Abstract
Background
Heart rate variability (HRV) measures homeostatic regulation of the autonomic nervous system in response to perturbation, and has been previously shown to quantify risk for cardiac events. In spite of known interactions between stress vulnerability, psychiatric illness, and cardiac health, however, to our knowledge this is the first study to directly compare the value of laboratory HRV in predicting autonomic modulation of “real-world” emotional stress.
Methods
We recorded ECG on 56 subjects: first, within the laboratory, and then during an acute emotional stressor: a first-time skydive. Laboratory sessions included two five-minute ECG recordings separated by one ambulatory 24-hour recording. To test the efficacy of introducing a mild emotional challenge, during each of the five-minute laboratory recordings subjects viewed either aversive or benign images. Following the laboratory session, subjects participated in the acute stressor wearing a holter ECG. Artifact-free ECGs (N=33) were analyzed for HRV, then statistically compared across laboratory and acute stress sessions.
Results
There were robust correlations (r=0.7-0.8) between the laboratory and acute stress HRV, indicating that the two most useful paradigms (long-term wake, followed by short-term challenge) also were most sensitive to distinct components of the acute stressor: the former correlated with the fine-tuned regulatory modulation occurring immediately prior and following the acute stressor, while the latter correlated with gross amplitude and recovery.
Conclusions
Our results confirmed the efficacy of laboratory-acquired HRV in predicting autonomic response to acute emotional stress, and suggest that ambulatory and challenge protocols enhance predictive value.
Keywords: heart rate variability, stress, emotion, fear, anxiety, autonomic, schizophrenia, depression
Introduction
The autonomic nervous system functions as a negative feedback loop, in which excitatory and inhibitory components are modulated by the sympathetic and parasympathetic nervous systems, respectively. The dynamic interplay between autonomic components enables efficient cardiovascular responses to both endogenous and exogenous influences, and can be quantified using heart rate variability analysis (HRV), which describes changes in the instantaneous heart rate over different time-scales. The most widely used method for HRV analysis is power spectrum density analysis, which measures heart rate changes in the frequency domain. Studies isolating the sympathetic and parasympathetic components using pharmacological blockers have established that the high frequency bandwidth (HF; 0.15-0.5 Hz) is predominantly associated with parasympathetic nervous system while the low frequency bandwidth (LF; 0.04-0.15 Hz) includes contributions from both the parasympathetic and sympathetic nervous systems(1-3). Since LF is not solely influenced by sympathetic activity, the LF/HF ratio is typically used as a measure of the sympathovagal balance(4). Clinical research has shown that HRV shows potential applications as a diagnostic instrument, predicting risk for sudden cardiac death(5) and hypertension(6).
Though less widely studied than physical stress reactivity, in recent years cardiology has displayed a renewed interest in the relationship between cardiovascular disease and emotional stress reactivity. Emotional arousal(7, 8), as well as psychiatric illnesses associated with dysregulation of emotional arousal, such as trait (9, 10) and pathological (11, 12) anxiety and depression, panic disorder(13, 14), and paranoid schizophrenia(15), are known to be associated with lowered HRV. This association has direct implications for cardiovascular health; for example, emotional stress reactivity has been linked to risk of angina(16), myocardial ischemia in patients with and without coronary artery disease (17-19), and left ventricular dysfunction(20). Yet despite the presumed ubiquity and deleterious effects of emotional stress in modern life, to our knowledge this is the first study that directly measures the degree to which HRV calculated from ECG signals obtained in a clinician’s office can actually predict autonomic reactivity to emotional stress.
This may be a consequence of the fact that emotional stress is significantly more difficult to experimentally induce than its physical counterpart, exercise. Emotion studies in non-patient populations typically rely upon three different types of paradigms: personality measures such as trait anxiety, anger, depression, or perceived stress; naturalistic studies that monitor individuals already undergoing a “real-world” stressor such as a natural disaster; or experimentally inducing emotional stress under laboratory conditions, such as with timed computer tasks. Each of these is far from ideal from both scientific and clinical perspectives. Personality measures depend upon a patient’s self-report, “real-world” stressors are by their very nature uncontrolled and therefore differences in the stressor severity can bias interpretation of individual variability, and laboratory stressors—while affording strong experimental control—normally have no genuine life consequences and therefore may not be a realistic proxy for even mild psychological trauma. Our general experimental aim was therefore to test the degree to which laboratory-obtained HRV correlates with “real-world” emotional stress reactivity, using a paradigm that reliably induced a powerful emotional response without sacrificing experimental control. Within this framework, we also sought to establish more specifically which types of acquisition parameters were most predictive.
Researchers have used both “short-term” (five to 30 minute) and “long-term” (24 to 48 hour) measurements to assess the diagnostic accuracy of HRV. Yet studies have shown conflicting results with respect to which of these is the more valuable in analyzing HRV as a diagnostic tool. Clinically, this is a question with direct practical importance, since short-term measurements can be easily obtained within an office visit, while long-term measurements require either use of an ambulatory holter-monitor or overnight hospitalization, both of which can incur significant expense and potential patient discomfort. Frequency domains of a spectral analysis yielded similar results in long and short-term HRV for patients with hypertrophic cardiomyopathy(21) and different study, testing HRV in heart-transplanted children, found that short term spectral HRV recordings were as reliable as a 24-hour ECG(22). On the other hand, other studies have reported that while long term and short term HRV values often correlate, 24-hour HRV is significantly more accurate in assessing cardiac risk(23, 24). A study on exercise and HRV revealed that short-term HRV recovery depended on the type of exercise, unlike long-term HRV, which was dependent on the total work of the body and uniquely revealed abnormalities up to 48 hours from the event(25). Some studies therefore argue that the two methods are equivalent, while other studies point to enhanced sensitivity and accuracy in using long-term HRV. A reasonable theoretical justification for greater accuracy with 24-hour HRV is that longer-term ambulatory measurement provides increased diversity of inputs, challenging the system sufficiently to obtain a more complete assessment of homeostatic regulation. But, if so, it also suggests the possibility that short-term HRV enhanced with perturbations (i.e., an emotional “stress test”) might deliver many of the same benefits as long-term HRV without the latter’s inconvenience.
Methods and Materials
Study Design
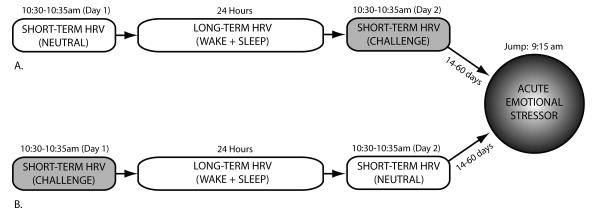
The purpose of this study was to determine, using a within-subjects design, the degree to which laboratory HRV measurements correlate with autonomic response to a real-world acute emotional stressor in healthy adults. In order to compare whether long and short-term HRV measurements capture the same information with the same sensitivity, the laboratory HRV measurement included both seated five-minute and ambulatory 24-hour data. In order to compare whether short-term HRV measurements could be made more sensitive by introducing a challenge condition, the five-minute HRV component was performed twice for each subject. These were performed on consecutive days counter-balanced for order between subjects: once during a emotionally neutral condition in which subjects were passively presented benign images, and once undergoing a mildly stressful condition in which the same subjects watched aversive images. The “real-world” acute emotional stressor, to which the subjects’ laboratory measures were compared, was a first-time tandem skydive. Subjects achieved altitude in 15 minutes, jumped at 4km (13,000 ft), and were in free-fall for one full minute, at which point the parachute opened and subjects landed four minutes later. Our previous studies confirmed this protocol’s efficacy in providing a reliably powerful, temporally-uniform, and well-controlled stressor, inducing marked increases of both cortisol and self-reported state anxiety(26, 27). Unlike a solo skydive, which can be physically demanding, in a tandem skydive the tandem-master assumes full responsibility for all stabilization and steering. Therefore for our subjects, who were attached to the tandem-master, the skydive produced a predominantly emotional, rather than physical, stressor. As illustrated by Figure 1, ECG for each subject was thus recorded during four conditions: two five-minute laboratory measurements (neutral and challenge), one 24-hour laboratory measurement, and throughout subjects’ participation in a real-world acute emotional stressor (first-time tandem skydive, with ECG recorded from two hours pre-jump until one hour post-jump, by which time heart rate had achieved full recovery). In order to maintain as much control as possible over the subjects’ physical and emotional environments, the first three components of the study occurred while subjects were continuously hospitalized at the Stony Brook University Hospital’s General Clinical Research Center for 48 hours. Wake/sleep times as well as ECG data recording times for all four components were standardized across subjects to minimize individual variance due to diurnal variability.
Figure 1. Schematic of Experiment.
Each subject (N=33 artifact-free data) participated in four laboratory sessions during which we recorded ECG and subsequently calculated heart rate variability, a measure of sympathetic dominance. These included two five-minute sessions (neutral and emotional challenge) separated by 24-hour ambulatory recording. Session order (a versus b) was counterbalanced between subjects. Following laboratory sessions, subjects participated in a first-time tandem skydive, to determine the degree to which laboratory ECG predicted autonomic response to an acute emotional stressor.
Subjects
Subjects were recruited from a sample of individuals who had contacted an areas skydiving school in order to schedule their first-time tandem skydives. All subjects were consented as per procedures formally approved by the State University of New York at Stony Brook. We tested 56 healthy adult subjects with no history of cardiac or mental illness, as determined by physical exam, medical history, and screening using the screening portion of the Structured Clinical Interview for DSM-IV(28). Of these, 33 data sets met our quality control criteria of being at least 95% artifact-free for all four study components, including the skydive (30% female; Age: 18-48, μ=24, s.d.=7.82, n=33; Body Mass Index: 18-34, μ=24, s.d.=3.69, n=33). Of note, although our subjects self-selected to participate in a recreational tandem skydive, as a whole they actually were more anxious than the general population, with mean trait anxiety scores in the 76th percentile (Spielberger Trait Anxiety Scale(29): 27-55, μ=41.63, s.d.=10.62, n=33). This had the advantage of providing a sufficiently diverse sample with which to test emotional stress reactivity. There were no differences between the included and excluded subjects with respect to gender (F=2.26, df=1, p=0.14), age (F=0.15, df=1, p=0.70), or trait anxiety (F=0.71, df=1, p=0.41).
Short-Term HRV
To control for diurnal variability, each subject participated in this portion of the protocol during two sessions at identical times (10:30—10:35am) on consecutive days. On each day, subjects viewed 48 images (six seconds each(30)) obtained from the International Affective Picture Scale(31), a well-validated research-based inventory of images designed for inducing emotional responses. Subjects sat two meters from a 1.23m (48″) plasma screen in an otherwise completely dark sound-proof chamber. On one day, subjects viewed pictures whose content was rated “neutral” (pictures with median ratings for pleasure and dominance and lowest ratings for arousal). On the other day, subjects viewed pictures whose content was rated “negatively arousing” (pictures with lowest ratings for pleasure and dominance and highest ratings for arousal). To control for order effects, the order of condition presentation was counter-balanced for order between subjects (of the n=33 with > 95% artifact-free ECGs for all four components of the study, 56% performed the study as described by Figure 1a and 44% performed the study as described by Figure 1b). ECG was acquired using the Biopac Systems MP-150 unit with ECG100-C amplifiers and Acknowledge software (Goleta, CA) with a 200 Hz sampling rate.
Long-Term HRV
Subjects’ ECG was monitored continuously for 24 hour immediately following the first day’s laboratory testing using the Aria holter monitor (Del Mar Reynolds Medical, Irvine, CA) with a sampling rate of 128 samples per second. Participants were hospitalized during the experiment, and asked to maintain wake-times of 7:30am and sleep-times of 11:30pm. In addition, both participants and nurses kept logs of sleep and wake times, which were confirmed using group mean changes in heart rate.
Acute Stressor
An ECG holter (Vivometrics, Ventura CA) was attached upon arrival to the airfield. The timing (9:00am plane takeoff, 9:15am jump time) as well as skydive durations (five minutes, of which one minute was in freefall and four minute were under the parachute) were equivalent across all subjects. Subjects’ skydive ECGs were divided into six different portions: 120-30 minutes pre-jump, 30-15 minutes pre-jump, 15-0 minutes pre-jump, 0-5 minutes post-jump, 5-30 minutes post-jump, and 30-60 minutes post-jump.
Heart Rate Variability Analyses
Records were reviewed and edited using the Impresario Holter Analysis system (Del Mar Reynolds Medical, Irvine, CA). The ECG was manually reviewed to check for any misclassifications or errors. Identified erroneous R-R intervals were replaced with values obtained by cubic spline. The R-R intervals were further filtered using an adaptive filtering technique(32) in order to remove artifacts and misclassifications that were not identified during the manual scan. The R-R intervals were converted to instantaneous heart rate with a sampling rate of 4 Hz using previously validated(33) methods; they were low-pass filtered at 0.5 Hz, down-sampled to 1 Hz and zero-meaned. Power spectrum density was estimated using 256 point FFT, with a Hanning window the same size. The HR data were divided into consecutive five-minute segments (one segment for the short-term HRV conditions, which only lasted five minutes). For the long-term HRV and acute stress HRV study components we then estimated the average power spectrum density for each of the relevant time-segments: sleep and wake durations for long-term HRV and six sampling periods during the acute stress HRV. For each, the power spectrum density was calculated within two frequency bands: LF band (0.04 – 0.15 Hz) and HF band (0.15-0.5 Hz). The sympathovagal balance was estimated by taking the ratio LF/HF.
Statistical Analyses
To permit comparison between acquisition parameters’ sensitivities in capturing subjects’ global reactivity to the emotional stressor, we performed a Repeated Measures ANOVA (six time-segments as within-subjects factor) with four laboratory measures (short-term neutral and challenge, long-term wake and sleep) as covariates. To investigate whether different laboratory measures predicted distinct anticipatory, peak, and recovery components of the stressor, we additionally performed bivariate Pearson correlations between LF/HF for each of the laboratory conditions as compared to the real world acute stressor. Because of the problem of Type I error inflation due to multiple comparisons, we confined our correlational analyses to 24 tests: six HRV measurements during the acute stressor and the four laboratory measures. After Bonferroni or Sidak correction, this yielded an adjusted threshold for statistical significance from p≤0.05 to p≤0.002.
Results
Global comparison of acquisition parameters
Of the four laboratory conditions, long-term wake LF/HF provided the strongest covariation (F=11.46, df=1, n=33, p=0.005) with the emotional stress response over all time-points, followed by short-term challenge (F=9.01, df=1, n=33, p=0.01), long-term sleep (F=8.52, df=1, n=33, p=0.01), and short-term neutral (F=6.41, df=1, n=33, p=0.03) conditions.
Correlations between short-term HRV and real-world acute emotional stressor
LF/HF during the neutral laboratory period correlated with LF/HF during the jump portion of the skydive (r=0.66, n=33, p=0.006), a relationship that was enhanced for the challenge condition, (r=0.69, n=33, p=0.001). There was no significant correlation between either of the short-term LF/HF conditions and the first 30 minutes of the recovery period. However, LF/HF during both the neutral and challenge laboratory periods correlated strongly with the second 30 minutes of the recovery period (respectively, r=0.73, n=33, p=0.002 and r=0.76, n=33, p=0.000).
Correlations between long-term HRV and real-world acute emotional stressor
LF/HF during the wake portions of the 24 hour HRV correlated strongly with the sympathetic dominance during the 15 minutes prior to the jump (r=0.78, n=33, p=0.000) and also with both of the recovery periods post-landing, with the first 30 minutes’ recovery being more strongly correlated (r=0.84, n=33, p=0.000) than the second (r=0.65, n=33, p=0.005). Sleep HRV correlated only with the second 30 minutes’ recovery (r=0.60, n=33, p=0.01).
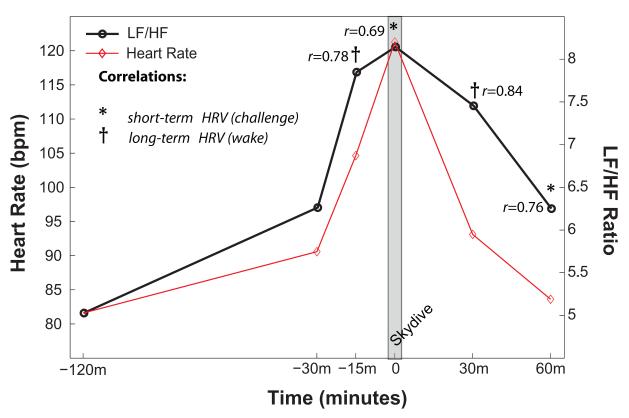
Results for all correlations that passed statistical thresholds for Bonferroni correction are provided in Figure 2. Neither variation in elapsed time between laboratory and skydive measurements nor menstrual cycle phase (for females) were found to be significant covariates for any results.
Figure 2. Correlations of Laboratory Heart Rate Variability with Heart Rate Variability During Acute Emotional Stressor.
Short-term emotional challenge and long-term wake measurements both strongly predicted heart rate variability during the first-time tandem skydive but were sensitive to distinct components of the anticipatory, peak, and recovery response. Short-term neutral and long-term sleep measurements did not meet thresholds for statistical significance after Bonferroni correction, and therefore results are not presented here.
Discussion
Heart-rate variability is a technique with demonstrated clinical utility; however, one of the critical assumptions underlying its use is that measurements taken in the laboratory provide an accurate snapshot of the autonomic regulation that occurs as a part of everyday stressors, both physical and emotional. This study focused on a specific type of stressor (emotional rather than physical, acute rather than chronic) in order to address several clinically relevant questions. Do laboratory measurements provide reasonable predictive accuracy for emotional stressors that occur within “real-world” environments? If so, does the more clinically and analytically-taxing 24-hour HRV increase that accuracy as compared to short-term laboratory HRV? Finally, does the introduction of a challenge condition for short-term laboratory HRV improve its sensitivity?
Our analyses made clear that the broad choice between five-minute versus 24-hour HRV may not the most appropriate way to analyze the question of predictive power. While long-term HRV provided a more robust association with the acute emotional stress, this was true only for wake values; after correction for multiple comparisons, long-term sleep HRV was not predictive for any component of the response. Likewise, while short-term neutral HRV—the version that would typically administered in a clinician’s office—showed a 44% decrease in effect-size for covariance with the acute stressor as compared to long-term wake HRV (and, like long-term sleep HRV, did not pass thresholds for statistical significance after multiple comparisons correction), its effect size increased by nearly 30% with the introduction of a mild challenge. Our results additionally suggest that the two most useful paradigms also seem to be most sensitive to distinct components of the stressor, with short-term challenge HRV correlating most strongly with the gross amplitude and recovery of the stressor’s autonomic response, and long-term wake HRV correlating most strongly with the more fine-tuned regulatory modulation occurring immediately prior and following the stressor.
Our results therefore suggest three conclusions for using HRV to predict autonomic response to acute emotional stress. First, laboratory HRV does appear to be able to provide a marker for future reactivity to a “real-world” acute emotional stressor: we found a number of robust correlations between the two, with coefficients in ranges (r=0.7-0.8) large enough to be considered strongly predictive. Second, for non-challenge conditions, long-term wake measurements provide additional information as compared to short-term wake measurements; however, at least in our sample, wearing a holter ECG during sleep delivered no additional benefits. Third, for clinical environments where only short-term ECG is feasible, introduction of an “emotional stress test,” conceptually analogous to the “physical stress test” commonly used diagnostically, may be a relatively straightforward way to perturb homeostatic regulation sufficiently to gain some of the benefits associated with longer-term ambulatory data collection. Finally, challenge versus rest conditions may provide distinct types of information with respect to the dynamics of stress resilience, and therefore a comprehensive protocol may benefit from including both.
The ability for laboratory-based HRV to predict both reactivity to and recovery from real-world stressors has important implications for the study of psychopathology. Interactions between emotional stress and underlying vulnerability to it have been suggested as critical mediators of signs and symptoms in various psychiatric disorders, including schizophrenia (34-36) as well as anxiety disorders and depression (37-45). The neural pathways that link emotional arousal and HRV are the limbic outputs to the autonomic nervous system; indeed, limbic and autonomic dysregulation have been shown to be coupled in trait anxiety (9, 10). As such, HRV has the potential to provide an inexpensive and noninvasive, if relatively coarse-grained, instrument for inferring limbic regulation in response to acute stress for patients with mental illness. The ability to fine-tune our interpretation of laboratory-based HRV measures, by increasing their sensitivity as well being able to optimize for either the amplitude of the stress response or its modulation, may increase detection power for individual components of the stress response that differ in ways that are disorder-specific. Just as importantly, it may inform cognitive-behavioral therapeutic strategies tailored toward addressing distinct types of stress vulnerabilities.
Table 1.
Mean Heart-Rate Variability Values for All Laboratory and Acute Stress Conditions
| Cardiac Variables (N=33) | |||||
|---|---|---|---|---|---|
| LF μ / s.d. |
HF μ / s.d. |
norm LF μ / s.d. |
norm HF μ / s.d. |
LF/HF μ / s.d. |
|
| Laboratory Conditions | |||||
| short-term HRV (neutral) | 3.933/3.288 | 5.280/8.786 | 0.538/0.224 | 0.462/0.224 | 2.062/2.433 |
| short-term HRV (challenge) | 3.570/3.472 | 4.654/4.766 | 0.483/0.218 | 0.507/0.218 | 1.549/1.701 |
| long-term HRV (wake) | 5.864/2.920 | 1.787/1.066 | 0.764/0.088 | 0.236/0.088 | 4.383/2.196 |
| long-term HRV (sleep) | 2.891/1.734 | 1.872/1.643 | 0.633/0.121 | 0.368/0.119 | 1.997/1.181 |
| Acute Stressor | |||||
| −120 minutes | 7.189/4.530 | 1.875/1.273 | 0.793/0.091 | 0.207/0.091 | 5.028/2.737 |
| −30 minutes | 8.031/3.487 | 1.856/1.453 | 0.822/0.082 | 0.178/0.024 | 6.263/3.690 |
| −15 minutes | 6.074/3.921 | 1.283/1.732 | 0.846/0.077 | 0.154/0.077 | 7.848/4.837 |
| Skydive | 12.863/9.932 | 2.716/3.152 | 0.850/0.093 | 0.151/0.093 | 8.145/4.808 |
| 30 minutes | 6.512/3.036 | 1.475/1.178 | 0.823/0.091 | 0.177/0.091 | 7.456/5.120 |
| 60 minutes | 7.736/4.302 | 1.667/1.128 | 0.808/0.102 | 0.192/0.102 | 6.251/4.321 |
Acknowledgments
The authors wish to acknowledge the technical assistance of Katherine Bertman, Chaithra Rajendra, Tsafrir Greenberg, and Mark Sedler in the collection and processing of data.
Funding Sources: This research was supported by the Office of Naval Research #N0014-04-1-005 (LRMP) and the National Institutes of Health # 5MO1-RR-10710 (General Clinical Research Center).
Footnotes
Disclosures: The authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto Y, Hughson RL, Peterson JC. Autonomic control of heart rate during exercise studied by heart rate variability spectral analysis. J Appl Physiol. 1991;71:1136–1142. doi: 10.1152/jappl.1991.71.3.1136. [DOI] [PubMed] [Google Scholar]
- 3.Ori Z, Monir G, Weiss J, Sayhouni X, Singer DH. Heart rate variability. Frequency domain analysis. Cardiol Clin. 1992;10:499–537. [PubMed] [Google Scholar]
- 4.Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Res. 2000;96:1–13. doi: 10.1016/s0165-1781(00)00195-5. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz PJ, De Ferrari GM. The influence of the autonomic nervous system on sudden cardiac death. Cardiology. 1987;74:297–309. doi: 10.1159/000174215. [DOI] [PubMed] [Google Scholar]
- 6.Abboud FM. The sympathetic system in hypertension. State-of-the-art review. Hypertension. 1982;4:208–225. [PubMed] [Google Scholar]
- 7.Weinstein AA, Deuster PA, Kop WJ. Heart rate variability as a predictor of negative mood symptoms induced by exercise withdrawal. Med Sci Sports Exerc. 2007;39:735–741. doi: 10.1249/mss.0b013e31802f590c. [DOI] [PubMed] [Google Scholar]
- 8.Hallas CN, Thornton EW, Fabri BM, Fox MA, Jackson M. Predicting blood pressure reactivity and heart rate variability from mood state following coronary artery bypass surgery. Int J Psychophysiol. 2003;47:43–55. doi: 10.1016/s0167-8760(02)00092-2. [DOI] [PubMed] [Google Scholar]
- 9.Mujica-Parodi LR, Korgaonkar M, Ravindranath B, Greenberg T, Tomasi D, Wagshul M, et al. Limbic dysregulation is associated with lowered heart rate variability and increased trait anxiety in healthy adults. Hum Brain Mapp. 2009;30:47–58. doi: 10.1002/hbm.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolkunov D, Rubin D, Mujica-Parodi LR. Power spectrum scale invariance quantifies limbic dysregulation in trait anxious adults using fMRI: adapting methods optimized for characterizing autonomic dysregulation to neural dynamic timeseries. Neuroimage. doi: 10.1016/j.neuroimage.2009.12.021. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorman JM, Sloan RP. Heart rate variability in depressive and anxiety disorders. Am Heart J. 2000;140:77–83. doi: 10.1067/mhj.2000.109981. [DOI] [PubMed] [Google Scholar]
- 12.Monk C, Kovelenko P, Ellman LM, Sloan RP, Bagiella E, Gorman JM, et al. Enhanced stress reactivity in paediatric anxiety disorders: implications for future cardiovascular health. Int J Neuropsychopharmacol. 2001;4:199–206. doi: 10.1017/S146114570100236X. [DOI] [PubMed] [Google Scholar]
- 13.Garakani A, Martinez JM, Aaronson CJ, Voustianiouk A, Kaufmann H, Gorman JM. Effect of medication and psychotherapy on heart rate variability in panic disorder. Depress Anxiety. 2009;26:251–258. doi: 10.1002/da.20533. [DOI] [PubMed] [Google Scholar]
- 14.McCraty R, Atkinson M, Tomasino D, Stuppy WP. Analysis of twenty-four hour heart rate variability in patients with panic disorder. Biological psychology. 2001;56:131–150. doi: 10.1016/s0301-0511(01)00074-6. [DOI] [PubMed] [Google Scholar]
- 15.Mujica-Parodi LR, Yeragani V, Malaspina D. Nonlinear complexity and spectral analyses of heart rate variability in medicated and unmedicated patients with schizophrenia. Neuropsychobiology. 2005;51:10–15. doi: 10.1159/000082850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholson A, Fuhrer R, Marmot M. Psychological distress as a predictor of CHD events in men: the effect of persistence and components of risk. Psychosom Med. 2005;67:522–530. doi: 10.1097/01.psy.0000171159.86446.9e. [DOI] [PubMed] [Google Scholar]
- 17.Jiang W, Babyak M, Krantz DS, Waugh RA, Coleman RE, Hanson MM, et al. Mental stress--induced myocardial ischemia and cardiac events. Jama. 1996;275:1651–1656. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 18.Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318:1005–1012. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- 19.Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105:1780–1784. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- 20.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 21.Morner S, Wiklund U, Rask P, Olofsson BO, Kazzam E, Waldenstrom A. Parasympathetic dysfunction in hypertrophic cardiomyopathy assessed by heart rate variability: comparison between short-term and 24-h measurements. Clin Physiol Funct Imaging. 2005;25:90–99. doi: 10.1111/j.1475-097X.2004.00595.x. [DOI] [PubMed] [Google Scholar]
- 22.Pozza R Dalla, Fuchs A, Bechtold S, Kozlik-Feldmann R, Daebritz S, Netz H. Short-term testing of heart rate variability in heart-transplanted children: equal to 24-h ECG recordings? Clin Transplant. 2006;20:438–442. doi: 10.1111/j.1399-0012.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- 23.Fei L, Statters DJ, Anderson MH, Malik M, Camm AJ. Relationship between short- and long-term measurements of heart rate variability in patients at risk of sudden cardiac death. Pacing Clin Electrophysiol. 1994;17:2194–2200. doi: 10.1111/j.1540-8159.1994.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 24.Faber TS, Staunton A, Hnatkova K, Camm AJ, Malik M. Stepwise strategy of using short- and long-term heart rate variability for risk stratification after myocardial infarction. Pacing Clin Electrophysiol. 1996;19:1845–1851. doi: 10.1111/j.1540-8159.1996.tb03238.x. [DOI] [PubMed] [Google Scholar]
- 25.Mourot L, Bouhaddi M, Tordi N, Rouillon JD, Regnard J. Short- and long-term effects of a single bout of exercise on heart rate variability: comparison between constant and interval training exercises. Eur J Appl Physiol. 2004;92:508–517. doi: 10.1007/s00421-004-1119-0. [DOI] [PubMed] [Google Scholar]
- 26.Mujica-Parodi LR, Renelique R, Taylor MK. Higher body fat percentage is associated with increased cortisol reactivity and impaired cognitive resilience in response to acute emotional stress. Int J Obes (Lond) 2009;33:157–165. doi: 10.1038/ijo.2008.218. [DOI] [PubMed] [Google Scholar]
- 27.Mujica-Parodi LR, Strey HH, Frederick B, Savoy R, Cox D, Botanov Y, et al. Chemosensory cues to conspecific emotional stress activate amygdala in humans. PLoS ONE. 2009;4:e6415. doi: 10.1371/journal.pone.0006415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- 29.Spielberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) Consulting Psychologists Press; Palo Alto (CA): 1983. [Google Scholar]
- 30.Sutton SK, Davidson RJ, Donzella B, Irwin W, Dottl DA. Manipulating affective state using extended picture presentations. Psychophysiology. 1997;34:217–226. doi: 10.1111/j.1469-8986.1997.tb02135.x. [DOI] [PubMed] [Google Scholar]
- 31.Bradley MM, Cuthbert BN, Lang PJ. Picture media and emotion: effects of a sustained affective context. Psychophysiology. 1996;33:662–670. doi: 10.1111/j.1469-8986.1996.tb02362.x. [DOI] [PubMed] [Google Scholar]
- 32.Wessel N, Voss A, Malberg H, Ziehmann C, Voss HU, Schirdewan A, et al. Nonlinear analysis of complex phenomena in cardiological data. Herzschrittmachertherapie und Elektrophysiologie. 2000;11:159–173. [Google Scholar]
- 33.Berger RD, Akselrod S, Gordon D, Cohen RJ. An Efficient Algorithm for Spectral-Analysis of Heart-Rate-Variability. Ieee Transactions on Biomedical Engineering. 1986;33:900–904. doi: 10.1109/TBME.1986.325789. [DOI] [PubMed] [Google Scholar]
- 34.Ventura J, Nuechterlein KH, Lukoff D, Hardesty JP. A prospective study of stressful life events and schizophrenic relapse. J Abnorm Psychol. 1989;98:407–411. doi: 10.1037//0021-843x.98.4.407. [DOI] [PubMed] [Google Scholar]
- 35.Mujica-Parodi LR, Corcoran C, Greenberg T, Sackeim HA, Malaspina D. Are cognitive symptoms of schizophrenia mediated by abnormalities in emotional arousal? CNS Spectr. 2002;7:58–60. 65–59. doi: 10.1017/s1092852900022276. [DOI] [PubMed] [Google Scholar]
- 36.Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29:671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- 37.Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–143. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riskind JH, Black D, Shahar G. Cognitive vulnerability to anxiety in the stress generation process: interaction between the Looming Cognitive Style and Anxiety Sensitivity. J Anxiety Disord. 24:124–128. doi: 10.1016/j.janxdis.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Connor KM, Vaishnavi S, Davidson JR, Sheehan DV, Sheehan KH. Perceived stress in anxiety disorders and the general population: a study of the Sheehan stress vulnerability scale. Psychiatry Res. 2007;151:249–254. doi: 10.1016/j.psychres.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 40.de Rooij SR, Schene AH, Phillips DI, Roseboom TJ. Depression and anxiety: Associations with biological and perceived stress reactivity to a psychological stress protocol in a middle-aged population. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Molecular psychiatry. 2009 doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, et al. 5-Hydroxytryptamine 2C Receptors in the Basolateral Amygdala Are Involved in the Expression of Anxiety After Uncontrollable Traumatic Stress. Biological psychiatry. 2009 doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 44.Claes S. Glucocorticoid receptor polymorphisms in major depression. Ann N Y Acad Sci. 2009;1179:216–228. doi: 10.1111/j.1749-6632.2009.05012.x. [DOI] [PubMed] [Google Scholar]
- 45.Dohrenwend BP. Adversity, Stress, and Psychopathology. Oxford University Press; New York: 1998. [Google Scholar]