Abstract
Little is known of the associations of endogenous fatty acids with sudden cardiac arrest (SCA). We investigated the associations of SCA with red blood cell membrane fatty acids that are end products of de novo fatty acid synthesis: myristic acid (14:0), palmitic acid (16:0), palmitoleic acid (16:1 n7), vaccenic acid (18:1 n7), stearic acid (18:0), oleic acid (18:1 n9) and a related fatty acid cis-7 hexadecenoic acid (16:1 n9). We used data from a population-based case-control study, where cases, aged 25-74 years, were out-of-hospital sudden cardiac arrest patients, attended by paramedics in Seattle, Washington (n=265). Controls, matched to cases by age, sex and calendar year, were randomly identified from the community (n=415). All participants were free of prior clinically-diagnosed heart disease. We observed associations of higher red blood cell membrane levels of 16:0, 16:1n-7, 18:1n-7 and 16:1n-9 with higher risk of SCA. In analyses adjusted for traditional SCA risk factors and trans- and n-3 fatty acids, a one-standard-deviation-higher level of 16:0 was associated with 38% higher risk of SCA (odds ratio [OR] 1.38, 95% confidence interval [CI]: 1.12-1.70) and a one-standard deviation-higher level of 16:1n-9 with 88% higher risk (OR 1.88, 95% CI: 1.27-2.78). Several fatty acids that are end products of fatty acid synthesis are associated with SCA risk. Further work is needed to investigate if conditions that favor de novo fatty acid synthesis, such as high carbohydrate/low fat diets, might also increase the risk of SCA.
Sudden cardiac arrest (SCA) is the leading cause of death from coronary heart disease (CHD). 1 We and others have shown that several red blood cell (RBC) membrane and whole blood fatty acids are associated with the risk of SCA. 2-5
Fatty acids in cell membranes are either derived from the diet (exogenous fatty acids), or from endogenous synthesis, or both. To date, our studies of SCA in King County, Washington, have focused on membrane fatty acids derived primarily from the diet (i.e. biomarkers of dietary fatty acid intake). In particular, we explored associations of RBC membrane docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), trans-fatty acids and recently α-linolenic acid with SCA risk 3-5. We now turn to fatty acids that are not direct biomarkers of dietary fatty acid intake, but instead derive from endogenous synthesis.
We were particularly interested in membrane fatty acids that are endogenously synthesized from dietary carbohydrates. Prior studies have suggested that dietary carbohydrates are associated with higher risk of CHD. 6 7 Diets that are low in fat and high in carbohydrates lead to de novo synthesis of specific fatty acids, and to higher levels of these synthesized fatty acids in RBC membranes. 8-12 Given the known influence of membrane fatty acids on SCA risk, we hypothesized that membrane fatty acids endogenously synthesized from carbohydrates are associated with higher risk of SCA.
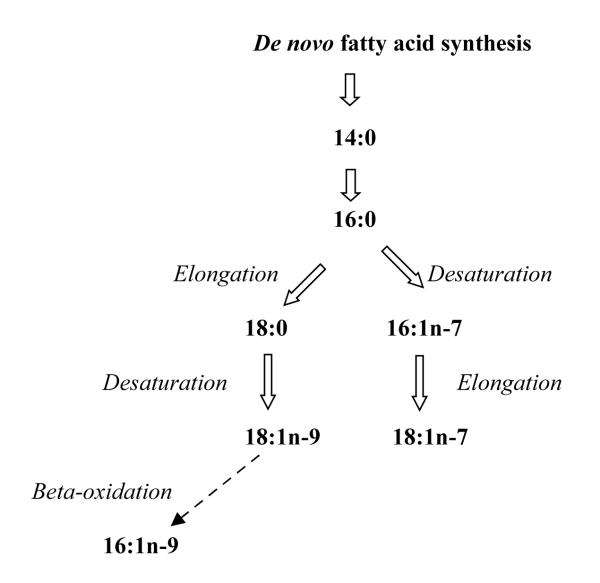
The main product of fatty acid synthase is palmitic acid (16:0) which can be processed by delta-9 desaturation and/or elongation to palmitoleic acid (16:1n-7), vaccenic acid (18:1n-7), stearic acid (18:0) or oleic acid (18:1n-9) depending upon cellular requirements (Figure). We used data from our study of SCA in King County, Washington to assess the association of these RBC membrane fatty acids with the risk of SCA. In addition, we included myristic acid (14:0), another possible product of fatty acid synthase and cis-7 hexadecenoic acid (16:1n-9), a related fatty acid that is also increased in the setting of a low fat, high carbohydrate diet. 9
FIGURE.
Endogenous Synthesis of Saturated and Monounsaturated Fatty Acids
MATERIALS AND METHODS
The study is a population-based case-control study which has been described in detail in earlier reports of polyunsaturated fatty acids and SCA risk. 3-5
Study Subjects
Cases, aged 25-74 years, were out-of-hospital sudden cardiac arrest patients, attended by paramedics in Seattle and suburban King County, Washington, USA, between October 1988 and September 2005. SCA cases were identified from emergency service incident reports. We excluded patients with cardiac arrest due to a non-cardiac cause, cases with a history of clinically-recognized heart disease or life-threatening co-morbidities, and users of fish oil supplements. We further restricted SCA cases to married residents of King County, Washington. The response rate among the spouses of cases was 73%.
Married control subjects, recruited concurrently with cases and individually matched to cases on age (within 7 years), sex, and calendar year, were a random sample from the community. The response rate among controls and their spouses was 55%. Controls were excluded using the same eligibility criteria as the cases. The University of Washington Human Subject Review Committee approved the study protocol and all study subjects or their proxy signed an informed consent.
Red blood cell membrane fatty acid measurements
Paramedics obtained blood specimens from the cases in the field after essential emergency medical care had been provided and either the patient was clinically stable, or resuscitation had proven ineffective, usually within 30 to 45 min of the cardiac arrest. Blood specimens from controls were obtained at the time of an interview.
Blood specimens were processed 5 and submitted to gas chromatography 13 according to published methods. Fatty acid identifications and levels were standardized with the National Institute of Health’s Fatty Acid Standards A, B, C, D, F, and GLC87 (Nu-Check-Prep, Elysian, MN). Identification, precision, and accuracy were evaluated using model mixtures of known fatty acid methyl esters and an established in-house control pool. For the fatty acids that have no standards available, such as cis 16:1n-9, we sent out samples for independent analysis. Our identification has been confirmed by GC – MS at the USDA lipid laboratory in Peoria, IL. In addition, identification of cis and trans-fatty acids has been verified by silver ion TLC. 14 Specimens from each case and its individually-matched controls were submitted to gas chromatography in the same batch. Laboratory analyses were conducted by technicians blinded to case and control status. Fatty acid levels were expressed as percentages of total fatty acids.
Other risk factors assessment
We collected information on demographic factors, medical conditions, life-style characteristics and dietary habits during a spouse interview. Controls themselves were interviewed to assess the validity of spouse information. Dietary saturated fat intake was assessed with the Northwest Lipid Research Clinic Fat Intake Scale, an index that correlates with total fat and saturated fat intake. 15
Statistical Analysis
Statistical analyses were carried out using Stata/SE 10.1 (StataCorp LP, College Station, Texas). To assess the associations of fatty acid levels with SCA while taking into account the individual matching of cases and controls and SCA risk factors, we performed conditional logistic regression.16 For the main analyses, separate models were fit, one for each fatty acid. In the models, fatty acids were included as a continuous linear term and odds ratios (estimates of relative risks) and 95% confidence intervals corresponding to a one-standard-deviation difference are presented. Quadratic terms were not included as they did not improve the fit of the models. Statistical significance was assessed with the likelihood ratio test. The covariates included in the models were from spouse interviews for both cases and controls. Potential interactions between fatty acid levels and subject characteristics were evaluated by testing whether addition of cross-products between fatty acids and a subject characteristic improved the fit of the model.
To assess which characteristics were independently associated with RBC level of specific fatty acids among controls, we used multiple linear regression with one fatty acid as outcome and the characteristics of study subjects as potential covariates, including levels of n-3 and trans fatty acids. Covariates significantly associated with each fatty acid were retained. Associations among fatty acid levels were assessed using Pearson correlations.
Information on some covariates was missing on less than 3% of cases and 5% of controls. 3 The missing values were imputed by a multivariate technique using sequential regressions, 17 and the results obtained with imputed values are presented in this report. Similar results were obtained when the analyses were restricted to those matched case-control pairs without missing values.
RESULTS
The study included 265 SCA cases and 415 individually-matched controls. Age and gender were matching factors and similarly distributed in cases and controls (Table 1). Other traditional risk factors for SCA were more prevalent among cases than among controls, as expected in this study design.
Table 1.
Characteristics of cases and controls
| Characteristic | Cases, N=265 | Controls, N=415 | p-value |
|---|---|---|---|
| Age, yr, mean (SD) | 58.4 (10.5) | 57.1 (10.4) | * |
| Women, % | 18.5 | 18.9 | * |
| White, % | 88.7 | 92.1 | 0.14 |
| Education, high school graduate, % | 71.7 | 79.5 | 0.02 |
| Current smokers, % | 28.9 | 8.6 | < 0.001 |
| Hypertension, % | 24.9 | 15.2 | 0.002 |
| Diabetes, % | 12.6 | 6.3 | 0.004 |
| Family history of MI or sudden death, % | 52.7 | 43.2 | 0.02 |
| Weight, kg, mean (SD) | 85.0 (18.2) | 83.2 (15.9) | 0.18 |
| Body mass index, kg/m2, mean (SD) | 27.0 (4.9) | 26.4 (4.0) | 0.09 |
| Physical activity, kcal/week, mean (SD) | 966.8 (1263.0) | 1301.8 (1403.8) | 0.002 |
| Fat index score, mean (SD) | 21.2 (3.8) | 21.4 (3.7) | 0.38 |
| Caffeine, mg/d, mean (SD) | 350.2 (476.0) | 297.1 (442.7) | 0.14 |
| Alcohol consumption, % | 64.2 | 69.2 | 0.18 |
| RBC membrane fatty acid, mean (SD)† | |||
| End products of fatty acid synthesis: | |||
| 14:0 | 0.25 (0.08) | 0.26 (0.07) | 0.37 |
| 16:0 | 18.93 (1.09) | 18.74 (0.93) | 0.01 |
| 16:1n-7 | 0.29 (0.13) | 0.27 (0.10) | 0.002 |
| 18:1n-7 | 0.92 (0.20) | 0.89 (0.11) | 0.006 |
| 18:0 | 14.26 (0.78) | 14.19 (0.71) | 0.24 |
| 18:1n-9 | 10.93 (0.85) | 10.90 (0.81) | 0.70 |
| 16:1n-9 | 0.079 (0.05) | 0.068 (0.05) | 0.005 |
| Exogenous fatty acids: | |||
| Linoleic acid | 9.26 (1.27) | 9.10 (1.11) | 0.08 |
| α-linolenic acid | 0.14 (0.04) | 0.13 (0.04) | 0.01 |
| EPA ¥ | 0.51 (0.24) | 0.57 (0.30) | 0.005 |
| DHA ¥ | 3.71 (1.06) | 4.06 (1.10) | < 0.001 |
| Trans 18:2 fatty acids | 0.19 (0.07) | 0.17 (0.06) | 0.01 |
| Trans 18:1 fatty acids | 1.60 (0.53) | 1.54 (0.48) | 0.14 |
Matching factor
Percent of total fatty acids
The long chain n-3 fatty acids EPA and DHA may also be synthesized endogenously from α-linolenic acid
Mean RBC membrane levels of 16:0, 16:1n-7, 18:1n-7 and 16:1n-9 were higher among cases than controls (Table 1), whereas levels of 14:0, 18:0 and 18:1n-9 did not differ. As reported previously, mean levels of DHA and EPA were lower and mean levels of trans-18:2 fatty acids and α linolenic acid were higher among cases than controls. 3-5
In multiple logistic regression analyses, RBC membrane levels of 16:0, 16:1n-7, 18:1n-7 and 16:1n-9 were associated with a higher risk of SCA (Table 2). The odds ratios associated with a standard deviation difference in the fatty acid levels were as follows: for 16:0, 1.28 (95% confidence interval [CI] 1.05-1.55); for 16:1n-7, 1.24 (95% CI 1.05-1.46); for 18:1n-7, 1.18 (95% CI 1.02-1.37); and for 16:1n-9, 1.95 (95% CI 1.33-2.86) after adjustment for smoking, diabetes, hypertension, education, leisure-time physical activity, fat intake index, weight and height. The associations changed minimally with further adjustment for RBC membrane levels of DHA+EPA, trans-fatty acids and α linolenic acid (Table 2). Further adjustments for alcohol and caffeine consumption, and RBC membrane levels of linoleic acid and arachidonic acid altered the results only slightly (not shown).
Table 2.
Association of RBC membrane products of fatty acid synthesis with sudden cardiac arrest*
| Fatty acid | Risk-factor adjusted † odds ratio for one higher SD (95% CI) |
p-value | Risk-factor and fatty- acid adjusted ⊥ odds ratio for one higher SD (95% CI) |
p-value |
|---|---|---|---|---|
| 14:0 | 1.10 (0.89-1.37) | 0.37 | 1.02 (0.81-1.29) | 0.85 |
| 16:0 | 1.28 (1.05-1.55) | 0.01 | 1.38 (1.12-1.70) | 0.002 |
| 16:1n-7 | 1.24 (1.05-1.46) | 0.01 | 1.18 (0.99-1.41) | 0.07 |
| 18:1n-7 | 1.18 (1.02-1.37) | 0.03 | 1.15 (0.98-1.34) | 0.08 |
| 18:0 | 0.98 (0.80-1.19) | 0.82 | 1.11 (0.90-1.37) | 0.33 |
| 18:1n-9 | 0.97 (0.81-1.17) | 0.77 | 0.88 (0.72-1.07) | 0.20 |
| 16:1n-9 | 1.95 (1.33-2.86) | 0.001 | 1.88 (1.27-2.78) | 0.002 |
Separate models were considered, one for each fatty acid
Adjusted for age, gender, smoking, diabetes, hypertension, education, kilocalories of physical activity, index of fat intake, body weight and height
Additionally adjusted for RBC membrane levels of DHA+EPA, trans-18:2 fatty acids and α-linolenic acid.
We explored if the observed fatty acid associations were independent of each other. RBC membrane levels of 16:0 and 16:1n-7 were correlated (r = 0.50); and in logistic regression models including both 16:0 and 16:1n-7, the association of each fatty acid with SCA was slightly diminished and not significant (not shown). A multiple logistic regression model including several fatty acids simultaneously demonstrated that 16:0, 16:1n-9, DHA+EPA, α linolenic acid and trans-18:2 fatty acids were all independently associated with SCA (Table 3). When substituted for 16:0 in the table 3 model, 16:1n-7 and 18:1n-7 were not significantly associated with SCA (not shown).
Table 3.
Independent associations of RBC membrane fatty acids with sudden cardiac arrest*
| Fatty acid | OR for one higher SD (95% CI) | p-value |
|---|---|---|
| 16:0 | 1.31 (1.06-1.62) | 0.01 |
| 16:1n-9 | 1.71 (1.15-2.54) | 0.008 |
| Trans 18:2 | 1.83 (1.30-2.59) | 0.001 |
| α-linolenic acid | 1.24 (0.99-1.56) | 0.06 |
| DHA+EPA | 0.80 (0.67-0.94) | 0.008 |
Adjusted for age, gender, smoking, diabetes, hypertension, education, kilocalories of physical activity, index of fat intake, body weight and height and all the fatty acid levels in the table.
We found no evidence that the association of RBC membrane 16:0 and 16:1n-9 levels with SCA risk was influenced by subject characteristics, including age, gender, smoking, diabetes, hypertension, body weight and RBC membrane levels of DHA+EPA, trans-fatty acids and α-linolenic acid (not shown). In analyses restricted to women, use of hormone replacement therapy altered only slightly the associations (not shown).
Among controls, RBC membrane levels of 16:0 were inversely associated with the Northwest Lipid Research Clinic index of fat intake and positively associated with age, gender, education, alcohol consumption, RBC membrane levels of EPA and time period of the study (Table 4). Together, these characteristics accounted for 23% of the variation in 16:0. In comparison, 16:1n-7 was associated with gender, alcohol consumption, body weight, levels of its precursor 16:0 and levels of EPA among controls (Table 5). Among controls, the levels of 18:1n-7 was positively associated with the level of its precursor 16:1n-7 and inversely related to the index of fat intake (not shown). The level of 16:1n-9 among controls was not associated with any study subject characteristic (not shown).
Table 4.
Characteristics associated with RBC membrane levels of 16:0*
| Characteristic | Mean difference in 16:0† | p-value |
|---|---|---|
| Age (years) | 0.009 | 0.02 |
| Gender (female vs male) | 0.442 | < 0.001 |
| Fat intake (one point increase out of 20 possible) |
− 0.023 | 0.05 |
| Consumption of alcohol (average level vs no consumption) |
0.187 | 0.05 |
| Alcohol consumption among drinkers (g) | 0.013 | < 0.001 |
| High school education, (Yes vs No) | 0.286 | 0.009 |
| Time period: 1989-1994 1995-1999 2000-2005 |
Ref 0.051 0.335 |
0.003 |
| RBC membrane levels of EPA (% of total fatty acids) |
0.716 | < 0.001 |
Adjusted for all other characteristics in the table. R-squared of full model: 0.23.
16:0 as % of total fatty acids; difference associated with a unit increase or indicated difference in characteristic.
Table 5.
Characteristics associated with RBC membrane levels of 16:1n-7*
| Characteristic | Mean difference in 16:1n-7† | p-value |
|---|---|---|
| 16:0 (% of total fatty acids) | 0.046 | < 0.001 |
| Gender (female vs male) | 0.055 | < 0.001 |
| Consumption of alcohol (average level vs no consumption) |
− 0.0044 | 0.64 |
| Alcohol consumption among drinkers (g) | 0.0011 | < 0.001 |
| Body weight (kg) | 0.001 | 0.003 |
| RBC membrane levels of EPA (% of total fatty acids) |
0.044 | 0.01 |
Adjusted for all other characteristics in the table. R-squared of full model: 0.33.
16:1n-7 as % of total fatty acids; difference associated with a unit increase or indicated difference in characteristic.
DISCUSSION
In this investigation of seven endogenous fatty acids, RBC membrane levels of four fatty acids, 16:0, 16:1n-7, 18:1n-7 and 16:1n-9, were associated with higher risk of SCA. These associations were independent of traditional risk factors. The associations of 16:0 and 16:1n-9 were independent of membrane levels of DHA+EPA, trans-18:2 fatty acids and α-linolenic acid and consistent across subgroups.
We chose to focus on these seven fatty acids because of evidence that their levels reflect de novo fatty acid synthesis in response to high carbohydrate-low fat diets. 8-12 Specifically, we recently conducted a dietary trial to compare the effects on tissue fatty acids of a moderate fat, moderate carbohydrate diet to that of an isocaloric low fat, high carbohydrate diet.9 After six weeks on the diets, RBC membrane levels of 16:0, 16:1n-7, 18:1n-7, 18:1n-9, 16:1n-9 and 14:0 were noticeably higher on the low fat, high carbohydrate diet than on the moderate fat, moderate carbohydrate diet. The observation of higher membrane levels of these fatty acids when their dietary intake was lower strongly suggested that they originated from de novo fatty acid synthesis. Interestingly, levels of the fatty acid 18:0 was similar in both diets 9 and 18:0 was not associated with higher risk of SCA in the present study. Membrane levels of 18:1n-9 and 14:0 were not associated with SCA either. The fatty acid 18:1n-9 is a major component of vegetable oil and 14:0 is found in dairy products. It is possible that diet contributed to membrane 18:1n-9 and 14:0 in the present study.
The fatty acid 16:0 is a major component of membranes and it is abundant in the diet. Therefore red blood cell membrane 16:0 could originate from the diet. However, membrane saturated fatty acids correlate poorly with levels of saturated fatty acids in the diet. 18, 19 In fact membrane 16:0 was inversely related with the index of total fat intake and positively associated with alcohol consumption in the present study, suggesting that 16:0 originated at least in part from fatty acid synthesis. In addition, we and others have shown heritability of RBC membrane 16:0, 20 21 suggesting that a genetic component also contributes to individual variation in 16:0 levels.
The fatty acid 16:1n-7 is in low amount in the diet and membrane 16:1n7 originates largely from endogenous synthesis. Genetic factors also contribute to 16:1n-7 levels. 21 Interestingly, membrane levels of 16:1n-7 in the present study was also related to body weight, possibly reflecting the effects of low-fat high-carbohydrate diets on de novo synthesis and hypertriglyceridemia. 10, 11 The fatty acid 16:1n-7 was highly correlated with its precursor 16:0 and the association of 16:0 with SCA risk appeared more robust than that of 16:1n-7. However, this difference in level of association cannot be concluded from the study data. Membrane 16:1n-7 is in much smaller amount and measured with a larger measurement error than 16:0. Measurement error could reduce the apparent association of 16:1n-7 with risk.
The origin of the fatty acid 16:1n-9 is not entirely clear. Conversion of 18:1n-9 to 16:1n-9 by beta-oxidation in peroxisomes has been shown to occur in cultured human liver cells. 22 Whether dietary 18:1n-9 can be converted to 16:1n-9 is not known. In our randomized dietary trial, 16:1n-9 was elevated in response to low fat, high carbohydrate diet, when dietary 18:1n-9 was lower, 9 suggesting that 16:1n-9 was formed from endogenously synthesized 18:1n-9. In the present study, we did not find any subject characteristics that were predictive of 16:1n-9 levels. Potential heritability of 16:1n-9 was not assessed in studies of heritability of fatty acid levels. 20, 21 Given its strong association with SCA risk, further studies are needed to explore conditions that result in higher levels of 16:1n-9 in membranes.
The mechanism by which 16:0, 16:1n-9 and possibly 16:1n-7 and 18:1n-7 might influence the risk of SCA is not known. Since the association of these fatty acids with risk of SCA remained after adjustment for membrane DHA+EPA levels, it was not explained by replacement of n-3 fatty acids by these fatty acids. Saturated and monounsaturated fatty acids usually occupy the sn1 position on membrane phospholipids; polyunsaturated fatty acids usually localize in the sn2 position. 23 During ischemia, activation of phospholipase A2 leads to the release of a polyunsaturated fatty acid from the sn2 position and the formation of lysophospholipids with a single fatty acid.24 The levels of polyunsaturated n-3 fatty acids in membrane phospholipids are known to influence the risk of SCA, 2, 5 and DHA or EPA released from the sn2 position during ischemia might protect from the effects of lysophospholipids on arrhythmogenesis.25, 26 It is also possible that the nature of the remaining fatty acid on the lysophospholipids affects the risk of arrhythmia. There is evidence that lysophospholipids influence the Kv11.1 potassium ion channel function leading to arrhythmias, 25 and the enhancement appears specific to lysophospholipids with a 16 carbon fatty acid. 27
De novo synthesis in humans occurs mainly in the liver where synthesis and oxidation of fatty acids are controlled simultaneously; when energy requirements are met and fatty acid oxidation stops, fatty acid synthesis resumes. 28 In addition, constitutional expression at low levels of the factor sterol regulatory element-binding protein 1-c, which activates the transcription of all the genes in the de novo synthesis pathway, may maintain basal levels of fatty acid synthesis. 29 The occurrence of fatty acid synthesis from dietary carbohydrates in the absence of excess caloric intake is supported by dietary trials. 8, 9 10-12 Further studies are needed to investigate dietary and genetic factors that promote fatty acid synthesis. The associations of end products of fatty acid synthesis with SCA risk raise the possibility that dietary carbohydrates in the setting of low fat intake might also be associated with SCA risk.
The strengths of this study include the use of population-based cases and controls, the objective assessment of fatty acid levels in RBC membranes, and adjustment of results for other known risk factors. To address the possibility that cases might have changed their diet or lifestyle as a consequence of poor health leading to SCA, we restricted the study to cases with no history of clinically-recognized heart disease and no life-threatening co-morbidities.
Several limitations are noteworthy. Due to the observational nature of the study, the possibility of residual confounding cannot be eliminated. The use of surrogate respondents inevitably introduced some misclassification in assessment of potential confounders; however, the exposure of interest was measured objectively. The participation rate among controls was 60 % and the odds ratio estimates could be biased if controls who declined participation in the study had different fatty acid patterns from the controls who participated. Despite sharing this limitation, however, our previously reported findings on the inverse association of dietary intake and cell-membrane levels of DHA and EPA with the risk of SCA in this same study population have been replicated in prospective cohort studies of other populations. 2, 30, 31
In conclusion, we observed an association between SCA and levels of several fatty acids that are end products of fatty acid synthesis. Further work is needed to confirm the study findings and to determine if characteristics that raise membrane levels of these fatty acids, such as genetic factors and dietary carbohydrates in the setting of low fat intake, also raise SCA risk.
Acknowledgments
The research reported in this article was supported by grants from the National Heart, Lung, and Blood Institute (HL41993), the University of Washington Clinical Nutrition Research Unit (DK-35816), and the Medic One Foundation, Seattle, WA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Zipes DP, Wellens HJJ. Sudden cardiac death. Circulation. 1998;98:2334–51. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 2.Albert CM, Campos H, Stampfer MJ, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–18. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 3.Lemaitre R, King I, Sotoodehnia N, et al. Red blood cell membrane alpha-linoleic acid and the risk of sudden cardiac arrest. Metabolism. 2009;58:534–40. doi: 10.1016/j.metabol.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemaitre RN, King IB, Raghunathan TE, et al. Cell membrane trans-fatty acids and the risk of primary cardiac arrest. Circulation. 2002;105:697–701. doi: 10.1161/hc0602.103583. [DOI] [PubMed] [Google Scholar]
- 5.Siscovick DS, Raghunathan TE, King I, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. Jama. 1995;274:1363–67. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 6.Halton TL, Willett WC, Liu S, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–9. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 8.Hudgins LC, Hellerstein M, Seidman C, et al. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest. 1996;97:2081–91. doi: 10.1172/JCI118645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King IB, Lemaitre RN, Kestin M. Effect of a low-fat diet on fatty acid composition in red cells, plasma phospholipids, and cholesterol esters: investigation of a biomarker of total fat intake. Am J Clin Nutr. 2006;83:227–36. doi: 10.1093/ajcn/83.2.227. [DOI] [PubMed] [Google Scholar]
- 10.Knopp RH, Retzlaff B, Walden C, et al. One-year effects of increasingly fat-restricted, carbohydrate-enriched diets on lipoprotein levels in free-living subjects. Proc Soc Exp Biol Med. 2000;225:191–9. doi: 10.1046/j.1525-1373.2000.22524.x. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr. 2003;77:43–50. doi: 10.1093/ajcn/77.1.43. [DOI] [PubMed] [Google Scholar]
- 12.Marques-Lopes I, Ansorena D, Astiasaran I, et al. Postprandial de novo lipogenesis and metabolic changes induced by a high-carbohydrate, low-fat meal in lean and overweight men. Am J Clin Nutr. 2001;73:253–61. doi: 10.1093/ajcn/73.2.253. [DOI] [PubMed] [Google Scholar]
- 13.Lemaitre RN, King IB, Patterson RE, et al. Assessment of trans-fatty acid intake with a food frequency questionnaire and validation with adipose tissue levels of trans-fatty acids. Am J Epidemiol. 1998;148:1085–93. doi: 10.1093/oxfordjournals.aje.a009586. [DOI] [PubMed] [Google Scholar]
- 14.Ulberth F, Henninger M. Simplified method for the determination of trans monoenes in edible fats by TLC-GLC. J Am Oil Chem Soc. 1992;69:829–31. [Google Scholar]
- 15.Retzlaff BM, Dowdy AA, Walden CE, et al. The Northwest Lipid Research Clinic Fat Intake Scale: validation and utility. Am J Public Health. 1997;87:181–5. doi: 10.2105/ajph.87.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breslow N, Day N. Statistical Methods in Cancer Research. Vol 1. IARC Scientific Publications; Lyon: 1980. The analysis of case-control studies. [PubMed] [Google Scholar]
- 17.Raghunathan T, Lepkowski J, Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27:85–95. [Google Scholar]
- 18.Fuhrman BJ, Barba M, Krogh V, et al. Erythrocyte membrane phospholipid composition as a biomarker of dietary fat. Ann Nutr Metab. 2006;50:95–102. doi: 10.1159/000090496. [DOI] [PubMed] [Google Scholar]
- 19.McMurchie EJ, Gibson RA, Charnock JS, McIntosh GH. Mitochondrial membrane fatty acid composition in the marmoset monkey following dietary lipid supplementation. Lipids. 1986;21:315–23. doi: 10.1007/BF02535693. [DOI] [PubMed] [Google Scholar]
- 20.Lemaitre RN, Siscovick DS, Berry EM, et al. Familial aggregation of red blood cell membrane fatty acid composition: the Kibbutzim Family Study. Metabolism. 2008;57:662–8. doi: 10.1016/j.metabol.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Kunesova M, Hainer V, Tvrzicka E, Phinney SD, Stich V, Parizkova J, Zak A, Stunkard AJ. Assessment of dietary and genetic factors influencing serum and adipose fatty acid composition in obese female identical twins. Lipids. 2002;37:27–32. doi: 10.1007/s11745-002-0860-z. [DOI] [PubMed] [Google Scholar]
- 22.Lee WN, Lim S, Bassilian S, et al. Fatty acid cycling in human hepatoma cells and the effects of troglitazone. J Biol Chem. 1998;273:20929–34. doi: 10.1074/jbc.273.33.20929. [DOI] [PubMed] [Google Scholar]
- 23.Lands WE, Merkl I. Metabolism of glycerolipids. III. Reactivity of various acyl esters of coenzyme A with alpha’-acylglycerophosphorylcholine, and positional specificities in lecithin synthesis. J Biol Chem. 1963;238:898–904. [PubMed] [Google Scholar]
- 24.Corr PB, Yamada KA. Selected metabolic alterations in the ischemic heart and their contributions to arrhythmogenesis. Herz. 1995;20:156–68. [PubMed] [Google Scholar]
- 25.Bai Y, Wang J, Lu Y, et al. Phospholipid lysophosphatidylcholine as a metabolic trigger and HERG as an ionic pathway for extracellular K accumulation and “short QT syndrome” in acute myocardial ischemia. Cell Physiol Biochem. 2007;20:417–28. doi: 10.1159/000107526. [DOI] [PubMed] [Google Scholar]
- 26.Kang JX, Leaf A. Protective effects of free polyunsaturated fatty acids on arrhythmias induced by lysophosphatidylcholine or palmitoylcarnitine in neonatal rat cardiac myocytes. Eur J Pharmacol. 1996;297:97–106. doi: 10.1016/0014-2999(95)00701-6. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Zhang Y, Wang H, et al. Potential mechanisms for the enhancement of HERG K+ channel function by phospholipid metabolites. Br J Pharmacol. 2004;141:586–99. doi: 10.1038/sj.bjp.0705646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster DW. The role of the carnitine system in human metabolism. Ann N Y Acad Sci. 2004;1033:1–16. doi: 10.1196/annals.1320.001. [DOI] [PubMed] [Google Scholar]
- 29.McPherson R, Gauthier A. Molecular regulation of SREBP function: the Insig-SCAP connection and isoform-specific modulation of lipid synthesis. Biochem Cell Biol. 2004;82:201–11. doi: 10.1139/o03-090. [DOI] [PubMed] [Google Scholar]
- 30.Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–21. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 31.Mozaffarian D, Lemaitre RN, Kuller LH, et al. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation. 2003;107:1372–77. doi: 10.1161/01.cir.0000055315.79177.16. [DOI] [PubMed] [Google Scholar]