Abstract
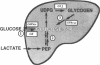
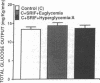
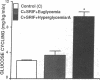
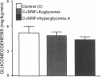
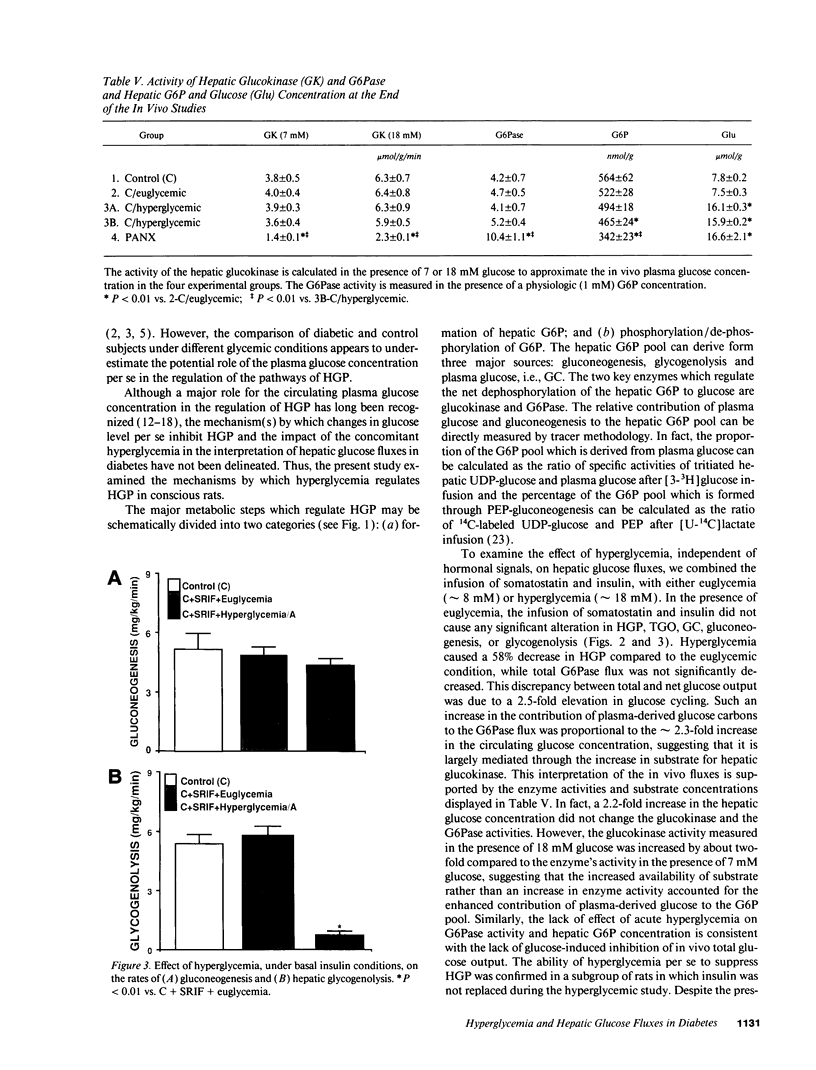
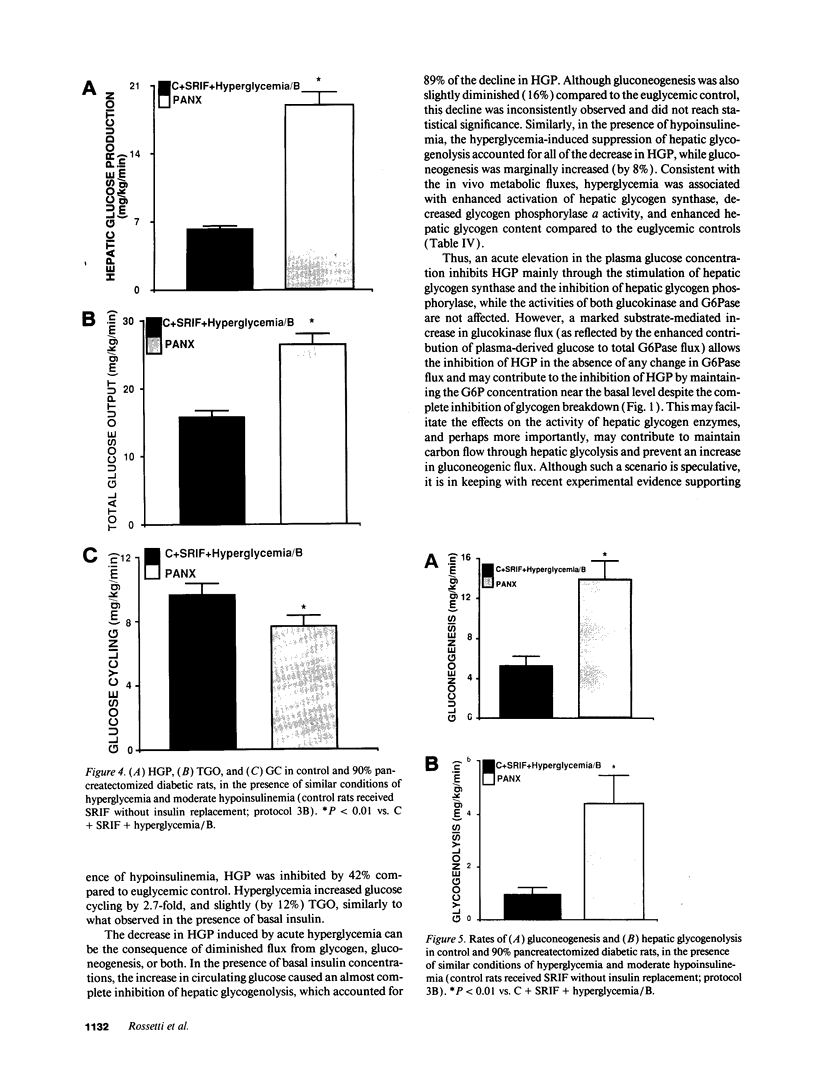
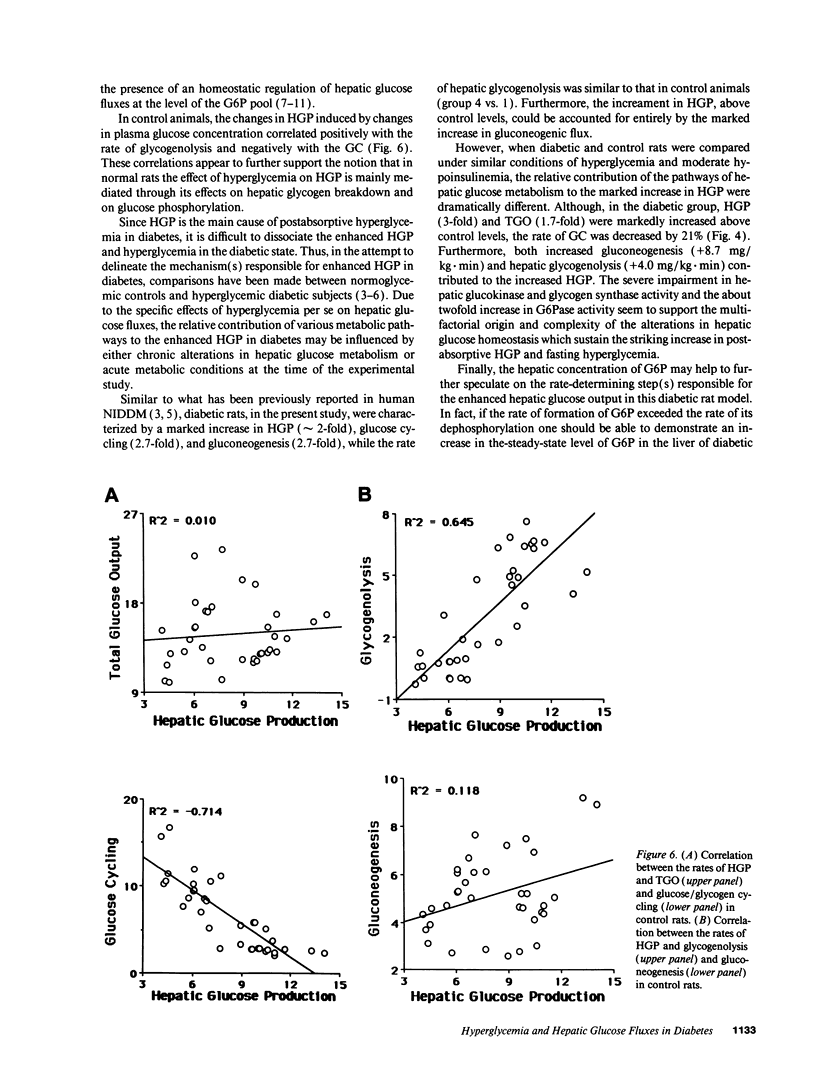
To examine the relationship between the plasma glucose concentration (PG) and the pathways of hepatic glucose production (HGP), five groups of conscious rats were studied after a 6-h fast: (a) control rats (PG = 8.0 +/- 0.2 mM); (b) control rats (PG = 7.9 +/- 0.2 mM) with somatostatin and insulin replaced at the basal level; (c) control rats (PG = 18.1 +/- 0.2 mM) with somatostatin, insulin replaced at the basal level, and glucose infused to acutely raise plasma glucose by 10 mM; (d) control rats (PG = 18.0 +/- 0.2 mM) with somatostatin and glucose infusions to acutely reproduce the metabolic conditions of diabetic rats, i.e., hyperglycemia and moderate hypoinsulinemia; (e) diabetic rats (PG = 18.4 +/- 2.3 mM). All rats received an infusion of [3-3H]glucose and [U-14C]lactate. The ratio between hepatic [14C]UDP-glucose sp act (SA) and 2X [14C]-phosphoenolpyruvate (PEP) SA (the former reflecting glucose-6-phosphate SA) measured the portion of total glucose output derived from PEP-gluconeogenesis. In control rats, HGP was decreased by 58% in hyperglycemic compared to euglycemic conditions (4.5 +/- 0.3 vs. 10.6 +/- 0.2 mg/kg.min; P < 0.01). When evaluated under identical glycemic conditions, HGP was significantly increased in diabetic rats (18.9 +/- 1.4 vs. 6.2 +/- 0.4 mg/kg.min; P < 0.01). In control rats, hyperglycemia increased glucose cycling (by 2.5-fold) and the contribution of gluconeogenesis to HGP (91% vs. 45%), while decreasing that of glycogenolysis (9% vs. 55%). Under identical plasma glucose and insulin concentrations, glucose cycling in diabetic rats was decreased (by 21%) and the percent contribution of gluconeogenesis to HGP (73%) was similar to that of controls (84%). These data indicate that: (a) hyperglycemia causes a marked inhibition of HGP mainly through the suppression of glycogenolysis and the increase in glucokinase flux, with no apparent changes in the fluxes through gluconeogenesis and glucose-6-phosphatase; under similar hyperglycemic hypoinsulinemic conditions: (b) HGP is markedly increased in diabetic rats; however, (c) the contribution of glycogenolysis and gluconeogenesis to HGP is similar to control animals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ader M., Pacini G., Yang Y. J., Bergman R. N. Importance of glucose per se to intravenous glucose tolerance. Comparison of the minimal-model prediction with direct measurements. Diabetes. 1985 Nov;34(11):1092–1103. doi: 10.2337/diab.34.11.1092. [DOI] [PubMed] [Google Scholar]
- Bell P. M., Firth R. G., Rizza R. A. Assessment of insulin action in insulin-dependent diabetes mellitus using [6(14)C]glucose, [3(3)H]glucose, and [2(3)H]glucose. Differences in the apparent pattern of insulin resistance depending on the isotope used. J Clin Invest. 1986 Dec;78(6):1479–1486. doi: 10.1172/JCI112739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P. M., Firth R. G., Rizza R. A. Effects of hyperglycemia on glucose production and utilization in humans. Measurement with [23H]-, [33H]-, and [614C]glucose. Diabetes. 1986 Jun;35(6):642–648. doi: 10.2337/diab.35.6.642. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Trent D. F., Weir G. C. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983 Jun;71(6):1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell A., Hume R., Burchell B. A new microtechnique for the analysis of the human hepatic microsomal glucose-6-phosphatase system. Clin Chim Acta. 1988 Apr 15;173(2):183–191. doi: 10.1016/0009-8981(88)90256-2. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Capani F., Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989 May;38(5):550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Reilly J. J., Jr, Bier D. M., Gerich J. E. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus. Role of alterations in systemic, hepatic, and muscle lactate and alanine metabolism. J Clin Invest. 1990 Dec;86(6):2038–2045. doi: 10.1172/JCI114940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A. L., Arion W. J. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: physiological implications of higher cellular activity. Arch Biochem Biophys. 1987 Feb 15;253(1):156–167. doi: 10.1016/0003-9861(87)90648-5. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Diamond M. P., Rollings R. C., Steiner K. E., Williams P. E., Lacy W. W., Cherrington A. D. Effect of alanine concentration independent of changes in insulin and glucagon on alanine and glucose homeostasis in the conscious dog. Metabolism. 1988 Jan;37(1):28–33. doi: 10.1016/0026-0495(88)90025-x. [DOI] [PubMed] [Google Scholar]
- Efendic S., Karlander S., Vranic M. Mild type II diabetes markedly increases glucose cycling in the postabsorptive state and during glucose infusion irrespective of obesity. J Clin Invest. 1988 Jun;81(6):1953–1961. doi: 10.1172/JCI113543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrace S., Rossetti L. Hyperglycemia markedly enhances skeletal muscle glycogen synthase activity in diabetic, but not in normal conscious rats. Diabetes. 1992 Nov;41(11):1453–1463. doi: 10.2337/diab.41.11.1453. [DOI] [PubMed] [Google Scholar]
- Giaccari A., Rossetti L. Isocratic high-performance liquid chromatographic determination of the concentration and specific radioactivity of phosphoenolpyruvate and uridine diphosphate glucose in tissue extracts. J Chromatogr. 1989 Dec 29;497:69–78. doi: 10.1016/0378-4347(89)80006-4. [DOI] [PubMed] [Google Scholar]
- Giaccari A., Rossetti L. Predominant role of gluconeogenesis in the hepatic glycogen repletion of diabetic rats. J Clin Invest. 1992 Jan;89(1):36–45. doi: 10.1172/JCI115583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinsmann W. H., Hern E. P., Lynch A. Intrinsic regulation of glucose output by rat liver. Am J Physiol. 1969 Apr;216(4):698–703. doi: 10.1152/ajplegacy.1969.216.4.698. [DOI] [PubMed] [Google Scholar]
- Granner D. K., Andreone T. L. Insulin modulation of gene expression. Diabetes Metab Rev. 1985;1(1-2):139–170. doi: 10.1002/dmr.5610010108. [DOI] [PubMed] [Google Scholar]
- Jahoor F., Peters E. J., Wolfe R. R. The relationship between gluconeogenic substrate supply and glucose production in humans. Am J Physiol. 1990 Feb;258(2 Pt 1):E288–E296. doi: 10.1152/ajpendo.1990.258.2.E288. [DOI] [PubMed] [Google Scholar]
- Jenssen T., Nurjhan N., Consoli A., Gerich J. E. Failure of substrate-induced gluconeogenesis to increase overall glucose appearance in normal humans. Demonstration of hepatic autoregulation without a change in plasma glucose concentration. J Clin Invest. 1990 Aug;86(2):489–497. doi: 10.1172/JCI114735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlander S., Roovete A., Vranić M., Efendić S. Glucose and fructose 6-phosphate cycle in humans. Am J Physiol. 1986 Nov;251(5 Pt 1):E530–E536. doi: 10.1152/ajpendo.1986.251.5.E530. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Rothman D. L., Katz L. D., Shulman R. G., Shulman G. I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992 Oct;90(4):1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H., Shulman G. I., Peters E. J., Wolfe M. H., Elahi D., Wolfe R. R. Hormonal control of substrate cycling in humans. J Clin Invest. 1988 May;81(5):1545–1555. doi: 10.1172/JCI113487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhakainen I., Koivisto V. A., Yki-Järvinen H. No reduction in total hepatic glucose output by inhibition of gluconeogenesis with ethanol in NIDDM patients. Diabetes. 1991 Oct;40(10):1319–1327. doi: 10.2337/diab.40.10.1319. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Farrace S., Choi S. B., Giaccari A., Sloan L., Frontoni S., Katz M. S. Multiple metabolic effects of CGRP in conscious rats: role of glycogen synthase and phosphorylase. Am J Physiol. 1993 Jan;264(1 Pt 1):E1–10. doi: 10.1152/ajpendo.1993.264.1.E1. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Giaccari A. Relative contribution of glycogen synthesis and glycolysis to insulin-mediated glucose uptake. A dose-response euglycemic clamp study in normal and diabetic rats. J Clin Invest. 1990 Jun;85(6):1785–1792. doi: 10.1172/JCI114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Lauglin M. R. Correction of chronic hyperglycemia with vanadate, but not with phlorizin, normalizes in vivo glycogen repletion and in vitro glycogen synthase activity in diabetic skeletal muscle. J Clin Invest. 1989 Sep;84(3):892–899. doi: 10.1172/JCI114250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G. I., Papachristou D., DeFronzo R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987 May;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacca L., Hendler R., Sherwin R. S. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab. 1978 Nov;47(5):1160–1163. doi: 10.1210/jcem-47-5-1160. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Lacy W. W., Liljenquist J. E., Keller U., Williams P. E., Cherrington A. D. Effect of glucose, independent of changes in insulin and glucagon secretion, on alanine metabolism in the conscious dog. J Clin Invest. 1980 Feb;65(2):496–505. doi: 10.1172/JCI109693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G. I., Liljenquist J. E., Williams P. E., Lacy W. W., Cherrington A. D. Glucose disposal during insulinopenia in somatostatin-treated dogs. The roles of glucose and glucagon. J Clin Invest. 1978 Aug;62(2):487–491. doi: 10.1172/JCI109150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Wajngot A., Khan A., Giacca A., Vranic M., Efendic S. Dexamethasone increases glucose cycling, but not glucose production, in healthy subjects. Am J Physiol. 1990 Nov;259(5 Pt 1):E626–E632. doi: 10.1152/ajpendo.1990.259.5.E626. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Jahoor F., Shaw J. H. Effect of alanine infusion on glucose and urea production in man. JPEN J Parenter Enteral Nutr. 1987 Mar-Apr;11(2):109–111. doi: 10.1177/0148607187011002109. [DOI] [PubMed] [Google Scholar]
- Zawadzki J. K., Wolfe R. R., Mott D. M., Lillioja S., Howard B. V., Bogardus C. Increased rate of Cori cycle in obese subjects with NIDDM and effect of weight reduction. Diabetes. 1988 Feb;37(2):154–159. doi: 10.2337/diab.37.2.154. [DOI] [PubMed] [Google Scholar]