPS 2003 smoking prevalence to have comparable estimates.
We projected the effect of changes quit attempts, treatment use, and treatment effectiveness from the year 2009 onward, assuming that changes in the quitting behaviors were sustained through the year 2020. These changes were compared with a status quo scenario, in which quit attempts, treatment use, and treatment effectiveness stayed at their 2008 levels in future years. Specifically, we considered the following strategies:
Increasing quit attempts relative to their current levels by 25%, by 50%, and by 100%;
Increasing the percentage who use each of the evidence-based treatment options (pharmacotherapy, behavioral therapy, or combination pharmacotherapy and behavioral therapy) by 50% and by 100%; and
Improving treatment effectiveness such that the percentage of smokers using evidence-based treatment were able to increase their 1-year success rates by 50% and by 100%.
We analyzed these strategies when implemented in isolation and then in combination.
The effect of the strategies on quit rates may weaken in future years, because those who are most amenable to quitting have already quit and those who have already tried to quit and failed may be less likely to try and more likely to fail in future attempts. To capture these decaying effects, we assumed a geometric decline in the effect of the strategy on the quit rate, i.e., for an annual decay rate of x%, PQRstatus quo + [PQRwith policy − PQRstatus quo] * (1 − x)i − 1 in the ith year that the strategy has been in place. We initially assumed a 10% decay rate based on evidence of the time pattern effects of policies on smoking prevalence,54 but also considered 0% and 25% decay rates.
RESULTS
With use of the TUS-CPS data, the quit attempt rate averaged 42% for all smokers aged 18 years and older and decreased with age from 52% for those aged 18 to 24 years to 31% for those aged 75 years and older. Treatment use rates from the TUS-CPS averaged 24.9% for pharmacotherapy only, 1.2% for behavioral therapy only, 2.0% for combined pharmacotherapy and behavioral therapy, and 72% for self-assists, with usage of evidence-based treatment generally increasing to age 45 and then decreasing after age 65.
The average initial quit rates from the model were 4.4% for those aged 18 years and older compared with 4.5% by use of the Burns measure53 (4.1% and 4.3%, respectively, for ages 24 years and older). By age group, the model rates were higher than the Burns measure for those aged 35 to 64 years and were lower for those younger than 35 years and older than 64 years. To calibrate the model, we adjusted the quit rate by age (120% for ages 18 to 24, 110% for ages 24 to 35, 90% for ages 35 to 64, 120% for ages 65 to 74, and 130% for ages 75 and older). The average age-adjusted quit rates from the model were then 4.5% for smokers 18 2years and older and 4.2% for those 24 years and older.
The model was initialized with adult (18 years and older) smoking prevalence at 21.6% in 2003, which fell to 20.1% by 2008. Similarly, the National Health Interview Survey smoking rate3 fell to 20.6% (95% CI = 19.9%, 20.4%) in 2008. Our predictions thus fell within the National Health Interview Survey confidence intervals for all years.
The smoking prevalences from 2008 to 2020 generated by the model for each of the different scenarios are shown in Table 1. In the absence of changes to existing tobacco control policy (i.e., the status quo), the model predicted that smoking prevalence would drop to 20.1% in 2008, 18.6% in 2015, and 17.5% in 2020.
TABLE 1.
Model-Generated Smoking Prevalences, by Year and by Quit Attempts, Treatment Use, and Treatment Effectiveness Scenarios, 2008–2020
| 2008, % | 2009, % | 2010, % | Change From Status Quo, % | 2015, % | Change From Status Quo, % | 2020, % | Change From Status Quo, % | |
| Status quo | 20.1 | 19.9 | 19.6 | 18.6 | 17.5 | |||
| Increase in QAs | ||||||||
| By 25% | 20.1 | 19.6 | 19.2 | −2.1 | 17.7 | −4.7 | 16.5 | −5.8 |
| By 50% | 20.1 | 19.4 | 18.8 | −4.2 | 16.9 | −9.2 | 15.6 | −11.1 |
| By 100% | 20.1 | 19.0 | 18.0 | −8.3 | 15.3 | −17.4 | 13.9 | −20.8 |
| Increase in TxUse | ||||||||
| By 50% | 20.1 | 19.8 | 19.4 | −0.9 | 18.2 | −2.1 | 17.1 | −2.5 |
| By 100% | 20.1 | 19.7 | 19.3 | −1.9 | 17.8 | −4.1 | 16.7 | −4.8 |
| Increase in QAs and TxUse | ||||||||
| By 50% and 50% | 20.1 | 19.3 | 18.5 | −5.6 | 16.3 | −11.9 | 15.0 | −14.3 |
| By 100% and 100% | 20.1 | 18.6 | 17.3 | −11.9 | 14.1 | −23.9 | 12.6 | −27.9 |
| Increase in TxEffa | ||||||||
| By 50% | 20.1 | 19.7 | 19.3 | −1.8 | 17.8 | −4.0 | 16.7 | −4.7 |
| By 100% | 20.1 | 19.5 | 18.9 | −3.6 | 17.1 | −7.8 | 15.9 | −9.2 |
| Increase in QAs and TxEff | ||||||||
| By 50% and 50% | 20.1 | 19.1 | 18.3 | −6.9 | 15.9 | −14.5 | 14.5 | −17.2 |
| By 100% and 100% | 20.1 | 18.2 | 16.6 | −15.2 | 13.1 | −29.5 | 11.6 | −33.9 |
| Increase in TxUse and TxEff | ||||||||
| By 50% and 50% | 20.1 | 19.5 | 18.9 | −3.7 | 17.1 | −7.8 | 15.9 | −9.2 |
| Increase in QAs, TxUse, and TxEff | ||||||||
| By 50%, by 50%, and by 50% | 20.1 | 18.8 | 17.8 | −9.6 | 14.9 | −19.5 | 13.5 | −22.8 |
| By 100%, by 100%, and by 100% | 20.1 | 17.0 | 14.7 | −25.1 | 10.4 | −44.1 | 9.0 | −48.9 |
| Increase in QAs, TxUse, and TxEff, with 0% decay rate | ||||||||
| By 50%, by 50%, and by 50% | 20.1 | 18.8 | 17.7 | −10.0 | 14.0 | −24.7 | 11.7 | −33.5 |
| By 100%, by 100%, and by 100% | 20.1 | 17.0 | 14.5 | −26.1 | 8.7 | −53.0 | 6.3 | −63.8 |
| Increase in QAs, TxUse, and TxEff with 25% decay rate | ||||||||
| By 50%, by 50%, and by 50% | 20.1 | 18.8 | 17.9 | −8.9 | 15.9 | −14.2 | 14.9 | −14.9 |
| By 100%, by 100%, and by 100% | 20.1 | 17.0 | 15.0 | −23.5 | 12.3 | −33.6 | 11.5 | −34.2 |
Note. QA = quit attempts; TxEff = treatment effectiveness; TxUse = treatment use.
Such as reduced relapse.
Under scenario 1, even a modest 25% increase in quit attempts would lead to a reduction in the smoking rate to 16.5% by 2020 (compared with 17.5% for the status quo), or 5.8% below that of the status quo rate in 2020 in relative terms. A 50% increase in quit attempts would lead to a 15.6% smoking prevalence by 2020, 11.1% below the status quo. A 100% increase in quit attempts would lead to a 13.9% rate by 2020, 20.8% below the status quo.
Under scenario 2, a 50% increase in treatment use would lead to smaller annual reductions in smoking prevalence compared with a 50% reduction in quit attempts, because only a portion of the smokers making quit attempts would be affected. A 50% increase in the use of all evidence-based treatments led to a 17.1% smoking rate in 2020. A 100% increase in treatment use would lead to a 16.7% smoking rate, or 4.8% below the status quo.
Scenario 3 revealed that increases in treatment effectiveness led to larger reductions in smoking prevalence compared with the second scenario, where the increase in evidence-based treatment users was offset by the reduction among quitters using no evidence-based treatment. By 2020, a 50% increase in treatment effectiveness for evidence-based treatments would lead to a 16.7% smoking rate in 2020. A doubling of treatment effectiveness would lead to a 15.9% smoking rate in 2020, or 9.2% below the status quo.
Although each simulation yielded major reductions, smoking prevalence in all scenarios was projected to be above the Healthy People 2010 goal of 12% in the year 2020. Combining strategies leads to synergies, because increasing quit attempts and treatment use means that the larger number of smokers making a quit attempt would also have higher rates of quit success. For example, a 100% increase in quit attempts and treatment use resulted in a 12.6% smoking rate in 2020, or 27.9% below the status quo.
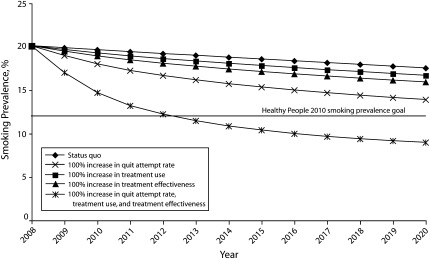
As shown in Figure 1, greater reductions in prevalence are accomplished faster when all 3 strategies are combined. For example, when 50% increases in quit attempts, treatment use, and treatment effectiveness were entered into the model simultaneously, a 13.5% smoking prevalence rate was predicted in 2020. With a 100% increase in all components, the Healthy People 2010 goal of 12% is reached by 2012.
FIGURE 1.
Effects of a 100% reduction in the quit attempt rate, treatment use, and treatment effectiveness on smoking prevalence from 2008–2020.
To reflect that the strategies may lose potency over time, we reduced each strategy's impact by 10% each year (i.e., a 10% per year decay rate) in the analyses in the previous paragraph. When a decay rate of 25% per year was applied, a 50% increase in each of quit attempts, treatment use, and treatment effectiveness led to a 14.9% smoking prevalence in 2020 compared with 13.5% with a 10% decay rate. With a 0% decay rate, smoking prevalence was projected to be 11.7% in 2020.
DISCUSSION
Our model focused on the elements of successful cessation to provide a framework for reaching the Healthy People 2010 goal in the foreseeable future. Although our results echo the concerns of a recent Institute of Medicine report6 that the 2010 goal will not be reached by the year 2010, our simulations suggest that the goal of a 12% smoking prevalence may be reached as early as 2012 if substantial and simultaneous changes in the rate of quit attempts, treatment use, and treatment effectiveness can be induced.
Our findings suggest that it is important to focus on each of these key indicators to achieve significant declines in smoking prevalence. Important synergies are created by increasing quit attempts along with treatment use and treatment effectiveness, because those who make a quit attempt are more likely to remain quitters.
Before calibration, the model underpredicted quit rates for smokers aged 65 years and older, which may have been because of higher quit success rates by those diagnosed with health problems. The data indicated low rates of treatment use and quit attempts for the higher age groups. The model also underpredicted rates for those aged younger than 35 years, which may have been the result of different smoking patterns in the more recent cohort, for example, more some-day smokers or a shorter smoking history. Treatment use was low, whereas quit attempt rates were high, among those aged younger than 25 years, suggesting room for improvement.
As in any simulation study, it is important to remember that the validity of the model depends on the assumptions inherent in the model. Every model is by necessity a simplification of the real world. We elected to set our model boundaries around quitting behavior; therefore, except where noted, other influences on smoking prevalence (e.g., initiation, tobacco industry behavior, use of other tobacco products) were not considered. The model also did not distinguish quitting by gender or socioeconomic status.
The validity of the model also depends on the data used to estimate key parameters. Treatment use was based on the last quit attempt, because information was not available on the history of treatment use for multiple quit attempts even for the last year. Treatments were categorized into 4 broad groups of pharmacotherapy and behavioral therapy and not specific types within a category or simultaneous use of multiple forms of treatment within each category. The data also did not gauge adherence to recommended intensity and duration of treatment, thus, initial treatment effectiveness reflects the average rate of adherence of those treated at baseline.
We focused on the first year of cessation. Although most relapse occurs in the first year, as many as 35% of smokers relapse over the following 15 years.12 When relapse rates after the first year were reduced under the different strategies, smoking prevalence in the year 2020 changed by less than 2% with a 10% reduction in relapse and by 5% with a 25% reduction.
The analyses in this article were based on hypothetical changes in quit attempts, treatment use, and treatment effectiveness. The next challenge is to develop policies to induce the desired increases in cessation behaviors. Other modeling work by our group indicates that cessation treatment policies, such as removing barriers to treatment (especially that of cost), better publicizing quit lines, and encouraging brief interventions, may go a long way toward this goal.55 When all of those policies were fully combined, we estimated that the percentage of smokers who quit would increase from 4.3% to 7.6%. Whereas most discussion of cessation treatment policies focuses on ways to induce more quit attempts or increase the use of evidence-based treatment, our analysis highlights the importance of treatment effectiveness, an often overlooked strategy. Potential avenues to increase treatment success include better involvement of health care providers, improved treatments, improved adherence to treatment, and tailoring treatments to smokers on the basis of individual characteristics.55
In addition to policies specifically directed at cessation treatment, studies also find that increases in cigarette prices (e.g., through taxes),18,56,57 worksite smoking bans,58–60 and antismoking media campaigns61 increase quit attempts and quit success. Increasing cigarette prices also boosts sales of nicotine-replacement therapy.62,63 When tax, clean air, and media policies are combined with cessation treatment policies (as described in the preceding paragraph), we estimate an increase in the use of evidence-based treatments by 130% (in relative terms), in the rate of quit attempts by 90%, and in the percentage of smokers who quit by 180%.54
We examined the mechanisms through which tobacco policies must work if they are to move us close to the Healthy People 2010 smoking prevalence goal in the next 15 years. Our model demonstrates the power of increasing cessation in reducing smoking prevalence, but also indicates that the greatest impact requires simultaneous attention to treatment use, treatment effectiveness, and quit attempts. Careful tracking of these indicators can be used to continually improve the success of policies, thereby moving us closer to the Healthy People 2010 goal for smoking prevalence.
Acknowledgments
This article was conducted under the auspices of the National Tobacco Cessation Collaborative's Consumer Demand Roundtable and was supported by funds provided by the National Institutes of Health, Office of Behavioral and Social Sciences Research (OBSSR, contract # HHSN 276200700294P) and the Robert Wood Johnson Foundation (RWJF). David T. Levy also received funding from the Cancer Intervention and Surveillance Modeling Network (CISNET) of the Division of Cancer Control and Population Sciences, National Cancer Institute (grant UO1-CA97450-02).
Note. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the American Legacy Foundation, the University of Baltimore, OBSSR, or RWJF.
Human Participant Protection
No approval was required because data were obtained from secondary sources.
References
- 1.Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Atlanta, GA: US Department of Health, Education and Welfare; 1964. DHHS publication 1103 [Google Scholar]
- 2.Percentage of Adults Who Were Current, Former, or Never Smokers, Overall and by Sex, Race, Hispanic Origin, Age, Education, and Poverty Status; National Health Interview Surveys, Selected Years—United States, 1965–2006 Atlanta, GA: Centers for Disease Control and Prevention; 2007 [Google Scholar]
- 3.Cigarette smoking among adults—United States, 2007. MMWR Morb Mortal Wkly Rep 2008;57(45):1221–1226 [PubMed] [Google Scholar]
- 4.Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep 2008;57(45):1226–1228 [PubMed] [Google Scholar]
- 5.Healthy People 2010: Understanding and Improving Health Washington, DC: US Department of Health and Human Services; 2000 [Google Scholar]
- 6.Institute of Medicine Ending the Tobacco Problem: A Blueprint for the Nation Washington, DC: The National Academies Press; 2007 [Google Scholar]
- 7.Preventing Tobacco Use Among Young People: a Report of the Surgeon General Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1994 [Google Scholar]
- 8.Levy DT, Cummings KM, Hyland A. A simulation of the effects of youth initiation policies on overall cigarette use. Am J Public Health 2000;90(8):1311–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cigarette smoking among adults—United States, 2006. MMWR Morb Mortal Wkly Rep 2007;56(44):1157–1161 [PubMed] [Google Scholar]
- 10.Cokkinides VE, Ward E, Jemal A, Thun MJ. Under-use of smoking-cessation treatments: results from the National Health Interview Survey, 2000. Am J Prev Med 2005;28(1):119–122 [DOI] [PubMed] [Google Scholar]
- 11.Cigarette smoking among adults: United States, 2000. MMWR Morb Mortal Wkly Rep 2002;51(29):642–645 [PubMed] [Google Scholar]
- 12.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004;99(1):29–38 [DOI] [PubMed] [Google Scholar]
- 13.Fiore M, Jaén C, Baker T, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008 [Google Scholar]
- 14.Gilman SE, Abrams DB, Buka SL. Socioeconomic status over the life course and stages of cigarette use: initiation, regular use, and cessation. J Epidemiol Community Health 2003;57(10):802–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagan P, Shavers V, Lawrence D, Gibson J, O'Connell M. Employment characteristics and socioeconomic factors associated with disparities in smoking abstinence and former smoking among U.S. workers. J Health Care Poor Underserved 2007;18(4 Suppl):52–72 [DOI] [PubMed] [Google Scholar]
- 16.Levy DT, Romano E, Mumford E. The relationship of smoking cessation to socio-demographic characteristics, smoking intensity and tobacco control policies. Nicotine Tob Res 2005;7(3):387–396 [DOI] [PubMed] [Google Scholar]
- 17.Gollust SE, Schroeder SA, Warner KE. Helping smokers quit: understanding the barriers to utilization of smoking cessation services. Milbank Q 2008;86(4):601–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orleans CT. Increasing the demand for and use of effective smoking-cessation treatments reaping the full health benefits of tobacco-control science and policy gains–in our lifetime. Am J Prev Med 2007;33(6 Suppl):S340–S348 [DOI] [PubMed] [Google Scholar]
- 19.Homer JB, Hirsch GB. System dynamics modeling for public health: background and opportunities. Am J Public Health 2006;96(3):452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy DT, Bauer JE, Lee HR. Simulation modeling and tobacco control: creating more robust public health policies. Am J Public Health 2006;96(3):494–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendez D, Warner KE, Courant PN. Has smoking cessation ceased? Expected trends in the prevalence of smoking in the United States. Am J Epidemiol 1998;148(3):249–258 [DOI] [PubMed] [Google Scholar]
- 22.Mendez D, Warner KE. Adult cigarette smoking prevalence: declining as expected (not as desired). Am J Public Health 2004;94(2):251–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tengs TO, Osgood ND, Lin TH. Public health impact of changes in smoking behavior: results from the Tobacco Policy Model. Med Care 2001;39(10):1131–1141 [DOI] [PubMed] [Google Scholar]
- 24.Tengs TO, Ahmad S, Moore R, Gage E. Federal policy mandating safer cigarettes: a hypothetical simulation of the anticipated population health gains or losses. J Policy Anal Manage 2004;23(4):857–872 [DOI] [PubMed] [Google Scholar]
- 25.Ahmad S. Increasing excise taxes on cigarettes in California: a dynamic simulation of health and economic impacts. Prev Med 2005;41(1):276–283 [DOI] [PubMed] [Google Scholar]
- 26.Ahmad S, Billimek J. Estimating the health impacts of tobacco harm reduction policies: a simulation modeling approach. Risk Anal 2005;25(4):801–812 [DOI] [PubMed] [Google Scholar]
- 27.Levy DT, Nikolayev N, Mumford EA. Recent trends in smoking and the role of public policies: results from the SimSmoke tobacco control policy simulation model. Addiction 2005;100(10):1526–1537 [DOI] [PubMed] [Google Scholar]
- 28.Levy DT, Nikolayev N, Mumford EA. The Healthy People 2010 smoking prevalence and tobacco control objectives: results from the SimSmoke tobacco control policy simulation model. Cancer Causes Control 2005;16(4):359–371 [DOI] [PubMed] [Google Scholar]
- 29.Levy DT, Chaloupka F, Gitchell J, Mendez D, Warner KE. The use of simulation models for the surveillance, justification and understanding of tobacco control policies. Health Care Manage Sci 2002;5(2):113–120 [DOI] [PubMed] [Google Scholar]
- 30.Levy DT, Cummings KM, Hyland A. Increasing taxes as a strategy to reduce cigarette use and deaths: results of a simulation model. Prev Med 2000;31(3):279–286 [DOI] [PubMed] [Google Scholar]
- 31.Levy D, Mumford E, Pesin B. Tobacco control policies, and reductions in smoking rates and smoking-related deaths: results from the SimSmoke model. Expert Rev Pharmacoeconomics Outcomes Res 2003;3(4):457–468 [DOI] [PubMed] [Google Scholar]
- 32.Levy DT, Friend K. A simulation model of policies directed at treating tobacco use and dependence. Med Decis Making 2002;22(1):6–17 [DOI] [PubMed] [Google Scholar]
- 33.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993;13(4):322–338 [DOI] [PubMed] [Google Scholar]
- 34.Levy DT, Friend K. Examining the effects of tobacco treatment policies on smoking rates and smoking related deaths using the SimSmoke computer simulation model. Tob Control 2002;11(1):47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Current Population Survey, February, June, and November 2003: Tobacco Use Supplement conducted by the U.S. Census Bureau for the Bureau of Labor Statistics Washington, DC: US Census Bureau; 2006 [Google Scholar]
- 36.Hughes JR, Peters EN, Naud S. Relapse to smoking after 1 year of abstinence: a meta-analysis. Addict Behav 2008;33(12):1516–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilpin EA, Pierce JP, Farkas AJ. Duration of smoking abstinence and success in quitting. J Natl Cancer Inst 1997;89(8):572–576 [DOI] [PubMed] [Google Scholar]
- 38.McWhorter WP, Boyd GM, Mattson ME. Predictors of quitting smoking: the NHANES I followup experience. J Clin Epidemiol 1990;43(12):1399–1405 [DOI] [PubMed] [Google Scholar]
- 39.The Health Benefits of Smoking Cessation: a report of the Surgeon General Atlanta, GA: US Department of Health and Human Services, Public Health Service, Centers for Disease Control, Office on Smoking and Health; 1990 [Google Scholar]
- 40.Cohen S, Lichtenstein E, Prochaska JO, et al. Debunking myths about self-quitting: evidence from 10 prospective studies of persons who attempt to quit smoking by themselves. Am Psychol 1989;44(11):1355–1365 [DOI] [PubMed] [Google Scholar]
- 41.Hughes JR. Four beliefs that may impede progress in the treatment of smoking. Tob Control 1999;8(3):323–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes JR, Gulliver SB, Fenwick JW, et al. Smoking cessation among self-quitters. Health Psychol 1992;11(5):331–334 [DOI] [PubMed] [Google Scholar]
- 43.Hughes JR, Shiffman S, Callas P, Zhang J. A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tob Control 2003;12(1):21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2004;(3):CD000146. [DOI] [PubMed] [Google Scholar]
- 45.Hughes JR, Stead LF, Lancaster T. Nortriptyline for smoking cessation: a review. Nicotine Tob Res 2005;7(4):491–499 [DOI] [PubMed] [Google Scholar]
- 46.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2007;(1):CD000031. [DOI] [PubMed] [Google Scholar]
- 47.Stead LF, Perera R, Lancaster T. A systematic review of interventions for smokers who contact quitlines. Tob Control 2007;16(Suppl 1):i3–i8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2005;(2):CD001292. [DOI] [PubMed] [Google Scholar]
- 49.Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2005;(2):CD001007. [DOI] [PubMed] [Google Scholar]
- 50.Fiore MC, Croyle RT, Curry SJ, et al. Preventing 3 million premature deaths and helping 5 million smokers quit: a national action plan for tobacco cessation. Am J Public Health 2004;94(2):205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foulds J, Steinberg MB, Williams JM, Ziedonis DM. Developments in pharmacotherapy for tobacco dependence: past, present and future. Drug Alcohol Rev 2006;25(1):59–71 [DOI] [PubMed] [Google Scholar]
- 52.Swan GE, McAfee T, Curry SJ, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Arch Intern Med 2003;163(19):2337–2344 [DOI] [PubMed] [Google Scholar]
- 53.Burns D, Anderson C, Johnson M, et al. Cessation and cessation measures among daily adult smokers: national- and state-specific data. : Population-Based Smoking Cessation: A Conference on What Works to Influence Smoking in the General Population Smoking and Tobacco Control Monograph no. 12 Bethesda, MD: National Cancer Institute, National Institutes of Health; 2000:113–304 [Google Scholar]
- 54.Levy DT, Mabry PL, Graham AL, Orleans CT, Abrams DB. Reaching Healthy People 2010 by 2013: A SimSmoke simulation. Am J Prev Med 2010;38(3 Suppl):S373–S381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levy DT, Graham AL, Mabry PL, Abrams DB, Orleans CT. Modeling the impact of smoking-cessation treatment policies on quit rates. Am J Prev Med 2010;38(3 Suppl):S364–S372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tauras J, Chaloupka F. Determinants of Smoking Cessation: An Analysis of Young Adult Men and Women Cheltenham, United Kingdom: Edward Elgar Publishing Limited; 2001 [Google Scholar]
- 57.Tauras JA, Chaloupka FJ. The demand for nicotine replacement therapies. Nicotine Tob Res 2003;5(2):237–243 [DOI] [PubMed] [Google Scholar]
- 58.Burns D, Shanks T, Major J, Gower K, Shopland D. Restrictions on smoking in the workplace. : Population Based Smoking Cessation Monograph No. 12 Bethesda, MD: National Institutes of Health; 2000 [Google Scholar]
- 59.Glasgow RE, Cummings KM, Hyland A. Relationship of worksite smoking policy to changes in employee tobacco use: findings from COMMIT. Community Intervention Trial for Smoking Cessation. Tob Control 1997;6(Suppl 2):S44–S48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farkas AJ, Gilpin EA, Distefan JM, Pierce JP. The effects of household and workplace smoking restrictions on quitting behaviours. Tob Control 1999;8(3):261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hyland A, Li Q, Bauer JE, Giovino GA, Bauer U, Cummings KM. State and community tobacco-control programs and smoking-cessation rates among adult smokers: what can we learn from the COMMIT intervention cohort? Am J Health Promot 2006;20(4):272–281 [DOI] [PubMed] [Google Scholar]
- 62.Tauras JA, Chaloupka FJ, Emery S. The impact of advertising on nicotine replacement therapy demand. Soc Sci Med 2005;60(10):2351–2358 [DOI] [PubMed] [Google Scholar]
- 63.Metzger KB, Mostashari F, Kerker BD. Use of pharmacy data to evaluate smoking regulations' impact on sales of nicotine replacement therapies in New York City. Am J Public Health 2005;95(6):1050–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]