Abstract
ATP-binding cassette (ABC) transporters are important, selective elements of the blood-brain barrier. They line the luminal plasma membrane of the brain capillary endothelium, facing the vascular space, both protecting the CNS from entry of neurotoxicants and limiting access of therapeutic drugs to the brain parenchyma. Recent studies highlight the multiple signaling pathways through which the expression and activity of P-glycoprotein and other ABC transporters are modulated in response to xenobiotics, stress and disease. They show that increased transporter expression occurs in response to signals that activate specific transcription factors including, PXR, CAR, NF-κB and AP-1, and reduced transporter activity occurs rapidly and reversibly in response to signaling through Src kinase, protein kinase C and estrogen receptors. A detailed understanding of such regulation can provide the basis for improved neuroprotection and enhanced therapeutic drug delivery to the brain.
Introduction
Delivery of drugs to the CNS is one of the “final frontiers” of pharmacotherapy. To a large extent this is due to the inability of so many candidate drugs to readily cross the blood-brain barrier (BBB) and reach sufficiently high concentrations at sites of action within the brain. This barrier resides within the 5-8 μm diameter microvessels that comprise the brain capillary endothelium (Box 1). One defining feature of the brain capillary phenotype is the expression of ATP-driven, drug efflux pumps (ATP-binding cassette (ABC) transporters) on the luminal, blood-facing plasma membrane of the endothelial cells (Fig. 1). These are members of the B, C and G families of ATP-binding cassette (ABC) transporters that collectively restrict the uptake of numerous lipophilic xenobiotics that, on the basis of structure, should readily diffuse across endothelial cell membranes. Of these BBB efflux transporters, we have the most complete picture of function and regulation for P-glycoprotein (ABCB1), which handles a surprisingly large number of therapeutic drugs (polyspecificity) and is expressed at high levels in the brain capillary endothelium [1, 2]. P-glycoprotein knockout mice have been available for over a decade, and for many drugs that are P-glycoprotein substrates, these animals show large increases in brain-to-plasma concentration ratios over wild-type controls. In addition, several animal studies show remarkably increased effectiveness of chemotherapeutics against implanted human tumors when P-glycoprotein inhibitors are co-administered [3-5]. P-glycoprotein has proved to be a primary obstacle to drug delivery to the brain.
Box 1. The blood-brain barrier (BBB).
Unlike peripheral capillaries, the brain capillary phenotype is distinguished by a lack of fenestrations, low pinocytotic activity and, critically, the presence of very high-resistance (low permeability) tight junctions between cells. These collectively present an effective physical barrier to the movement of macromolecules and smaller, more polar solutes both through the cells and between them. Brain capillary endothelial cells also express plasma membrane transport proteins and receptors, both of which provide selective routes of entry for polar nutrients (GLUT-1), ions (Na,K-ATPase and Na,K,Cl-cotransporter) and some macromolecules (insulin and transferrin receptors) and routes of exit for potentially toxic metabolic wastes and macromolecules (ABC transporters; Fig. 1). Through restrictive barrier properties and polarized expression of selective transport proteins, the BBB effectively regulates solute and fluid exchange between blood and brain parenchyma [50, 78]. The brain capillary endothelium is the central element of the neurovascular unit, which also includes astrocytes, pericytes and neurons. The components of the neurovascular unit working in concert influence the barrier’s properties, but the underlying mechanisms of communication have yet to be defined [78].
Experimental techniques used to study BBB transport function and its regulation run the full gamut from isolated brain endothelial cells (primary cells in culture and cell lines) to endothelial cell monolayers to isolated brain capillaries to intact animals (brain perfusion and brain efflux) and human subjects (positron emission spectroscopy and single photon emission computed tomography). Each approach has inherent strengths and weaknesses. In general, moving away from the in vivo situation increases the potential to bring powerful molecular tools to bear on underlying mechanisms of transport and their regulation. However, it raises concerns about altered expression of key proteins and loss of critical cell-cell interactions within the endothelium and the larger neurovascular unit, and thus physiological relevance. For all of these experimental systems, measuring ABC transporter activity is especially challenging. Because these transporters are unidirectional, drug efflux pumps, direct experimental measurements of substrate efflux rates are difficult to make in vitro using cells, monolayers and tissues and nearly impossible in vivo. In most cases, transport activity is defined by an absence of accumulation and this is often done at steady-state. Moreover, at least one of these transporters, P-glycoprotein, seems to extract substrates from the plasma membrane’s lipid bilayer and estimates of substrate affinities are dependent on substrate partitioning into the membrane and thus on the composition of that membrane.
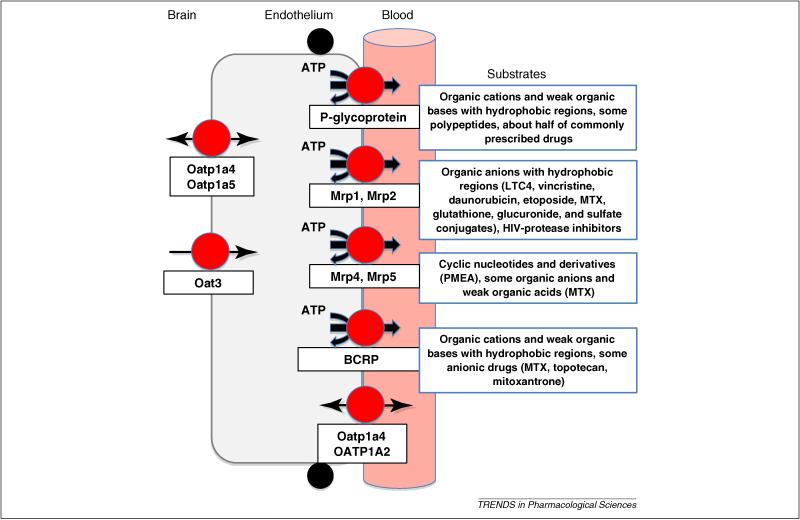
Figure 1.
Distribution of selected drug transporters at the BBB. ABC transporters are ATP-driven xenobiotic efflux pumps that can be highly polyspecific (P-glycoprotein is an extreme example), with overlapping substrate specificities that are briefly outlined for each in the boxes. There is considerable controversy over the localization of essentially all ABC transporters in brain capillaries [1, 6]. It is likely that species differences in protein expression levels and cellular transporter distribution as well as differences in detection techniques across laboratories underlie many of the conflicts that permeate the literature. Nevertheless, when expressed on the luminal plasma membrane, ABC transporters provide an active, structure-sensitive barrier to drugs within the vascular space. Other transporters shown are members of the SLC superfamily and handle primarily organic anions and weak organic acids. Substrates for these transporters include some drugs, for example NSAIDS, as well as drug metabolites and waste products of normal CNS metabolism. They, along with luminal ABC transporters, provide a two-stage system for active and efficient excretion of potentially toxic chemicals and metabolites from the CNS.
Other ATP-driven xenobiotic efflux pumps expressed at the luminal membrane, include multidrug resistance-associated proteins (Mrp1,2,4,5 [6]; ABC C family) and breast cancer-related protein (Bcrp, ABCG2 [7, 8]; Fig. 1). Experiments with Bcrp- or Mrp4-null mice and specific inhibitors of these transporters show increased brain accumulation of a restricted list of therapeutic drugs, such as imatinib (Bcrp [8]), topotecan (Bcrp [9] and Mrp4 [10]), whereas no such list is available for Mrp2 in spite of the long availability of two strains of Mrp2-null rats, and, more recently, of Mrp2-null mice. It is likely, however, that all of these transporters, along with basolateral uptake transporters, such as organic anion transporter 3 (Oat3), organic anion transporting polypeptides (Oatp1a4, Oatp1a5; Fig. 1), contribute to brain to blood transport of potentially toxic endogenous metabolic wastes, which are largely organic anions. This aspect of BBB function is certainly understudied.
There are four compelling reasons for wanting to understand regulation of these transporters. First, we lack a basic understanding of how environmental factors, including diet, therapy, and toxicant exposure, alter barrier properties and affect CNS pharmacotherapy. In certain situations, e.g., chemotherapy to the periphery, it may be advantageous to upregulate ABC transporter expression to increase CNS protection; we should devise strategies to do this in a safe manner. Second, ABC transporter expression in other barrier and excretory tissues seems to be affected by inflammatory and oxidative stress. Because inflammation and oxidative stress are factors in nearly all CNS disorders, it is important to understand how they affect ABC transporter expression at the BBB. Third, it is critical to know how transporter function is altered in specific CNS diseases, since alterations in BBB transporters will affect the efficacy of CNS-acting drugs. Fourth, so far the use of ABC transporter-specific inhibitors to improve drug delivery has not translated well to the clinic. Identifying and targeting signals that have the potential to rapidly modulate transporter activity could provide an alternative strategy.
Here I review recent findings on signals that regulate BBB ABC transporters in the context of the four reasons discussed above and attempt to integrate findings from in vitro and in vivo studies (Box 1). The material that follows is in three major sections: one dealing with signals that increase transporter protein expression, a second dealing with signals that reduce transporter activity without altering expression, and a third discussing the complexities of BBB signaling and transport.
Altered expression of ABC transporters
Xenobiotic Exposure
In peripheral barrier and excretory tissues, several ligand-activated intracellular receptors signal increase the expression of both xenobiotic metabolizing enzymes and excretory transporters. Prominent among these receptors are the former “orphan” receptors, pregnane-X receptor (PXR) and constitutive androstane receptor (CAR), which are considered to be a major part of our first line of defense against both endogenous toxicants and xenobiotics [11, 12]. Both receptors are activated by endogenous ligands, such as bile acids, and by numerous therapeutic drugs, many of which are handled by the enzymes and transporters that the receptors themselves regulate. Induction of metabolism and excretory transport provides one mechanistic basis for drug-metabolite and drug-drug interactions. For PXR and CAR, there is some overlap in activating ligands, target sequences in gene promoter regions and target genes; activation of one receptor can alter the expression of the other and, once in the nucleus, both partner with the retinoid-X receptor before binding to DNA. Extensive studies with hepatocytes and model systems suggest that target genes and thus the regulatory network can be extended to other ligand-activated receptors, for example farnesyl-X receptor, glucocorticoid receptor (GR) and possibly liver-X receptor; this also appears to be the case for the BBB (Box 2 and [13]).
Box 2. Signals that increase expression of ABC transporters at the BBB.
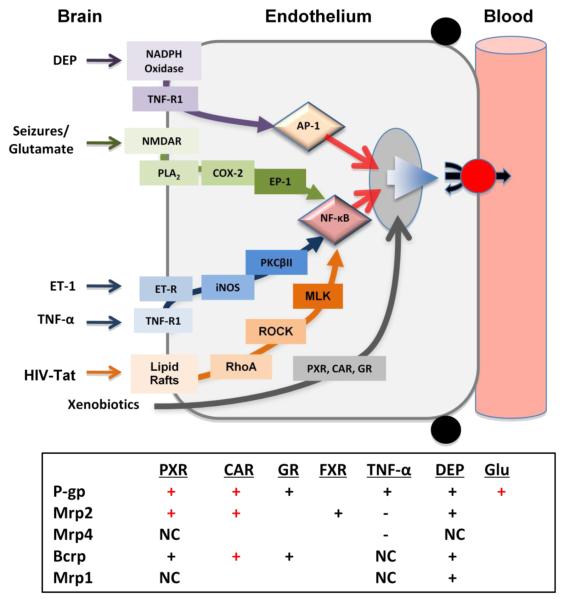
Xenobiotics, stress and disease act through specific signaling pathways to alter expression of multiple ABC transporter proteins in brain capillaries. We have the most complete picture of transcriptional regulation for P-glycoprotein. The figure in this Box maps known signals and key transcription factors that increase expression of this transporter. Xenobiotics, including therapeutic drugs and environmental toxicants, bind directly to ligand-activated transcription factors, CAR (Wang and Miller, unpublished data), PXR [14] and glucocorticoid receptor (GR) [19]), which then translocate to the nucleus to increase gene expression. The transcription factor NF-κB mediates the effects of three signaling pathways: 1) proinflammatory signaling initiated by tumor necrosis factor α (TNF-α) and endothelin-1 (ET-1) [24], 2) seizure-induced signaling through cyclo-oxygenase-2 (COX-2) [55], and 3) HIV-Tat protein signaling through RhoA, Rac and myosin light chain kinase (MLK) [49]. Diesel exhaust particles (DEPs) signal though a pathway that is both oxidative and proinflammatory to activate AP-1 [35]. Increased P-glycoprotein expression should selectively tighten the BBB, increasing CNS protection but reducing uptake of many CNS-active therapeutic drugs.
These signaling pathways also affect protein expression of other ABC transporters at the BBB. The table summarizes available Western blot data for p-glycoprotein (P-gp), Mrp1, Mrp2, Mrp4 and Bcrp, Plus (+) indicates increased expression, minus (−) indicates reduced expression and NC indicates no change in expression; symbols in red indicate in vivo confirmation of the in vitro findings. Note that PXR, CAR and DEP upregulate expression of multiple transporters found on the luminal membrane of the brain capillary endothelial cells ([13, 14, 18, 35] and Wang and Miller, unpublished data). In addition to these ABC transporters, PXR and CAR also increase expression of xenobiotic metabolizing enzymes in brain capillary endothelial cells. This coordinate upregulation enzymes and efflux transporters can be viewed as a primary defense mechanism, designed to protect the CNS from foreign chemicals.
Recent studies show that both PXR and CAR are expressed in brain capillaries or brain capillary endothelial cells from mouse, rat, pig and human [14-17]. Exposing rat and mouse brain capillaries to pregnenolone 16α-carbonitrile (PCN; PXR ligand) or phenobarbital or 1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP; both CAR ligands) increases transport activity and protein expression of P-glycoprotein, Mrp2 and Bcrp in addition to the phase II metabolizing enzyme glutathione transferase π ([13, 14, 18] and Wang and Miller unpublished data). Similarly, dexamethasone acting through GR increases expression and transport activity of P-glycoprotein and Bcrp [19]. Consistent with the in vitro findings for PXR and CAR, dosing rodents with PCN or TCPOBOP increases transport activity and protein expression of P-glycoprotein, Mrp2 and Bcrp in brain capillaries ([13, 14, 18] and Wang and Miller, unpublished data). Thus, two master regulators of xenobiotic defense, PXR and CAR, act in brain capillaries to increase expression of multiple ABC transporters (Box 2).
The pharmacodynamic consequences of P-glycoprotein induction at the BBB were first demonstrated in experiments with transgenic mice expressing human PXR (hPXR) [18]. The DNA-binding domain of PXR is conserved across species, but the ligand-binding domain is highly variable. This can be seen when comparing ligand affinities for rodent vs. human PXR: PCN is a ligand for rodent PXR but not hPXR; and rifampin, an antibiotic, and hyperforin, a component of the herbal remedy St. John’s wort, are high-affinity ligands for hPXR (and porcine PXR) but not rodent PXR. When transgenic mice expressing hPXR were given rifampin at a dose that resulted in free plasma drug levels equivalent to those seen in humans undergoing a course of rifampin treatment, P-glycoprotein and Mrp2 protein expression in liver, intestine and brain capillaries increased 2-3-fold. In these rifampin-dosed, hPXR mice, the antinociceptive effects of injected methadone was reduced by 70% [18]. These findings with a transgenic animal showed for the first time that decreases in the efficacy of CNS-acting drugs that are P-glycoprotein substrates is one consequence of transporter induction at the BBB.
There is no reason to expect that similar pharmacodynamic effects would not be seen when transporter expression is induced though other signals. Given that our diets contain ligands for PXR and CAR, that other signals increase P-glycoprotein expression and that polypharmacy exposes humans to additional ligands, it is likely that transporter expression in barrier and excretory tissues is already induced for a substantial portion of the population. This may contribute to patient-to-patient variability in response to CNS therapeutics.
Stress
Inflammation and the generation of reactive oxygen (and nitrogen) species are cofactors in nearly every CNS disease and there is substantial evidence that severe inflammatory and oxidative stress can acutely disrupt the BBB at the level of the tight junctions. Such disruption probably occurs in ischemia/reperfusion and in several neurodegenerative diseases [20, 21]. Recent studies with brain capillary endothelial cells and intact capillaries have mapped in detail several stress-related signaling pathways that target nuclear transcription factors to increase P-glycoprotein expression in brain capillaries and brain endothelial cells (Box 2). These pathways involve elements of the barrier’s response to inflammation and oxidative stress. Not surprisingly, some of the pathways converge on the transcription factor, NF-κB, which has been implicated in responses leading to both cell protection and cell death. In some instances, expression of other ABC transporters is altered (Box 2).
Inflammation, induced primarily but not exclusively by systemic administration of bacterial endotoxin (lipopolysaccharide, LPS), decreases the expression of multiple ABC transporters (P-glycoprotein, Mrp2, Mrp3 and bile salt export pump) in rodent liver and intestine [22]; proinflammatory cytokines produce cytokine- and transporter-specific effects in mouse liver. Recently, Roberts and Goralski [23] critically reviewed the effects of inflammation or infection on P-glycoprotein activity and expression in brain. In vivo effects of CNS and peripheral inflammation seem to be at least as complex as in liver, with the nature of the response being dependent on the inflammatory signal (endotoxin, bacterial infection, peripheral inflammatory hyperalgesia) and the time after exposure when measurements of P-glycoprotein expression and CNS drug accumulation were made. However, these in vivo experiments provide little information on the signals through which transporter expression is altered.
In vitro studies show a complex time course of response to proinflammatory signals (lipopolysaccharide (LPS), tumor necrosis factor-α (TNF-α), endothelin-1 (ET-1)) with initial loss of P-glycoprotein activity (no change in protein expression) followed by delayed increases in activity and expression above control levels [23]. The signaling pathways responsible for these effects have been mapped in isolated rat brain capillaries [24-26]. The mechanistic basis for the initial, rapid response is discussed later in the context of signals that alter transporter activity. Delayed induction of P-glycoprotein expression is signaled through a single pathway with entry points for TNF-α and ET-1 (Box 2). Critical to TNF-α signaling is the release of ET-1 from the endothelium and its paracrine or autocrine action through surface ET receptors. Downstream intracellular events include activation of inducible nitric oxide synthase (iNOS) and protein kinase C βII (PKCβII) as well as translocation of the transcription factor, NF-κB, into the nucleus. At the same time that TNF-α increases expression of P-glycoprotein, in brain capillaries it reduces expression of Mrp2 and Mrp4, increases expression of Glut-1 and Na+/K+-ATPase, but does not alter expression of Mrp1, Bcrp or tight junction proteins (Box 2) [24]. It is not clear whether the same chain of events drives changes in expression of all ABC transporters.
Indeed, two reports suggests that signaling in vivo may be substantially more complex., Salkeni et al [27] found that P-glycoprotein activity decreased in mice given a single dose of lipopolysaccharide while P-glycoprotein expression in whole brain lysates increased. These effects appeared to be independent of NOS, suggesting signaling through a distinct pathway. Separately, Seelbach et al [28] showed that peripheral inflammatory hyperalgesia increased P-glycoprotein activity and expression in the BBB of rats. This finding that peripheral inflammation can alter BBB efflux transporter expression is potentially very important, but at present the mechanism by which this occurs is unknown.
In peripheral tissues, certain ABC transporters act in concert with GSH to protect against oxidative damage. GSH depletion is known to upregulate expression of Mrp1, which mediates efflux of GSH conjugates [29]. Similar findings have been reported for brain capillary endothelial cells. Exposure of primary rat brain capillary endothelial cells to H2O2 or direct depletion of GSH increases P-glycoprotein expression through a complex signaling pathway involving intracellular reactive oxygen species (ROS [30, 31]); the signals involved are not well-characterized, but recent studies suggest involvement of a wnt/β-catenin pathway [32, 33]. Consistent with these in vitro findings, Wu et al have found upregulated P-glycoprotein expression at the BBB in an animal model of global GSH depletion [34].
Recent experiments with rat and mouse brain capillaries exposed to diesel exhaust particles (DEPs) implicate both oxidative (NADPH oxidase activation and superoxide production) and inflammatory (release of the pro-inflammatory cytokine, TNF-α, from the endothelium) signals in upregulation of expression of P-glycoprotein, Mrp1, Mrp2 and Bcrp, but not Mrp4 (Box 2 and [35]). This novel signaling pathway contained within the endothelial cells involves activation of the transcription factor AP-1. Diesel exhaust is the main particulate component of polluted air in the urban environment, and thus a public health concern. Autopsy samples from individuals living in air-polluted environments show deposition of DEPs within the brain, indicating that DEPs cross the BBB [36, 37]. DEPs induce oxidative and inflammatory responses in microglia, the primary immune responsive cells of the brain [38]. Thus, DEP-stimulated brain capillaries could serve as an additional source of both oxidative and inflammatory stress for the brain parenchyma, contributing to CNS pathology and disease. Altered BBB function in turn could contribute to DEP-exacerbated cerebrovascular disease and its treatment.
Because inflammatory and oxidative stress accompanies so many CNS diseases, stress-induced signaling pathways that affect BBB ABC transporter expression may be important modifiers of disease progression and therapy. Connecting specific pathways to diseases, where so many processes are changing, remains a challenge.
Disease
Altered expression of ABC transporters at the BBB accompanies several neuropathologies. Reduced expression or transport function for BBB P-glycoprotein is associated with Alzheimer’s disease [39], Jacob-Creutzfeld disease [40], Parkinson’s disease [41], HIV infection [42] and normal aging [43]; increased expression of P-glycoprotein, Mrp1, Mrp2 and Bcrp is associated with epileptic seizures [44]. Certainly, these findings have immediate and obvious implications for delivery of therapeutic drugs to the CNS. For HIV infection, Alzheimer’s disease and epilepsy, recent reports are beginning to disclose mechanisms underlying changes in ABC transporter expression.
In post-mortem samples from HIV infected individuals, P-glycoprotein expression in brain capillaries is reduced, but expression in astrocytes and microglia is increased [42]. These results are particularly interesting, since HIV protease inhibitors and nucleoside and nucleotide reverse transcriptase inhibitors are inducers of P-glycoprotein [45, 46] and many of the samples came from patients that had been treated using highly active antiretroviral therapy. Effects on glial cells appear to be consistent with such induction. However, it seems that P-glycoprotein expression at the BBB was reduced by the disease even though patients were receiving drugs that should have increased transporter expression in that tissue.
Subsequent studies with HIV-TAT protein suggest that the mechanisms underlying changes in endothelial cell transporter expression may be complex [47, 48]. In contrast to the patient data, exposure of rodent brain capillary endothelial cells to HIV-TAT protein in vitro increases P-glycoprotein expression and transport function; in vivo dosing studies with mice confirmed these results [47, 48]. In endothelial cells, prolonged exposure to HIV-TAT signals increases transporter expression through a complex pathway involving lipid rafts, RhoA/Rac and probably NF-κB ([49] Box 2).
For Alzheimer’s disease, recent studies suggest that ABC transporters may play more than a spectator role. Zlokovic has proposed that a cascade of neurovascular events alters BBB function and fuels disease progression in Alzheimer’s disease [50]. One element of this hypothesis is that reduced β-amyloid efflux from the brain increases brain accumulation of that pathological protein in Alzheimer’s disease [50]. P-glycoprotein and Bcrp have been implicated as possible efflux pumps for β-amyloid itself [51-53] and available data indicate that Alzheimer’s patients exhibit reduced expression of P-glycoprotein and increased expression of Bcrp in brain capillaries [39, 53]. Thus, upregulating expression of the former, perhaps through diet, may slow β-amyloid deposition and possibly disease progression. Hartz et al [54] have tested this hypothesis in an animal model of Alzheimer’s disease based on young, transgenic mice expressing human amyloid precursor protein. They found that brain capillaries from these mice show substantially reduced P-glycoprotein expression and that dosing mice with a PXR ligand upregulates P-glycoprotein expression and reduces brain β-amyloid deposition. At present, the relationship between β-amyloid clearance, disease progression and elevated Bcrp expression is less clear, and the mechanisms underlying changes in brain P-glycoprotein and Bcrp expression in this disease are unknown.
Limited drug delivery to the brain is a common cause of therapeutic failure in epilepsy, a disease that affects 50 million people worldwide. One suggested basis for pharmacoresistance is the overexpression of ATP-driven drug efflux pumps at the BBB including, P-glycoprotein, Mrp1, Mrp2 and Bcrp [44]. Evidence connecting transporter overexpression with pharmacoresistance to antiepileptic drugs (AEDs) is strongest for P-glycoprotein, and recent studies with animal models suggest both a mechanistic basis for such resistance and a therapeutic strategy for overcoming it. Bauer et al [55] used brain capillaries isolated from rat and mouse to demonstrate that the neurotransmitter glutamate signals through an NMDA receptor, cyclooxygenase-2 (COX-2), prostaglandin E2 and NF-κB to increase expression of P-glycoprotein (Box 2). They further showed in rats that microinjection of glutamate into the hippocampus locally increased brain capillary P-glycoprotein expression and that indomethacin, a non-selective COX inhibitor [55], abolished seizure-induced increases in capillary P-glycoprotein expression following pilocarpine-induced status epilepticus. Subsequent in vivo studies with rodent seizure models [56] have shown similar effects with a specific COX-2 inhibitor, an NMDA receptor antagonist and a prostaglandin E2 receptor (EP-1) antagonist, thus validating the major elements of the signaling system (Box 2).
Despite these encouraging findings from animal studies, the role of P-glycoprotein in AED resistance remains controversial. At issue are which commonly prescribed AEDs are substrates for human P-glycoprotein [57, 58], the pharmacokinetics of some AEDs [59], and the question of whether polymorphisms in the P-glycoprotein gene affect AED uptake and seizure frequency [60-62]. Indeed, it still remains to be demonstrated that signaling can be targeted in patients to reduce seizure-induced pharmacoresistance.
Thus, altered ABC transporter protein expression is associated with a number of CNS diseases, where changes in transport activity appear to parallel changes in expression. In vivo these changes probably take place over a time scale of hours to days. Recent evidence indicates that transporter activity in brain capillaries can also change over a time scale of minutes. The following section deals with the signals that cause such changes.
Altered Transport Activity
Despite the success of specific transport inhibitors in improving drug delivery to the brain in animal studies, the results have not been translated into the clinic. One problem is that we possess incomplete knowledge about transport function in the human BBB in situ. Indeed, it has been suggested that P-glycoprotein is present in excess in brain capillaries. As a result, more complete inhibition of transport activity would be needed in vivo than one would predict based in vitro dose response curves. Consistent with this view, limited experiments using positron emission spectroscopy in primates and human subjects showed more modest increases in brain levels than expected when the labeled drugs are given with P-glycoprotein inhibitors [63]. In addition, preliminary results from clinical trials suggest that systemic toxicity of specific P-glycoprotein inhibitors limits the ability to achieve free plasma concentrations sufficient to abolish transporter activity. Thus, for humans, the full impact of P-glycoprotein inhibition on drug accumulation in the brain is not known for any drug, making an understanding of the mechanisms that regulate basal transporter activity even more critical.
Rapid (minutes) and reversible changes in transport mediated by certain ABC transporters occurs in hepatocytes and renal proximal tubules [64, 65]. In rat brain capillaries, similar modulation of transport activity occurs in response to three separate signaling pathways. In the first pathway, exposing capillaries to low levels of LPS, TNF-α or endothelin-1 (ET-1) causes a rapid and fully reversible loss of P-glycoprotein transport function with no change in protein expression; inhibitors of protein synthesis are without effect and neither Mrp2-mediated transport nor tight junctional permeability is altered [14, 26]. As shown in Fig. 2, signaling involves ligand binding to toll-like receptor-4 (TLR4), tumor necrosis factor −α receptor 1 (TNFR1) or ETBR, followed by activation of NOS and PKC. All of these steps occur on or in capillary endothelial cells.
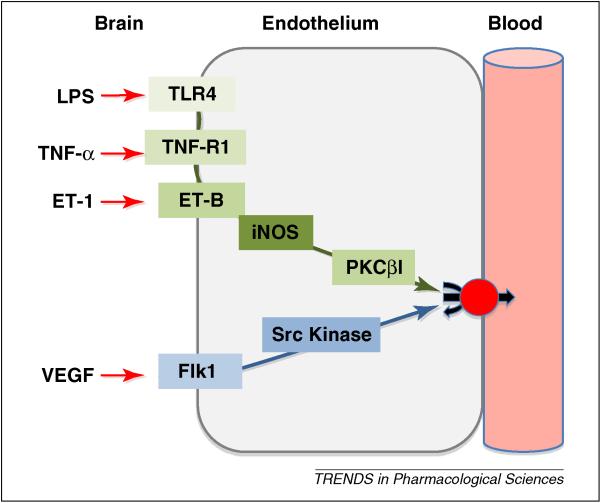
Figure 2.
Signals that rapidly decrease P-glycoprotein transport activity at the BBB. Two signaling pathways (proinflammatory and vascular endothelial growth factor (VEGF) – induced) were initially described in rat brain capillaries through the use of pharmacological tools. They have been validated in vivo, in that activating each of the pathways rapidly and substantially increases brain uptake of verapamil and morphine, both P-glycoprotein substrates [25, 26, 66, 68]. Because signaling through these pathways has the potential to rapidly and transiently reduce P-glycoprotein activity without altering expression, targeting downstream steps provide a strategy for improving delivery to the brain of drugs that are P-glycoprotein substrates. Abbreviations: lipopolysaccharide (LPS), tumor necrosis factor (TNF), endothelin (ET), toll-like receptor (TLR), inducible nitric oxide synthase (iNOS), protein kinase C (PKC)
In the first publication describing these findings, we speculated that this rapid and reversible loss of specific transport activity in the capillary endothelium could provide the time window needed to deliver P-glycoprotein-excluded drug to the CNS [25]. To that end, we have used pharmacological tools to identify the specific signals in the pathway downstream of ETBR. Recent experiments show that iNOS activation is the penultimate identified step in the pathway (Rigor and Miller, unpublished data) and that activation of PKCβI is the final identified step [66]. The latter result was validated in vivo using brain perfusion. Treatment of rats with 12-deoxyphorbol 13-phenylacetate 20-acetate (dPPA), a specific PKCβI agonist, rapidly and specifically increased cyclosporine A-sensitive brain uptake of 14C-verapamil, indicating loss of P-glycoprotein activity. dPPA did not affect uptake of 14C-Sucrose, a sensitive measure of changes in tight junction permeability [66]. Thus, targeted signaling through PKCβI has the potential to enhance delivery of therapeutic drugs to the brain. Although there would certainly be problems using phorbol ester derivatives like dPPA in the clinic, this PKCβI activator provided excellent proof-of-principle. One could envision an engineered peptide or small molecule PKCβI activator to enhance delivery of pharmacotherapeutics to treat brain tumors and other CNS diseases.
The second distinct pathway that signals rapid, reversible loss of P-glycoprotein activity in brain capillaries is initiated by vascular endothelial growth factor (VEGF; Fig. 2). Increased brain expression of this growth factor is associated with neurological disease, brain injury and BBB dysfunction [67]. Exposing isolated rat brain capillaries to VEGF acutely and reversibly decreases P-glycoprotein transport activity without decreasing transport protein expression or opening tight junctions [68]. This effect is blocked by inhibitors of the VEGF receptor, Flk-1 and Src kinase, but not by inhibitors of phosphatidylinostitol-3 (PI3)-kinase or PKC. VEGF also increases Tyr-14 phosphorylation of caveolin-1 and this phosphorylation is blocked by a Src kinase inhibitor (PP2). Previous studies using brain capillary endothelial cells suggested a role for caveolin-1 in regulation of P-glycoprotein activity [69, 70]. In vivo, intracerebroventricular injection of VEGF increases brain distribution of the P-glycoprotein substrates, morphine and verapamil, but not the tight junction marker, sucrose; this effect is blocked by systemic PP2 [68]. These findings imply that P-glycoprotein activity is acutely diminished in pathological conditions associated with increased brain VEGF expression. They also imply that once the more downstream elements of VEGF signaling to P-glycoprotein are identified, they could provide additional accessible targets that could be used to modulate P-glycoprotein activity acutely and thus improve brain drug delivery.
The third pathway involves estrogen regulation of Bcrp activity. Estrogen regulates BCRP expression in multiple tissues, where transporter expression is higher in males than in females [71, 72]. In brain capillaries from rats and mice, there are no such differences, presumably due to estrogen production by brain aromatase [73]. Brain capillaries from male and female rats and mice respond identically to nanomolar concentrations of 17-β-estradiol (E2) by rapidly and reversibly reducing transport mediated by Bcrp [73]. Such effects are independent of transcription and translation. Experiments with estrogen receptor (ER) agonists and antagonists and with brain capillaries from ER knockout mice indicate non-genomic E2 signaling through both ERα and ERβ. Initial experiments implicate PI3-kinase and AKT as downstream elements of ER signaling and as potential therapeutic targets [73]. On the one hand, these findings suggest a simple strategy to increase brain penetration of chemotherapeutics that are Bcrp substrates, e.g., topotecan and imatinib [7, 8]. On the other hand, they identify one potential mechanism by which potent environmental estrogens could alter BBB function, an area in need of further study.
Rapid and reversible loss of P-glycoprotein and Bcrp transport activity indicates modulation of transporter function over a time scale of minutes. Although the mechanistic basis for this is unclear, one could envision two general classes of underlying mechanism: first, trafficking of the transporter away from the cell surface and second, modification of the transporter or its immediate microenvironment in the plasma membrane. In hepatocytes, there is direct evidence for rapid trafficking of P-glycoprotein and other ABC transporters between intracellular sites and the canalicular membrane [64]. Both caveoli and lipid rafts have been implicated in regulation of P-glycoprotein in endothelial cells [49, 69, 70] and BCRP in tumor cells [74]. Our unpublished experiments with isolated rat brain capillaries suggest an essential cytoskeletal contribution to both TNF-α and VEGF signaling, it is not yet certain whether the cytoskeleton-dependent step is proximal to the loss of activity or whether it indicates translocation.
Conclusions
It is now clear from studies with animal models and with patient samples that expression/activity of P-glycoprotein and other ABC transporters at the BBB can be moving targets, affected by genetics, disease, pharmacotherapy and diet. Indeed, we are rapidly adding to maps of the signals and signaling pathways involved with a view towards improving both CNS protection and the delivery of small-molecule drugs to the brain. Thus, an understanding of signaling could provide opportunities to both selectively fine tune barrier function up or down and begin to identify the barrier-based and external factors that contribute to patient-to-patient variability in response to CNS-acting drugs. Although we are rapidly developing a very detailed picture of signaling to ABC transporters in animal models (Fig. 2, Box 2 and Box 3), it is still not clear to what extent these pathways operate in humans. An understanding transporter function and regulation at the human BBB is critical before we can determine to what extent signaling can be manipulated to improve drug delivery to the CNS and to enhance neuroprotection.
Box 3. Complexities.
Given that transporter function and its regulation are inherently non-linear, complex processes, we should not be surprised by results that are counter-intuitive. Before closing, I will briefly discuss two recent findings that should color how we think about ABC transporters and drug delivery to the brain. First, Box 2 shows that signaling through TNF-R1 is a common step in the P-glycoprotein response to DEP and to the pro-inflammatory cytokine, TNF-α. When signaling is initiated by TNF-α in the absence of ROS, downstream events include ET-1 release and binding to the ETA/B receptors, activation of NOS and PKC and NF-κB-driven increases in P-glycoprotein expression [24]. However, when signaling is initiated by DEP, NADPH oxidase is activated to produce ROS, stimulate TNF-α release, and activate TNF-R1, the increase in P-glycoprotein expression is signaled through the transcription factor, AP-1. This appears to be an example of context-dependent switching of signaling events. In the context of oxidative stress, TNF-R1 activates one transcription factor (AP-1) and in an inflammatory context it activates another (NF-κB). Context-dependent, switch-like behavior has been demonstrated for other receptors. Pathway switching is one consequence of signaling network complexity and we anticipate seeing additional examples as signaling maps for the transporters are developed further.
A second form of complexity was recently discovered when brain accumulation of certain drugs was measured in mice that are null for P-glycoprotein (Mdr1a and Mdr1b) and Bcrp (triple knockout mice). Consider the tyrosine kinase inhibitors, imatinib and dasatinib, which are modest substrates for both transporters. Comparisons of brain to plasma concentration ratios show increases in the triple knockout (relative to wild-type controls) are substantially greater than for the P-glycoprotein and BCRP knockouts individually and for what one might expect if the effects of knocking out these transporters were simply additive [75, 76]. Enokizono et al [77] recently proposed a kinetic basis for this phenomenon. Considering the magnitude of this effect, a full understanding of the underlying mechanism could be the basis for new strategies aimed at improving delivery of certain small drugs to the brain.
Acknowledgements
Supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. I thank current and past members of the Miller Laboratory for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Miller DS, et al. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizuno N, et al. Impact of drug transporter studies on drug discovery and development. Pharmacol Rev. 2003;55:425–461. doi: 10.1124/pr.55.3.1. [DOI] [PubMed] [Google Scholar]
- 3.Fellner S, et al. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest. 2002;110:1309–1318. doi: 10.1172/JCI15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kemper EM, et al. Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin Cancer Res. 2003;9:2849–2855. [PubMed] [Google Scholar]
- 5.Kemper EM, et al. Improved penetration of docetaxel into the brain by co-administration of inhibitors of P-glycoprotein. Eur J Cancer. 2004;40:1269–1274. doi: 10.1016/j.ejca.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Dallas S, et al. Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev. 2006;58:140–161. doi: 10.1124/pr.58.2.3. [DOI] [PubMed] [Google Scholar]
- 7.Xia CQ, et al. Breast cancer resistance protein in pharmacokinetics and drug-drug interactions. Expert Opin Drug Metab Toxicol. 2005;1:595–611. doi: 10.1517/17425255.1.4.595. [DOI] [PubMed] [Google Scholar]
- 8.Breedveld P, et al. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27:17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 9.de Vries NA, et al. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13:6440–6449. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]
- 10.Leggas M, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24:7612–7621. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohle C, Bock KW. Coordinate regulation of human drug-metabolizing enzymes, and conjugate transporters by the Ah receptor, pregnane X receptor and constitutive androstane receptor. Biochem Pharmacol. 2009;77:689–699. doi: 10.1016/j.bcp.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Pascussi JM, et al. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: crosstalk and consequences. Annu Rev Pharmacol Toxicol. 2008;48:1–32. doi: 10.1146/annurev.pharmtox.47.120505.105349. [DOI] [PubMed] [Google Scholar]
- 13.Bauer B, et al. Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metab. 2008;28:1222–1234. doi: 10.1038/jcbfm.2008.16. [DOI] [PubMed] [Google Scholar]
- 14.Bauer B, et al. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66:413–419. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 15.Dauchy S, et al. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J Neurochem. 2008;107:1518–1528. doi: 10.1111/j.1471-4159.2008.05720.x. [DOI] [PubMed] [Google Scholar]
- 16.Nannelli A, et al. Expression and distribution of CYP3A genes, CYP2B22, and MDR1, MRP1, MRP2, LRP efflux transporters in brain of control and rifampicin-treated pigs. Mol Cell Biochem. 2009 doi: 10.1007/s11010-009-0292-1. [DOI] [PubMed] [Google Scholar]
- 17.Ott M, et al. Pregnane X receptor (PXR) regulates P-glycoprotein at the blood-brain barrier: functional similarities between pig and human PXR. J Pharmacol Exp Ther. 2009;329:141–149. doi: 10.1124/jpet.108.149690. [DOI] [PubMed] [Google Scholar]
- 18.Bauer B, et al. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol. 2006;70:1212–1219. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- 19.Narang VS, et al. Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood-brain barrier. Am J Physiol Cell Physiol. 2008;295:C440–450. doi: 10.1152/ajpcell.00491.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 21.Pun PB, et al. Involvement of ROS in BBB dysfunction. Free Radic Res. 2009;43:348–364. doi: 10.1080/10715760902751902. [DOI] [PubMed] [Google Scholar]
- 22.Teng S, Piquette-Miller M. Regulation of transporters by nuclear hormone receptors: implications during inflammation. Mol Pharm. 2008;5:67–76. doi: 10.1021/mp700102q. [DOI] [PubMed] [Google Scholar]
- 23.Roberts DJ, Goralski KB. A critical overview of the influence of inflammation and infection on P-glycoprotein expression and activity in the brain. Expert Opin Drug Metab Toxicol. 2008;4:1245–1264. doi: 10.1517/17425255.4.10.1245. [DOI] [PubMed] [Google Scholar]
- 24.Bauer B, et al. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71:667–675. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- 25.Hartz AM, et al. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol. 2004;66:387–394. doi: 10.1124/mol.104.001503. [DOI] [PubMed] [Google Scholar]
- 26.Hartz AM, et al. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol. 2006;69:462–470. doi: 10.1124/mol.105.017954. [DOI] [PubMed] [Google Scholar]
- 27.Salkeni MA, et al. Lipopolysaccharide impairs blood-brain barrier P-glycoprotein function in mice through prostaglandin- and nitric oxide-independent pathways. J Neuroimmune Pharmacol. 2009;4:276–282. doi: 10.1007/s11481-008-9138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seelbach MJ, et al. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem. 2007;102:1677–1690. doi: 10.1111/j.1471-4159.2007.04644.x. [DOI] [PubMed] [Google Scholar]
- 29.Leslie EM, et al. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology. 2001;167:3–23. doi: 10.1016/s0300-483x(01)00454-1. [DOI] [PubMed] [Google Scholar]
- 30.Felix RA, Barrand MA. P-glycoprotein expression in rat brain endothelial cells: evidence for regulation by transient oxidative stress. J Neurochem. 2002;80:64–72. doi: 10.1046/j.0022-3042.2001.00660.x. [DOI] [PubMed] [Google Scholar]
- 31.Nwaozuzu OM, et al. Signalling pathways influencing basal and H(2)O(2)-induced P-glycoprotein expression in endothelial cells derived from the blood-brain barrier. J Neurochem. 2003;87:1043–1051. doi: 10.1046/j.1471-4159.2003.02061.x. [DOI] [PubMed] [Google Scholar]
- 32.Lim JC, et al. Activation of beta-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J Neurochem. 2008;106:1855–1865. doi: 10.1111/j.1471-4159.2008.05537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim JC, et al. Decreased expression of multidrug efflux transporters in the brains of GSK-3beta transgenic mice. Brain Res. 2009;1276:1–10. doi: 10.1016/j.brainres.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, et al. Glutathione depletion upregulates P-glycoprotein expression at the blood-brain barrier in rats. J Pharm Pharmacol. 2009;61:819–824. doi: 10.1211/jpp/61.06.0016. [DOI] [PubMed] [Google Scholar]
- 35.Hartz AM, et al. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. Faseb J. 2008;22:2723–2733. doi: 10.1096/fj.08-106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calderon-Garciduenas L, et al. Pediatric respiratory and systemic effects of chronic air pollution exposure: nose, lung, heart, and brain pathology. Toxicol Pathol. 2007;35:154–162. doi: 10.1080/01926230601059985. [DOI] [PubMed] [Google Scholar]
- 37.Calderon-Garciduenas L, et al. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn. 2008;68:117–127. doi: 10.1016/j.bandc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Block ML, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. Faseb J. 2004;18:1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- 39.Vogelgesang S, et al. Deposition of Alzheimer‘s beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics. 2002;12:535–541. doi: 10.1097/00008571-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Vogelgesang S, et al. Cerebrovascular P-glycoprotein expression is decreased in Creutzfeldt-Jakob disease. Acta Neuropathol (Berl) 2006;111:436–443. doi: 10.1007/s00401-006-0042-3. [DOI] [PubMed] [Google Scholar]
- 41.Vautier S, Fernandez C. ABCB1: the role in Parkinson‘s disease and pharmacokinetics of antiparkinsonian drugs. Expert Opin Drug Metab Toxicol. 2009;5:1349–1358. doi: 10.1517/17425250903193079. [DOI] [PubMed] [Google Scholar]
- 42.Langford D, et al. Altered P-glycoprotein expression in AIDS patients with HIV encephalitis. J Neuropathol Exp Neurol. 2004;63:1038–1047. doi: 10.1093/jnen/63.10.1038. [DOI] [PubMed] [Google Scholar]
- 43.Bauer M, et al. Age dependency of cerebral P-gp function measured with (R)-[11C]verapamil and PET. Eur J Clin Pharmacol. 2009;65:941–946. doi: 10.1007/s00228-009-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 45.Perloff MD, et al. Induction of P-glycoprotein expression and activity by ritonavir in bovine brain microvessel endothelial cells. J Pharm Pharmacol. 2007;59:947–953. doi: 10.1211/jpp.59.7.0006. [DOI] [PubMed] [Google Scholar]
- 46.Weiss J, et al. Comparison of the induction of P-glycoprotein activity by nucleotide, nucleoside, and non-nucleoside reverse transcriptase inhibitors. Eur J Pharmacol. 2008;579:104–109. doi: 10.1016/j.ejphar.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi K, et al. HIV-TAT protein upregulates expression of multidrug resistance protein 1 in the blood-brain barrier. J Cereb Blood Flow Metab. 2006;26:1052–1065. doi: 10.1038/sj.jcbfm.9600254. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi K, et al. HIV-Tat protein induces P-glycoprotein expression in brain microvascular endothelial cells. J Neurochem. 2005;93:1231–1241. doi: 10.1111/j.1471-4159.2005.03114.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhong Y, et al. Intact lipid rafts regulate HIV-1 Tat protein-induced activation of the Rho signaling and upregulation of P-glycoprotein in brain endothelial cells. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Kuhnke D, et al. MDR1-P-Glycoprotein (ABCB1) Mediates Transport of Alzheimer’s amyloid-beta peptides--implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Pathol. 2007;17:347–353. doi: 10.1111/j.1750-3639.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tai LM, et al. P-glycoprotein and breast cancer resistance protein restrict apical-to-basolateral permeability of human brain endothelium to amyloid-beta. J Cereb Blood Flow Metab. 2009;29:1079–1083. doi: 10.1038/jcbfm.2009.42. [DOI] [PubMed] [Google Scholar]
- 53.Xiong H, et al. ABCG2 is upregulated in Alzheimer’s brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood-brain barrier for Abeta(1-40) peptides. J Neurosci. 2009;29:5463–5475. doi: 10.1523/JNEUROSCI.5103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartz AM, et al. Restoring Blood-Brain Barrier P-glycoprotein Reduces Brain A{beta} in a Mouse Model of Alzheimer’s Disease. Mol Pharmacol. doi: 10.1124/mol.109.061754. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bauer B, et al. Seizure-induced up-regulation of P-glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Mol Pharmacol. 2008;73:1444–1453. doi: 10.1124/mol.107.041210. [DOI] [PubMed] [Google Scholar]
- 56.Hartz AM, et al. Signaling to P-glycoprotein-A new therapeutic target to treat drug-resistant epilepsy? Drug News Perspect. 2009;22:393–397. doi: 10.1358/dnp.2009.22.7.1401354. [DOI] [PubMed] [Google Scholar]
- 57.Baltes S, et al. Valproic acid is not a substrate for P-glycoprotein or multidrug resistance proteins 1 and 2 in a number of in vitro and in vivo transport assays. J Pharmacol Exp Ther. 2007;320:331–343. doi: 10.1124/jpet.106.102491. [DOI] [PubMed] [Google Scholar]
- 58.Baltes S, et al. Differences in the transport of the antiepileptic drugs phenytoin, levetiracetam and carbamazepine by human and mouse P-glycoprotein. Neuropharmacology. 2007;52:333–346. doi: 10.1016/j.neuropharm.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 59.Anderson GD, Shen DD. Where is the evidence that p-glycoprotein limits brain uptake of antiepileptic drug and contributes to drug resistance in epilepsy? Epilepsia. 2007;48:2372–2374. doi: 10.1111/j.1528-1167.2007.01260_3.x. [DOI] [PubMed] [Google Scholar]
- 60.Siddiqui A, et al. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003;348:1442–1448. doi: 10.1056/NEJMoa021986. [DOI] [PubMed] [Google Scholar]
- 61.Sills GJ, et al. Lack of association between the C3435T polymorphism in the human multidrug resistance (MDR1) gene and response to antiepileptic drug treatment. Epilepsia. 2005;46:643–647. doi: 10.1111/j.1528-1167.2005.46304.x. [DOI] [PubMed] [Google Scholar]
- 62.Tan NC, et al. Failure to confirm association of a polymorphism in ABCB1 with multidrug-resistant epilepsy. Neurology. 2004;63:1090–1092. doi: 10.1212/01.wnl.0000137051.33486.c7. [DOI] [PubMed] [Google Scholar]
- 63.Kannan P, et al. Imaging the function of P-glycoprotein with radiotracers: pharmacokinetics and in vivo applications. Clin Pharmacol Ther. 2009;86:368–377. doi: 10.1038/clpt.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kipp H, et al. Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J Biol Chem. 2001;276:7218–7224. doi: 10.1074/jbc.M007794200. [DOI] [PubMed] [Google Scholar]
- 65.Miller DS. Xenobiotic export pumps, endothelin signaling, and tubular nephrotoxicants--a case of molecular hijacking. J Biochem Mol Toxicol. 2002;16:121–127. doi: 10.1002/jbt.10030. [DOI] [PubMed] [Google Scholar]
- 66.Rigor RR, et al. Activation of PKC isoform beta(I) at the blood-brain barrier rapidly decreases P-glycoprotein activity and enhances drug delivery to the brain. J Cereb Blood Flow Metab. doi: 10.1038/jcbfm.2010.21. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 68.Hawkins BT, et al. Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J Neurosci. 2010;30:1417–1425. doi: 10.1523/JNEUROSCI.5103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barakat S, et al. Modulation of p-glycoprotein function by caveolin-1 phosphorylation. J Neurochem. 2007;101:1–8. doi: 10.1111/j.1471-4159.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- 70.Barakat S, et al. Regulation of brain endothelial cells migration and angiogenesis by P-glycoprotein/caveolin-1 interaction. Biochem Biophys Res Commun. 2008;372:440–446. doi: 10.1016/j.bbrc.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 71.Merino G, et al. Sex-dependent expression and activity of the ATP-binding cassette transporter breast cancer resistance protein (BCRP/ABCG2) in liver. Mol Pharmacol. 2005;67:1765–1771. doi: 10.1124/mol.105.011080. [DOI] [PubMed] [Google Scholar]
- 72.Tanaka Y, et al. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun. 2005;326:181–187. doi: 10.1016/j.bbrc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 73.Hartz AM, et al. 17-beta-Estradiol: a powerful modulator of blood-brain barrier BCRP activity. J Cereb Blood Flow Metab. doi: 10.1038/jcbfm.2010.36. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Storch CH, et al. Localization of the human breast cancer resistance protein (BCRP/ABCG2) in lipid rafts/caveolae and modulation of its activity by cholesterol in vitro. J Pharmacol Exp Ther. 2007;323:257–264. doi: 10.1124/jpet.107.122994. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y, et al. P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J Pharmacol Exp Ther. 2009;330:956–963. doi: 10.1124/jpet.109.154781. [DOI] [PubMed] [Google Scholar]
- 76.Polli JW, et al. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethy l]amino}methyl)-2-furyl]-4-quinazolinamine; GW572016) Drug Metab Dispos. 2009;37:439–442. doi: 10.1124/dmd.108.024646. [DOI] [PubMed] [Google Scholar]
- 77.Enokizono J, et al. Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug Metab Dispos. 2008;36:995–1002. doi: 10.1124/dmd.107.019257. [DOI] [PubMed] [Google Scholar]
- 78.Abbott NJ, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]