Abstract
Background
Traumatic experiences in early childhood are associated with increased risk for developing mood and anxiety disorders later in life. Low serotonin1A receptor (5-HT1AR) density during development has been proposed as a trait-like characteristic leading to increased vulnerability of stress-related neuropsychiatric disorders.
Methods
To assess the relationship between early-life stress and alterations in the serotonin system during development, we used positron emission tomography (PET) to measure in vivo 5-HT1AR density and apparent dissociation constant (KDapp) in the brain of juvenile rhesus monkeys exposed to the early-life stress of peer-rearing.
Results
In general, 5-HT1AR density and KDapp were decreased in peer-reared compared with control mother-reared animals. However, increase in receptor density was found in the dorsomedial prefrontal cortex of peer-reared females.
Conclusions
These findings suggest that exposure to an adverse early-life environment during infancy is associated with long-term alterations in the serotonin system and, support previous studies suggesting that reduced 5-HT1AR density during development may be a factor increasing vulnerability to stress-related neuropsychiatric disorders. Further, alterations in the serotonin system appeared to be gender- and region-specific, providing a biological basis for the higher prevalence of affective disorders in women.
Keywords: serotonin1A receptor, PET, early-life stress, nonhuman primate, development
Low 5-HT1AR binding, measured by PET and in vitro binding assays of postmortem brain tissue, is found in individuals with several anxiety-related disorders, including depression, social anxiety and panic disorders (1–8). Moreover, a spontaneous variation in the HTR1A gene promoter that results in lower levels of HTR1A expression (9) is also associated with anxiety-related personality traits (10).
Rodent studies have demonstrated that 5-HT1AR knockout −/− and −/+ heterozygous mice demonstrate high levels of anxiety-like behaviors, while mice over expressing this receptor show reduced anxiety levels (11). Importantly, Gross and colleagues reported that the expression of 5-HT1ARs early during development, but not in adulthood, is critical to rescue the anxiety-related phenotype in knockout −/− mice (12). The above evidence from human and animal studies has lead to the proposal that low 5-HT1AR density early in life may increase vulnerability for anxiety-related disorders (11, 13).
Epidemiological data indicate a higher prevalence of mood and anxiety disorders in women compared to men, with these gender differences appearing first during puberty (14, 15), suggesting that, in light of the above evidence, lower 5-HT1AR density might be expected in women. However, previous studies reported either increased 5-HT1AR availability in women (16, 17), or no gender differences (18). In addition, higher 5-HT1AR availability measured by PET (19, 20) and increased 5-HT1ARs in vitro binding (1) has also been reported in depressed patients.
These results seem in contrast with the hypothesis that low 5-HT1AR density increases vulnerability for affective disorders. However, at least three factors need to be considered when interpreting these data. First, the reported studies were all performed in adults, while low 5-HT1AR expression during development, not in the adulthood, is hypothesized to be a critical risk factor for anxiety-related disorders (12). Second, the primary outcome of PET studies is receptor availability, measured by binding potential. Although receptor availability is directly proportional to receptor density, it also reflects competition at the binding sites between the endogenous neurotransmitter and PET radioligand. This competition can be influenced by several factors, including variations in endogenous synaptic 5-HT levels, receptor internalization and/or changes in receptor affinity for 5-HT, all of which would affect receptor occupancy, and consequently, receptor availability for the exogenous PET radioligand. Finally, in vitro binding studies in humans are often conducted in post mortem tissue from suicide victims, which may not be representative of generalized affective disorders in the population or the in vivo characteristics of the receptor.
Among environmental factors, traumatic experiences during childhood are consistently linked to an increased risk for developing anxiety-related neuropsychiatric diseases (21). For example, early loss of a parent leads to increased risk for anxiety, depression, and abnormalities of the hypothalamic-pituitary-adrenal (HPA) axis (22, 23). In this respect, rhesus macaques deprived of their parents during infancy provide a particularly germane animal model to investigate the long-term consequences of early-life adversity on 5-HT1AR expression. Previous studies show that peer-reared (PR) monkeys exhibit dysfunctions of the 5-HT system, HPA axis, and high levels of anxiety-like behaviors compared to animals reared with their mothers (MR) (24–26). We employed this model to study the long-term effects of early-life stress on 5-HT1AR availability using PET and [18F]FPWAY, an analogue of WAY100636 and a selective 5-HT1AR antagonist (27, 28).
By definition, receptor availability is proportional to total receptor density (Bmax) and radioligand affinity for the receptor (1/KDapp, where KDapp is the apparent dissociation constant). We employed an experimental design that allowed us to calculate Bmax and KDapp of [18F]FPWAY to determine the contribution of each parameter to potential receptor availability alterations. Importantly, KDapp represents the dissociation constant of radioligand (KD) adjusted for occupancy by the endogenous neurotransmitter (29). We hypothesized that juvenile PR monkeys would show decreased 5-HT1AR density and availability compared to MR monkeys and that the effect would be more pronounced in females.
Methods and Materials
Subjects
Protocols for the care and use of experimental animals were approved by the Institutional Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism, National Institute on Drug Abuse (NIDA) and National Institute of Child Health and Human Development, National Institutes of Health (NIH).
Twenty-one rhesus macaques (Macaca mulatta), representing 2 birth cohorts, were born and housed at the NIH Animal Center in Poolesville, MD. At birth, subjects were randomly assigned into two groups with different social and rearing experiences. MR monkeys (n=11; 6 males) were reared for the first 6 months of life with their biological or adopted mothers and fathers in social groups comprised of 8 to 12 adult females (about half of whom had same-aged infants) and 2 adult males.
PR monkeys (n=10; 5 males) were separated from their mothers and housed in an incubator for the first 14 days of life. From day 14 to 37, they were placed alone in a nursery cage and provided a blanket and a terry cloth–covered, rocking surrogate. At day 37, they were placed in a cage with 3 other age-mates with whom they thereafter had continuous access. At 6 months of age, MR subjects were removed from their mother and social group and housed together with the PR monkeys. Thereafter, both groups received identical treatment (26).
The study was conducted when the monkeys were about 24 months old (Table 1), corresponding to childhood in terms of human brain development (6–8 years old) (30). The animals were transported in groups of four to the NIDA-IRP in Baltimore, where they were housed in pairs for about one month, during which time the study was performed. One week before their transport, cerebrospinal fluid (CSF) was collected and assayed for baseline levels of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) [(26) and Supplemental Methods and Materials in Supplement 1].
Table 1.
Demographic and physiological measures
| Measures | Female MR | Female PR | Male MR | Male PR |
|---|---|---|---|---|
| Age (months) | 25.80 ± .86 | 25.60 ± .25 | 24.17 ± .48 | 26.00 ± .78 |
| Body weight (kg) * | 3.32 ± .21 | 3.10 ± .07 | 3.61 ± .12 | 3.74 ± .09 |
| CSF 5-HIAA (pmol/ml) # | 281.66 ± 24.26 | 212.73 ± 8.68 | 232.76 ± 34.54 | 256.35 ± 11.60 |
Results are presented as mean ± s.e.m.
Body weight was significantly smaller in females compared to males (ANOVA, p<0.005).
Baseline CSF 5-HIAA concentrations were significantly lower in PR compared to MR monkeys (ANOVA, p<0.05).
PET Imaging
Radiochemistry
[18F]FPWAY was produced as described (31). There were no differences in the specific activity across groups [males: 1521.2 ± 341.4, females: 2014.8 ± 331.9 (p>0.3); MR: 1454.5 ± 206.9, PR: 2081.5 ± 419.0 (p>0.2)].
Data acquisition
PET data were acquired on a Siemens Exact ECAT HR+ whole body tomograph (63 slices, center to center spacing of 2.4mm, with an in-plane resolution, full width at half maximum (FWHM), of 4.7mm at the center of the field of view and axial spatial resolution (or slice thickness) of 4.2mm in 3D mode. Before each radioligand administration, transmission scans were acquired with three rotating 68Ge-68Ga sources. Transmission scans were used to correct the emission scans for the attenuation of 511keV photons by body tissue and face mask. PET images were reconstructed from the raw data with a standard filtered back-projection algorithm and a RAMP filter.
Each monkey was anesthetized with 1.5mg/kg alfadolone and alfaxolone acetate (Saffan®, Arnolds Veterinary Products, Shropshire, U.K.), given intramuscularly. The monkey was intubated, an arterial line inserted for subsequent blood sampling and an intravenous line was set for the continuous intravenous infusion of 9–14mg/kg/h Saffan® to allow transport of the animal from the Animal Facility to the PET Center. Anesthesia was subsequently maintained throughout the PET study by 1.5–2.0% isoflurane.
An individually molded thermoplastic face mask was secured to a custom-made monkey head-holder attached to a backboard. The monkey’s head was positioned in the gantry with the aid of orthogonal laser lines. [18F]FPWAY, 6–18mCi, was administered intravenously as a bolus followed by a constant infusion (B/I) to reach an equilibrium state of radioactivity distribution. The bolus component delivered a volume of radiotracer equivalent to the volume that would be administered in the subsequent 60min infusion period (Kbol=60min). Acquisition of dynamic PET scans started with the beginning of the bolus component administration and continued for 180–210min. At 120min after the bolus infusion, an unlabelled FPWAY injection started as B/I (Kbol=40min) corresponding to the equilibrium infusion rate of 0.15nmol/kg/min. Specific activity in the second period was calculated by the ratio of the radioactivity infusion rate to the mass infusion rate. Blood samples were drawn at predetermined times from the arterial catheter with the first sample before dosing and subsequent samples following attainment of equilibrium (70 to 210min); plasma radioactivity and concentration of non-metabolized [18F]FPWAY not bound to plasma proteins were measured (32). Vital signs, including heart rate, EKG, respiration rate, end tidal CO2 and blood oxygen saturation (maintained above 95%) were continuously monitored during the study.
Magnetic resonance imaging (MRI)
Structural brain images were acquired on a 3.0 Tesla Siemens Magnetom Allegra MRI (Siemens Medical Solutions, Inc., Malvern, PA, U.S.A.). MRI and PET acquisitions were separated by at least 2 days to allow full recovery between studies [(34) and Supplement 1].
Co-registration and region-of-interest (ROI) placement
A ROI analysis was performed on brain regions previously implicated in emotional regulation and stress reactivity (21, 33) and known to be vulnerable to stress exposure (21, 34): amygdala (AMY), hippocampus (HC), dorsomedial prefrontal cortex (dmPFC) and anterior and medial cingulate cortex (ACC and MCC). We also included the midbrain raphe nuclei (RN), an area where 5-HT1ARs are present in high density and are organized as somatodendritic autoreceptors that help regulate 5-HT neurotransmission in other brain regions (35). ROIs were drawn on the T1-weighted MRI images of each monkey with reference to a stereotaxic atlas (36) (in Supplement 1, see Methods and Materials and Figure S1), and were co-registered to the PET images. For co-registration, MRI images of each monkey were re-sliced to the PET voxel size (1.14mm × 1.14mm × 2.45mm) and averaged between 90 and 120min after the start of [18F]FPWAY administration. PET images were manually aligned to their corresponding T1 MRI images (Fig. 1) using Fusion mode in PMOD v.2.7, (PMOD Technologies Ltd., Zurich, Switzerland). The obtained transformation parameters were applied to the respective re-sliced dynamic PET images. Time–activity curves were calculated based on the mean radioactivity (kBq/ml) for each ROI (Fig. 2a).
Figure 1.
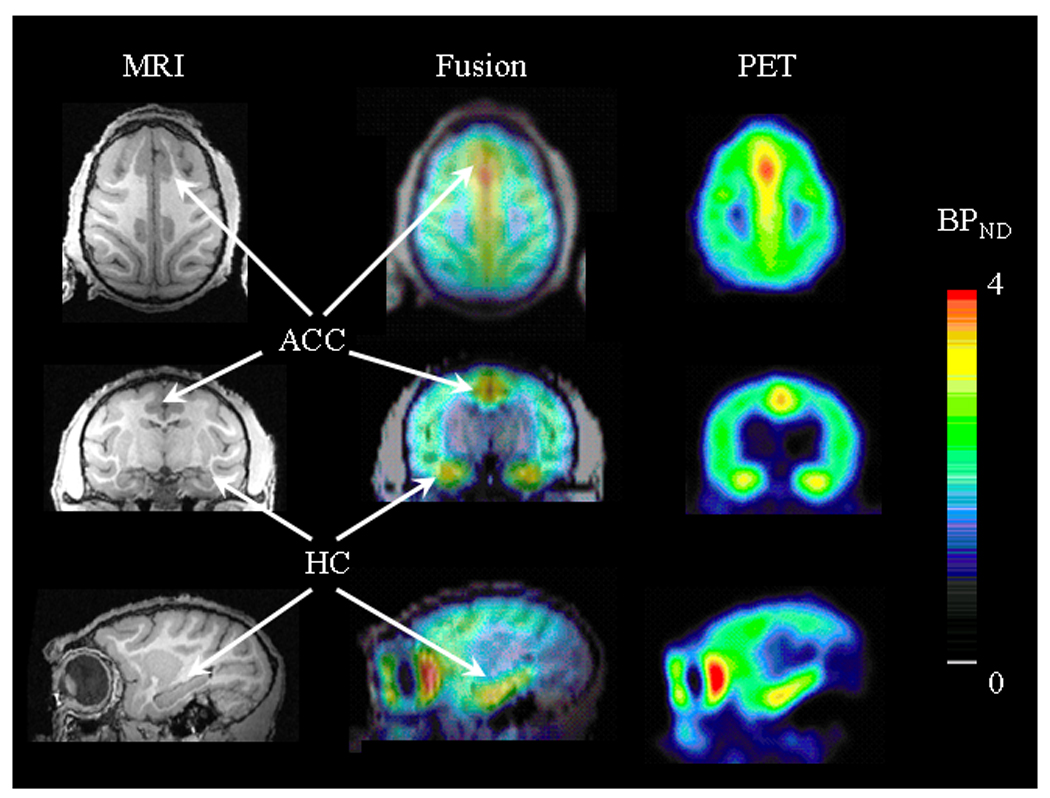
Representative images of Rhesus monkey brain in transaxial (top panel), coronal (middle panel) and sagittal (bottom panel) view. On the left – T1 MRI scans used for co-registration with parametric BPND maps for ROI placement (middle panel). Parametric BPND maps were calculated from bolus plus infusion PET studies with [18F]FPWAY and PMOD (right panel). Pseudocolor bar represents BPND values (arbitrary unit).
Figure 2.
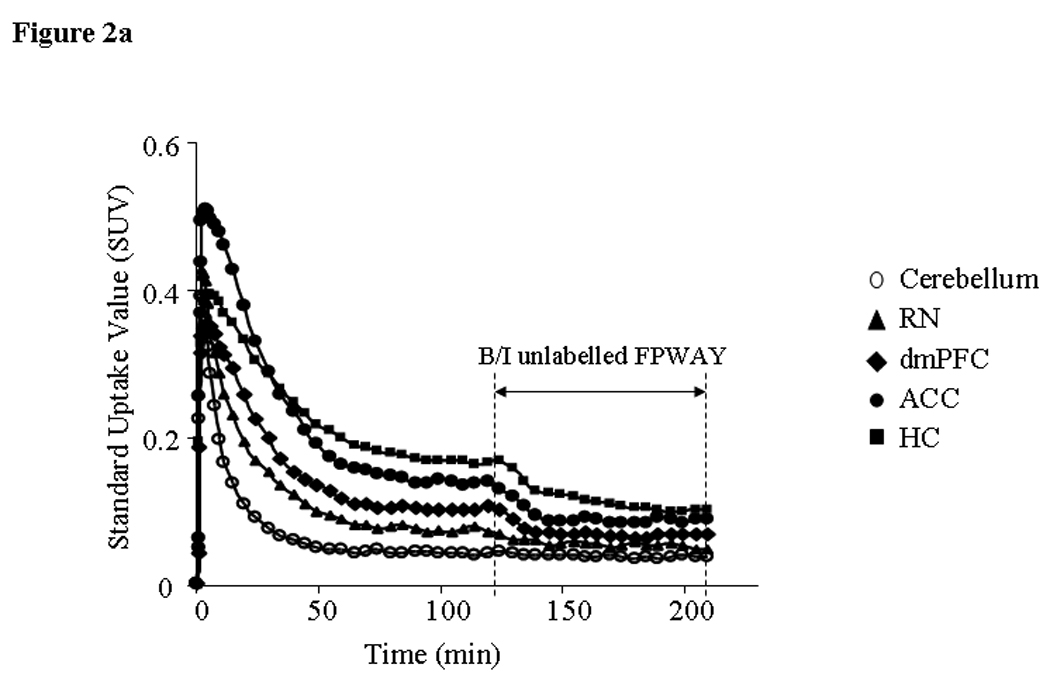
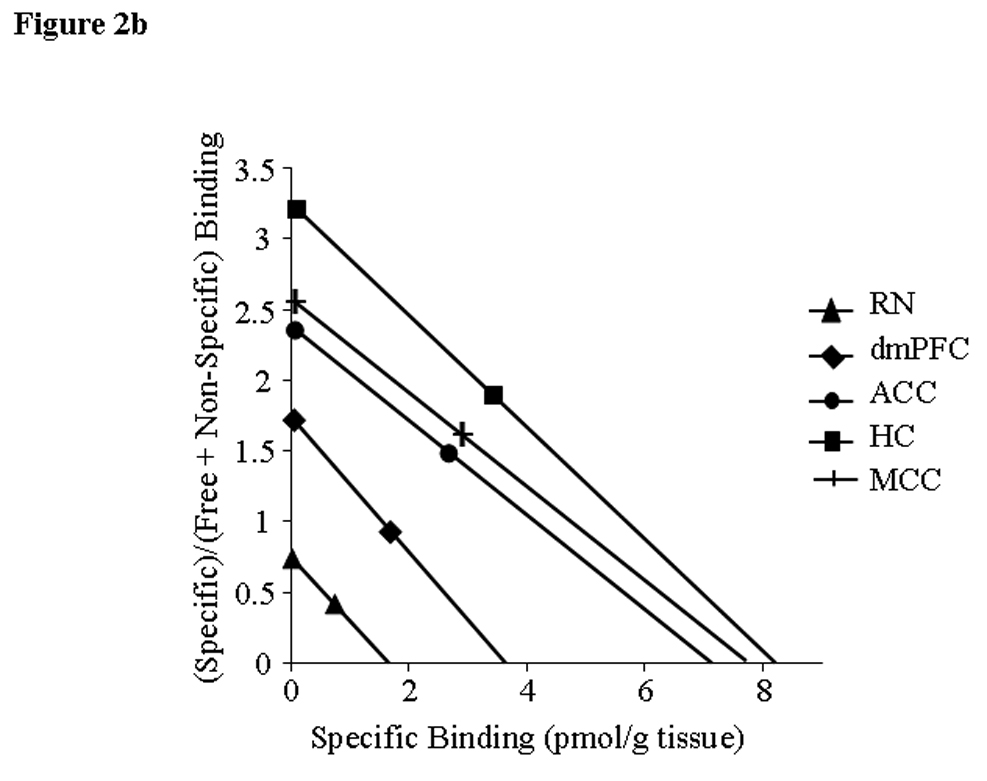
Time-activity curves (TAC) (a) and Scatchard plots (b) from brain areas of a single animal. A: Y-axis - standard uptake value (SUV) (fraction of injected dose/g body weight/g tissue), X-axis – time after [18F]FPWAY administration onset. [18F]FPWAY was injected as a bolus followed by a constant infusion (B/I) to reach radioactivity equilibrium distribution. 120 min after the B/I of [18F]FPWAY, unlabelled FPWAY was co-injected as bolus plus constant infusion (B/I) for another 90 min to reach a second equilibrium state. B: Two points for Scatchard analysis were obtained from TAC, averaging the data from 90 to 120 min after the start of [18F]FPWAY injection; second point - 50 to 90 min after the beginning of unlabelled FPWAY administration, respectively (see Methods for details). HC - hippocampus, MCC - medial cingulate cortex, ACC - anterior cingulate cortex, AMY - amygdala, dmPFC - dorsomedial prefrontal cortex, and RN - raphe nuclei (midbrain).
PET Data Analyses
Binding potential (BP) was calculated as BPND and BPF defined by consensus nomenclature for in vivo imaging of reversible radioligand binding (37). Here we report BPND calculated as the ratio of specifically bound radioligand concentration to that of non-displaceable radioligand concentration in tissue ((CROI − CND)/CND) at equilibrium (BPND = (CROI − CND)/CND = fNDBmax/KDapp) [results for BPF were similar and given in Supplement 1].
The 5-HT1AR Bmax and the KDapp of [18F]FPWAY in each brain region were calculated using Scatchard analysis (29). KDapp is defined as the in vivo KD adjusted for occupancy by 5-HT and by [18F]FPWAY non-displaceable fraction in tissue. After equilibrium was reached (first point: 90 to 120min after the start of [18F]FPWAY injection; second point 50 to 90min after the beginning of unlabelled [18F]FPWAY administration (Fig. 2b), specifically bound radioactivity was calculated as the difference between the total radioactivity measured in a brain ROI (CROI) and that in the cerebellum (CCB). (CROI − CCB) was plotted against (CROI − CCB)/ CCB. Due to technical problems, data acquisition for one male MR monkey was stopped after 120min and thus Bmax and KDapp could not be calculated for this animal.
Statistical Analyses
StatView 5.0.1 (SAS Institute, Inc., Cary, NC) was used for all statistical analyses. When ROIs were considered as a group, a multivariate analysis of variance (MANOVA) was conducted to assess the overall effects of rearing condition on Bmax, KDapp and BPND. Analyses were performed on the natural logarithm of the data to achieve homogeneity of variances, after adding 2.5 to all measures to ensure positivity. However, Tables and Figures report actual, not log-transformed values. A two-way analysis of variance (ANOVA) was conducted with rearing condition (MR or PR) and sex (male or female) as independent variables for BPND data in each ROI, as well as physiological measures. Significance was set at p<0.05 two-tailed alternatives, not adjusted for multiple comparisons.
Results
Effects of Rearing Conditions and Sex on Physiological Measures
ANOVA showed no difference in age across rearing group (F(1,17)=1.70, p>0.20) or sex (F(1,17)=0.34, p>0.33), as well as no difference in body weight across rearing conditions (F(1,17)=2.63, p>0.74). However, as expected, females had lower body weight than males (F(1,17)=12.62, p<0.003). CSF 5-HIAA concentrations were lower in PR animals (F(1,12)=5.23, p<0.05), but there was no difference between sexes (F(1,12)=1.42, p>0.26; Table 1).
Pattern of 5-HT1AR Distribution in the Brain and KDapp
Generally, the pattern of BPND values (Table 4) across studied brain areas within each of the four groups was similar to that previously reported in human PET experiments (18) and followed the pattern of 5-HT1AR brain distribution observed using in vitro binding assay of postmortem human brain tissue (38). BPND and Bmax values varied across studied brain areas. The HC and MCC showed the highest 5-HT1AR density and BPND values under both rearing conditions followed by MCC, ACC, AMY and DmPFC, respectively. The lowest BPND and Bmax values were determined in RN (Fig. 3, Table 2 and 4).
Table 4.
BPND values of [18F]FPWAY in brain regions in MR and PR juvenile rhesus monkeys
| ROI | Female MR | Female PR | % Difference in females |
Male MR | Male PR | % Difference in males |
% Difference MR-PR |
|---|---|---|---|---|---|---|---|
| HC | 3.05 ± .16 | 3.36 ± .19 | 10.16 | 3.08 ± .14 | 3.13 ± .13 | 1.62 | 5.91 |
| MCC | 3.03 ± .24 | 3.25 ± .19 | 7.26 | 3.31 ± .16 | 2.71 ± .15 | −18.13^ | −6.35 |
| ACC | 2.64 ± .22 | 2.95 ± .16 | 11.74 | 2.97 ± .14 | 2.47 ± .13 | −16.84^ | −4.04 |
| AMY | 1.59 ± .09 | 1.65 ± .09 | 3.77 | 1.67 ± .07 | 1.60 ± .12 | −4.19 | −0.86 |
| dmPFC | 1.43 ± .07 | 2.23 ± .22 | 55.94** | 1.67 ± .13 | 1.67 ± .16 | 0.00 | 24.82* |
| RN | .80 ± .09 | .80 ± .06 | 0.00 | .91 ± .09 | .77 ± .04 | −15.38 | −8.04 |
Abbreviations are as in Table 2. Results are presented as mean ± s.e.m. % Difference is calculated as (mean values for MR group − mean values for PR group)/mean values for MR group*100.
MANOVA across all ROI showed a significant rearing effect, p<0.01.
Rearing effect in the dmPFC, ANOVA, p<0.02.
Rearing effect in the female group, unpaired t-test, p<0.04.
Rearing effect in the male group, unpaired t-test, p<0.04.
Figure 3.
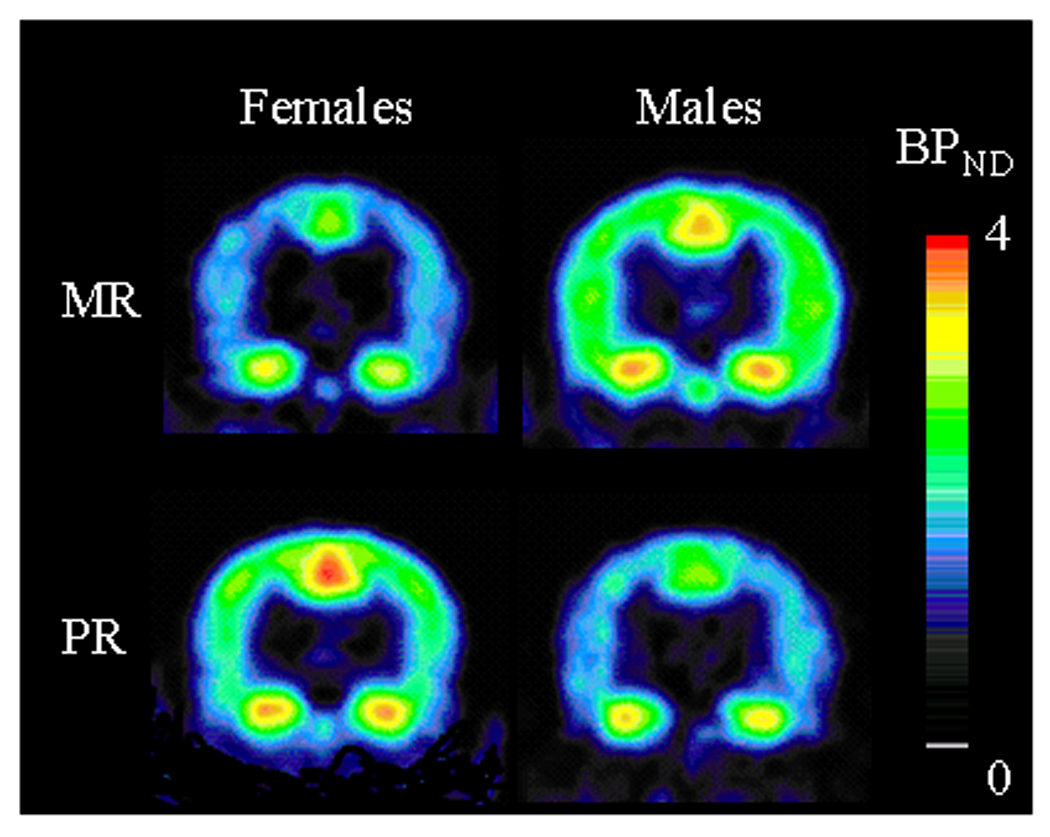
Coronal view of BPND parametric maps obtained with [18F]FPWAY in four representative animals. Top row: MR animals - bottom row: PR animals - left column: females - right column: males. Images correspond to coronal slices at the level 15–16 mm from the interaural line(36). Pseudocolor bar represents BPND values (arbitrary unit).
Table 2.
Bmax (pmol/mL) values measured by [18F]FPWAY in PR and MR juvenile rhesus monkeys
| ROI | Female MR | Female PR | % Difference in females |
Male MR | Male PR | % Difference in males |
% Difference MR - PR |
|---|---|---|---|---|---|---|---|
| HC | 8.00 ± 2.07 | 5.46 ± 1.64 | −31.75 | 13.30 ± 3.54 | 6.14 ± 2.15 | −53.83 | −45.44 |
| MCC | 5.98 ± 1.75 | 4.42 ± 1.07 | −26.09 | 9.80 ± 2.11 | 5.61 ± 1.68 | −42.76 | −36.44 |
| ACC | 5.14 ± 1.34 | 3.84 ± .92 | −25.29 | 8.20 ± 1.24 | 5.20 ± 1.62 | −36.59 | −32.22 |
| AMY | 4.34 ± 1.64 | 2.61 ± .96 | −39.86 | 7.32 ± 1.73 | 5.38 ± 2.27 | −26.50 | −31.41 |
| dmPFC | 2.26 ± 0.79 | 2.96 ± .81 | 30.97 | 4.36 ± .86 | 3.31 ± 1.23 | −24.08 | −5.32 |
| RN | 1.48 ± 0.50 | 1.00 ± .20 | −32.43 | 2.44 ± .44 | 1.38 ± .40 | −43.44 | −3.57 |
Data represent values averaged for right and left hemispheres for all animals in the group. HC - hippocampus, MCC - medial cingulate cortex, ACC - anterior cingulate cortex, AMY - amygdala, dmPFC - dorsomedial prefrontal cortex, RN – raphe nuclei (midbrain). Results are presented as mean ± s.e.m. % Difference is calculated as (mean MR values − mean PR values)/mean MR values*100. MANOVA across all ROIs showed a tendency for a significant rearing effect, p<0.09.
The AMY showed the highest KDapp values for both rearing conditions followed by HC (Table 3, but see PR males). Compared to the AMY, KDapp values were 30–50% lower in the other regions (MCC, ACC, dmPFC, RN), which also showed limited variability (2–12%) across brain areas within each experimental group.
Table 3.
KDapp values (pmol/mL) measured by [18F]FPWAY in PR and MR juvenile rhesus monkeys
| ROI | Female MR | Female PR | % Difference in females |
Male MR | Male PR | % Difference in males |
% Difference MR - PR |
|---|---|---|---|---|---|---|---|
| HC | 2.62 ± .70 | 1.50 ± .41 | −42.75 | 4.08 ± 1.07 | 1.96 ± .72 | −51.96 | −48.36* |
| MCC | 1.94 ± .56 | 1.22 ± .26 | −37.11 | 2.84 ± .65 | 2.06 ± .62 | −27.46 | −31.38 |
| ACC | 1.90 ± .53 | 1.20 ± .26 | −36.84 | 2.64 ± .47 | 1.98 ± .61 | −25.00 | −29.89 |
| AMY | 2.80 ± 1.17 | 1.69 ± .37 | −39.64 | 4.28 ± 1.15 | 3.61 ± 1.67 | −15.65 | −25.07 |
| dmPFC | 1.60 ± .44 | 1.23 ± .26 | −23.13 | 2.46 ± .46 | 1.84 ± .69 | −25.20 | −24.14 |
| RN | 1.72 ± .54 | 1.09 ± .17 | −36.63 | 2.46 ± .31 | 1.83 ± .55 | −25.61 | −30.05 |
Abbreviations are as in Table 2. Results are presented as mean ± s.e.m. % Difference is calculated as (mean values for MR group − mean values values for PR group)/mean values for MR group *100. MANOVA across all ROIs showed a significant rearing effect, p<0.05.
Rearing effect in the HC, ANOVA, p<0.05.
Effect of Rearing Condition and Sex on 5-HT1AR Bmax and KDapp
For the 10 MR and 10 PR juvenile monkeys analyzed ([18F]FPWAY specific activity was not available for one MR male monkey), a MANOVA for Bmax values across all ROIs revealed a trend for lower 5-HT1AR density (F(6,11)=2.60, p>0.08, Table 2) and significantly lower KDapp values (F(6,11)=4.27, p<0.02, Table 3) in PR animals. There was no effect of sex on Bmax or KDapp (F(6,11)=0.99, p>0.47 and F(6,11)=0.93, p>0.51, respectively) nor was there a rearing × sex interaction (F(6,11)=0.55, p>0.76 and F(6,11)=0.91, p>0.52 for Bmax and KDapp , respectively).
Similarly, MANOVA for KDapp across all ROIs revealed lower values in PR animals (F(6,11)=4.27, p<0.02, Table 3). Again, there was no effect of sex (F(6,11)=0.93, p>0.51) or rearing × sex interaction (F(6,11)=0.91, p>0.52). Two-way ANOVAs on each ROI revealed a significant rearing effect in the HC (F(1,16)=4.80, p<0.05); no sex (F(1,16)=1.20, p>0.28) or rearing × sex interaction (F(1,16)=0.32, p<0.57) effects were found. There was no significant correlation between mean KDapp (or Bmax values in any brain ROI) and 5-HIAA concentrations.
Effect of Rearing Condition and Sex on 5-HT1AR Availability
Peer-rearing affected BPND values in several brain regions as demonstrated by MANOVA (F(6,12)=5.74, p<0.006), in the absence of a main effect of sex (F(6,12)=0.38, p>0.87) or a rearing × sex interaction (F(6,12)=1.63, p>0.22). Two-way ANOVAs on each ROI revealed a significant rearing effect in the dmPFC (F(1,17)=6.79, p<0.02) but no sex effect (F(1,17)=0.97, p>0.33). There was a significant rearing × sex interaction in the following three brain regions: dmPFC (F(1,17)=6.81, p<0.02), MCC (F(1,17)=4.79, p<0.05) and ACC (F(1,17)=5.88, p<0.03), but no main effect of rearing or sex [MCC rearing (F(1,17)=0.96, p>0.34), sex (F(1,17)=0.49, p>0.49); ACC rearing (F(1,17)=0.32, p>0.58), sex (F(1,17)=0.18, p>0.68)]. Because of the rearing × sex interaction, we conducted an unpaired t-test for each sex separately.
In PR females, BPND values were greater in the dmPFC compared to control females (t(1,8)=−3.73, p<0.006; Table 4 and Fig. 3), while in males there was no significant effect of rearing condition in this area (t(1,9)=0.00, p>0.99). However in males, peer-rearing induced a significant reduction in BPND values in the MCC (t(1,9)=2.66, p<0.03) and the ACC (t(1,9)=2.55, p<0.04; Table 4 and Fig. 3), with no significant effect in female monkeys in either of these areas (t(1,8)=−0.74, p>0.48 and t(1,8)= −1.12, p>0.29, respectively). There was no significant correlation between 5-HIAA concentration and BPND.
Discussion
The present study shows that early-life stress as a result of parental deprivation affects 5-HT1ARs density and radioligand affinity. In both males and females, peer-rearing induced an overall decrease in Bmax and in KDapp. The exception was the dmPFC in females, where Bmax values were elevated. Our results are consistent with the hypothesis that exposure to an adverse environment during infancy has long-term consequences on the 5-HT system. To our knowledge, these findings provide the first evidence in nonhuman primates for such developmental changes in an aspect of the 5-HT system that has been strongly linked to anxiety and depression.
Overall our findings in nonhuman primates are consistent with and extend the data from human PET and in vitro binding studies of post mortem brain tissue suggesting a down-regulation of 5-HT1ARs associated with anxiety disorders (39, 40). The present results are also consistent with previous evidence indicating decreased 5-HT1AR availability using PET in adult females macaques with signs of behavioral “depression”(41), and decreased 5-HT1AR binding and mRNA expression in vitro in the HC in marmoset monkeys exposed to early-life stress (42). Moreover, low 5-HT1AR binding in the HC and ACC was found in male rats exposed to early-life stress (43).
Surprising, early-life stress was associated with increased 5-HT1AR density the in the dmPFC in female monkeys. Using structural MRI in the same cohort of monkeys, we recently reported (34) increased volume of the dmPFC and cingulate cortex in male and female PR animals. As such, it is possible that partial volume effect contributed to the present result. However, lower Bmax values in females PR monkeys were found in the ACC, a region that also showed increased gray matter volume in PR animals, suggesting that any structural changes in the dmPFC may not fully account for the present Bmax differences. Gender differences in PFC 5-HT1AR density have been observed in humans using in vitro binding assays from postmortem brain tissue (44). Moreover, specific changes in 5-HT1AR protein levels were found in PFC of depressed female patients (45). As a part of the ‘medial prefrontal network’, the dmPFC, together with the cingulate cortex, is thought to modulate activity in limbic structures (e.g. AMY and HC) known to be important for the processing of emotional information (46, 47). Taken together, these data suggest that gender differences in brain regions involved in the cognitive control of emotional regulation are present on a molecular level, and may contribute to the increased vulnerability to affective disorders in women.
In addition to the tendency for an overall decrease in Bmax values, we also found an increased radioligand affinity for 5-HT1AR in PR animals, as shown by the decreased KDapp values, particularly in the HC. Reduced KDapp values reflect less competition between radioligand and endogenous 5-HT at the receptor sites, which could be related to one or more of the following: lower baseline 5-HT levels, reduced 5-HT affinity for 5-HT1AR as a result of conformational receptor alterations, or such other factors as receptor internalization (48). Unfortunately, PET analysis limitations do not allow us to define the exact reason for the observed changes in radioligand affinity. Nevertheless, the decreased CSF 5-HIAA concentrations found in PR animals in the present study, and throughout development by earlier findings (24, 26), support the hypothesis of lower brain 5-HT levels in PR animals. However, since neither KDapp nor CSF 5-HIAA concentrations represent direct measures of 5-HT levels in the brain, future investigations using other techniques, such as microdialysis, are warranted to confirm that early-life stress affects endogenous 5-HT in PR animals. In rodents, early-life stress has been shown to affect both basal 5-HT and 5-HIAA levels in several brain regions (49), and previous data obtained by in vitro assays in rodents exposed to chronic stress showed decreased 5-HT1AR density and increased 5-HT1AR affinity in the HC (50), indicating that stress might induce transcriptional or post-transcriptional effects on 5-HT1AR in this region.
Our results on 5-HT1AR availability calculated as BPND (and BPF, see Supplement 1) indicate that the effect of peer-rearing on BPND was different in males and females. Bmax values were decreased in most brain regions in male and female PR animals. However, while overall lower 5-HT1AR availability was found in PR males, it was increased in PR females compared to control MR animals. These gender differences in receptor availability may be related to reported differences in SSRI efficacy between men and women (51). As mentioned in the Introduction, differences in 5-HT1AR availability can be linked to changes in receptor density, radioligand apparent affinity (1/KDapp) or both as demonstrated in the current study. Our results further emphasize the importance of considering changes in receptor availability along with concomitant changes in 5-HT1AR density and in vivo affinity.
Consequences of the findings
Overall these data suggest that early-life stress is associated with changes in the development of the 5-HT system in brain regions considered to be critically involved in major depression and to be modulated by antidepressant treatment (47). An impaired interaction between neural networks involved in emotional and cognitive processing is proposed as a key dysfunction in mood disorders (46, 47). Specifically, it is suggested that the dmPFC, dorsal ACC and posterior cingulate cortex are part of a “dorsal network” involved in emotional and cognitive deficits, while the HC and the AMY are part of a “ventral network” associated with vegetative and somatic symptoms of mood disorders; the (rostral) ACC, which is connected to both networks, is thought to have a more regulatory function (46). Moreover, antidepressant treatment, known to increase 5-HT levels, is known to modulate neural activity in these regions/networks (52).
In this context, our data indicate that peer-rearing stress affects 5-HT1AR density and in vivo affinity in brain regions known to play a key role in affective disorders. Affinity changes, particularly in the HC, may be important considering that the SSRI efficacy seems to be mediated by 5-HT1AR and by neurogenesis in the HC (53). It is also possible that region- and sex-specific changes in 5-HT1AR function during development have important consequences on cognitive abilities (54). The 5-HT system is implicated in the behavioral and brain responsiveness to punishment (55). Modulation of the 5-HT system has been shown to affect neural activity in dmPFC during negative feedback processing in humans (56) and a disrupted top-down control by dmPFC over AMY has been proposed to underlie some of the negative feedback deficits reported in depression (57). Recently, increased responding to threat (and reward) has been reported specifically in two-year old PR males (58), supporting the hypothesis that 5-HT changes may affect the processing of negative information. However, it is unclear if and how 5-HT1AR alterations during development may be related to cognitive differences in humans and potentially to increased vulnerability for affective disorders later in life.
Limitations of the study
We found no differences in Bmax values between male and female monkeys in either the MR or PR group. Although the number of subjects (n=21) was relatively high for a nonhuman primate study, it may still not have been sufficient to demonstrate more subtle differences between males and females. Previous PET studies in humans showed either no changes or elevated 5-HT1AR availability in healthy adult women (16–18). Conversely, there are as yet no data regarding the levels of brain 5-HT1AR in children, which due to issues of radioactivity administration, are not likely to be easily obtained. Gender differences in 5-HT1AR in the adult nonhuman primate brain is also not available at this point in time. However, since in vitro data from rodents show sex differences in 5-HT1AR binding both in adults and throughout development (59, 60), it is possible that sex specific differences are also present during development in nonhuman primates. Thus, future in vitro binding and microdialysis studies will be important to confirm our PET results and to investigate possible sex differences in 5-HT1AR density and 5-HT levels occurring during development.
In conclusion, we provide the first evidence in nonhuman primates that a model of early-life stress affects 5-HT1AR density and in vivo affinity in juvenile monkeys. Specifically, we found decreased 5-HT1AR density and KDapp in PR animals. However, in females, early-life stress induced an increase in 5-HT1AR density in the dmPFC, a brain region involved in emotional regulation. Overall these findings support the hypothesis that low 5-HT1AR density during development may be related to an increased risk for affective disorders. Moreover, sex difference may be present early in development and need to be considered in the context of the increased vulnerability for mood and anxiety disorders reported in women.
Supplementary Material
Acknowledgments
The authors thank Karen McCullough and Larry Reyes for providing excellent technical assistance, Eric Singley, Stephen Lindell, and Melanie Schwandt for physiological data collection and analyses and Eliscia Smith, Varughese Kurian and Ira Baum for assisting during MR and PET scanning. We gratefully acknowledge Carlo Contoreggi for financial support and early conversations during the study inception. Drs Carson, Jagoda, Lang and Stein were involved in designing and conducting the PET study. Drs Higley, Suomi, and Barr oversaw the rearing of the monkeys and the collection of physiological data. Dr Heilig oversaw the analyses of physiological data.
This work was supported by the Intramural Research Programs of the National Institute on Alcohol Abuse and Alcoholism, National Institute on Drug Abuse, National Institute of Child Health and Human Development and the National Institute of Biomedical Imaging and Bioengineering.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001 Dec;25(6):892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 2.Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004 Apr;9(4):386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- 3.Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007 Oct;34(7):865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. International Journal of Neuropsychopharmacology. 2008;11:465–476. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- 5.Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007 May 1;61(9):1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, et al. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008 Sep;193(3):229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- 7.Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, et al. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004 Jan 21;24(3):589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000 Feb;57(2):174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 9.Czesak M, Lemonde S, Peterson EA, Rogaeva A, Albert PR. Cell-specific repressor or enhancer activities of Deaf-1 at a serotonin 1A receptor gene polymorphism. J Neurosci. 2006 Feb 8;26(6):1864–1871. doi: 10.1523/JNEUROSCI.2643-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strobel A, Gutknecht L, Rothe C, Reif A, Mossner R, Zeng Y, et al. Allelic variation in 5-HT1A receptor expression is associated with anxiety- and depression-related personality traits. J Neural Transm. 2003 Dec;110(12):1445–1453. doi: 10.1007/s00702-003-0072-0. [DOI] [PubMed] [Google Scholar]
- 11.Toth M. 5-HT1A receptor knockout mouse as a genetic model of anxiety. Eur J Pharmacol. 2003 Feb 28;463(1–3):177–184. doi: 10.1016/s0014-2999(03)01280-9. [DOI] [PubMed] [Google Scholar]
- 12.Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002 Mar 28;416(6879):396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- 13.Lesch KP, Gutknecht L. Focus on The 5-HT1A receptor: emerging role of a gene regulatory variant in psychopathology and pharmacogenetics. Int J Neuropsychopharmacol. 2004 Dec;7(4):381–385. doi: 10.1017/S1461145704004845. [DOI] [PubMed] [Google Scholar]
- 14.Garber J. Depression in children and adolescents: linking risk research and prevention. Am J Prev Med. 2006 Dec;31(6) Suppl 1:S104–S125. doi: 10.1016/j.amepre.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Sloan DM, Kornstein SG. Gender differences in depression and response to antidepressant treatment. Psychiatr Clin North Am. 2003 Sep;26(3):581–594. doi: 10.1016/s0193-953x(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 16.Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, et al. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. 2008 Feb 1;39(3):1408–1419. doi: 10.1016/j.neuroimage.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002 Nov 8;954(2):173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- 18.Stein P, Savli M, Wadsak W, Mitterhauser M, Fink M, Spindelegger C, et al. The serotonin-1A receptor distribution in healthy men and women measured by PET and [carbonyl-(11)C]WAY-100635. Eur J Nucl Med Mol Imaging. 2008 Jun 10; doi: 10.1007/s00259-008-0850-x. [DOI] [PubMed] [Google Scholar]
- 19.Miller JM, Brennan KG, Ogden TR, Oquendo MA, Sullivan GM, Mann JJ, et al. Elevated serotonin 1A binding in remitted major depressive disorder: evidence for a trait biological abnormality. Neuropsychopharmacology. 2009 Sep;34(10):2275–2284. doi: 10.1038/npp.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006 Jan 15;59(2):106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003 Jan–Mar;27(1–2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs JR, Bovasso GB. Re-examining the long-term effects of experiencing parental death in childhood on adult psychopathology. J Nerv Ment Dis. 2009 Jan;197(1):24–27. doi: 10.1097/NMD.0b013e3181927723. [DOI] [PubMed] [Google Scholar]
- 23.Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008 Jun 15;63(12):1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry. 1992 Jul 15;32(2):127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- 25.Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, et al. Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. J Neurosci. 2006 Apr 26;26(17):4638–4643. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shannon C, Schwandt ML, Champoux M, Shoaf SE, Suomi SJ, Linnoila M, et al. Maternal absence and stability of individual differences in CSF 5-HIAA concentrations in rhesus monkey infants. Am J Psychiatry. 2005 Sep;162(9):1658–1664. doi: 10.1176/appi.ajp.162.9.1658. [DOI] [PubMed] [Google Scholar]
- 27.Jagoda EM, Lang L, Tokugawa J, Simmons A, Ma Y, Contoreggi C, et al. Development of 5-HT1A receptor radioligands to determine receptor density and changes in endogenous 5-HT. Synapse. 2006 May;59(6):330–341. doi: 10.1002/syn.20246. [DOI] [PubMed] [Google Scholar]
- 28.Carson RE, Lang L, Watabe H, Der MG, Adams HR, Jagoda E, et al. PET evaluation of [(18)F]FCWAY, an analog of the 5-HT(1A) receptor antagonist, WAY-100635. Nucl Med Biol. 2000 Jul;27(5):493–497. doi: 10.1016/s0969-8051(00)00118-9. [DOI] [PubMed] [Google Scholar]
- 29.Carson RE, Channing MA, Der MG, Herscovitch P, Eckelman WC. Scatchard analysis with bolus/infusion administration of [11C]raclopride; amphetamine effects in anesthetized monkeys. In: Senda M, Kimura Y, Herscovitch P, editors. Brain imaging using PET. San Diego: Academic; 2002. pp. 63–96. [Google Scholar]
- 30.Malkova L, Heuer E, Saunders RC. Longitudinal magnetic resonance imaging study of rhesus monkey brain development. Eur J Neurosci. 2006 Dec;24(11):3204–3212. doi: 10.1111/j.1460-9568.2006.05175.x. [DOI] [PubMed] [Google Scholar]
- 31.Lang L, Jagoda E, Schmall B, Sassaman M, Ma Y, Eckelman WC. Fluoro analogs of WAY-100635 with varying pharmacokinetics properties. Nucl Med Biol. 2000 Jul;27(5):457–462. doi: 10.1016/s0969-8051(00)00111-6. [DOI] [PubMed] [Google Scholar]
- 32.Ma Y, Lang L, Kiesewetter DO, Jagoda E, Sassaman MB, Der M, et al. Liquid chromatography-tandem mass spectrometry identification of metabolites of two 5-HT1A antagonists, N-[2-[4-(2-methoxylphenyl)piperazino]ethyl]-N-(2-pyridyl) trans- and cis-4-fluorocyclohexanecarboxamide, produced by human and rat hepatocytes. J Chromatogr B Biomed Sci Appl. 2001 May 5;755(1–2):47–56. doi: 10.1016/s0378-4347(00)00610-1. [DOI] [PubMed] [Google Scholar]
- 33.Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006 Jul;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- 34.Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein EA. Early life stress induces long-term morphological changes in primate brain. Arch Gen Psychiatry. 2009;66(6):658–665. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res. 2003 Sep–Oct;37(5):357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 36.Saleem KS, Logothetis NK. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in stereotaxic coordinates. Academic Press; 2006. p. 336. [Google Scholar]
- 37.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007 Sep;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 38.Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, et al. Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]way-100635. Brain Res. 1997 Jan 16;745(1–2):96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- 39.Akimova E, Lanzenberger R, Kasper S. The Serotonin-1A Receptor in Anxiety Disorders. Biol Psychiatry. 2009 May 6; doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009 May;88(1):17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, et al. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Arch Gen Psychiatry. 2006 Apr;63(4):396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- 42.Law AJ, Pei Q, Walker MP, Gordon-Andrews H, Weickert CS, Feldon J, et al. Early Parental Deprivation in the Marmoset Monkey Produces Long-Term Changes in Hippocampal Expression of Genes Involved in Synaptic Plasticity and Implicated in Mood Disorder. Neuropsychopharmacology. 2008 July;:1–14. doi: 10.1038/npp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leventopoulos M, Russig H, Feldon J, Pryce CR, Opacka-Juffry J. Early deprivation leads to long-term reductions in motivation for reward and 5-HT1A binding and both effects are reversed by fluoxetine. Neuropharmacology. 2009 Mar;56(3):692–701. doi: 10.1016/j.neuropharm.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995 Aug 7;688(1–2):121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- 45.Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, et al. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol. 2009 Mar;12(2):155–168. doi: 10.1017/S1461145708009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deckersbach T, Dougherty DD, Rauch SL. Functional imaging of mood and anxiety disorders. J Neuroimaging. 2006 Jan;16(1):1–10. doi: 10.1177/1051228405001474. [DOI] [PubMed] [Google Scholar]
- 47.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008 Sep;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmer L, Riad M, Rbah L, Belkacem-Kahlouli A, Le Bars D, Renaud B, et al. Toward brain imaging of serotonin 5-HT1A autoreceptor internalization. Neuroimage. 2004 Jul;22(3):1421–1426. doi: 10.1016/j.neuroimage.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 49.Arborelius L, Eklund MB. Both long and brief maternal separation produces persistent changes in tissue levels of brain monoamines in middle-aged female rats. Neuroscience. 2007 Mar 16;145(2):738–750. doi: 10.1016/j.neuroscience.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Daniels WM, Pietersen CY, Carstens ME, Daya S, Stein D. Overcrowding induces anxiety and causes loss of serotonin 5HT-1a receptors in rats. Metab Brain Dis. 2000 Dec;15(4):287–295. doi: 10.1023/a:1011123208674. [DOI] [PubMed] [Google Scholar]
- 51.Simon NM, Zalta AK, Worthington JJ, 3rd, Hoge EA, Christian KM, Stevens JC, et al. Preliminary support for gender differences in response to fluoxetine for generalized anxiety disorder. Depress Anxiety. 2006;23(6):373–376. doi: 10.1002/da.20184. [DOI] [PubMed] [Google Scholar]
- 52.Anand A, Li Y, Wang Y, Gardner K, Lowe MJ. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an FMRI study. J Neuropsychiatry Clin Neurosci. 2007 Summer;19(3):274–282. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003 Aug 8;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 54.Borg J. Molecular imaging of the 5-HT(1A) receptor in relation to human cognition. Behav Brain Res. 2008 Dec 16;195(1):103–111. doi: 10.1016/j.bbr.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008 Jan;12(1):31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Evers EA, Cools R, Clark L, van der Veen FM, Jolles J, Sahakian BJ, et al. Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology. 2005 Jun;30(6):1138–1147. doi: 10.1038/sj.npp.1300663. [DOI] [PubMed] [Google Scholar]
- 57.Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008 Sep 1;42(3):1118–1126. doi: 10.1016/j.neuroimage.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nelson EE, Herman KN, Barrett CE, Noble PL, Wojteczko K, Chisholm K, et al. Adverse Rearing Experiences Enhance Responding to Both Aversive and Rewarding Stimuli in Juvenile Rhesus Monkeys. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johns JM, Lubin DA, Lieberman JA, Lauder JM. Developmental effects of prenatal cocaine exposure on 5-HT1A receptors in male and female rat offspring. Dev Neurosci. 2002;24(6):522–530. doi: 10.1159/000069363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schiller L, Jahkel M, Oehler J. The influence of sex and social isolation housing on pre- and postsynaptic 5-HT1A receptors. Brain Res. 2006 Aug 4;1103(1):76–87. doi: 10.1016/j.brainres.2006.05.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


