Abstract
Cytotoxic chemotherapy remains the mainstay of treatment for triple negative breast cancer (TNBC) despite the promise of new targeted and biologic agents. Many studies have shown significant benefit of chemotherapy in the neoadjuvant, adjuvant and metastatic treatment of TNBC. Neoadjuvant chemotherapy studies have consistently reported higher response rates in TNBC than non-TNBC, and pathologic complete response has been shown to predict improved long term outcomes for TNBC. Although the specific adjuvant regimens that may be most effective for TNBC are still being determined, third generation chemotherapy regimens utilizing dose dense or metronomic polychemotherapy are among the most effective tools presently available. The role of specific chemotherapy agents in the treatment of TNBC remains incompletely defined and warrants careful review to ensure the most effective therapy is delivered while minimizing unnecessary toxicity. Platinum agents have seen renewed interest in TNBC based on a growing body of preclinical and clinical data suggesting encouraging activity. Taxanes and anthracyclines are active in TNBC and remain important agents, but have not shown specific benefit over non-TNBC. Capecitabine has limited reported data in TNBC, but some reports suggest differential activity in TNBC compared to hormone receptor positive breast cancer. TNBC is itself a heterogeneous group in which subgroups such as BRCA1 mutation carriers may have particular sensitivity to platinum agents and relatively less sensitivity to taxanes. Therefore, the identification of additional molecular biomarkers to predict response to specific chemotherapy is required to further improve treatment strategies with the current menu of chemotherapy options and future combinations with targeted therapies.
Keywords: triple negative breast cancer, basal-like breast cancer, chemotherapy, cisplatin, carboplatin, neoadjuvant, metastatic
Introduction
The defining characteristic of triple-negative breast cancer (TNBC) is the absence of staining for the estrogen receptor (ER), progesterone receptor (PR) and HER2. 1 This renders TNBC insensitive to some of the most effective therapies available for breast cancer treatment including HER2-directed therapy and endocrine therapy. The lack of known specific therapeutic targets results in a limited arsenal to attack TNBC, primarily consisting of standard cytotoxic chemotherapy. In the metastatic setting, TNBC presents with higher rates of visceral metastases, has a relatively shorter medial survival of 7-13 months, and has limited duration of response to successive lines of chemotherapy (median response duration of 12 weeks to first line, 9 weeks to second, and 4 weeks to third line). 2-4 Therefore, it is important to select the agents most likely to result in a meaningful benefit.
Despite the promise of new targets and new agents such as PARP inhibitors, the treatment of TNBC today demands a critical review of whether TNBC is particularly sensitive to specific types of chemotherapy. This review will focus on the role of standard cytotoxic chemotherapy agents to treat TNBC both for early stage and advanced disease. Herein the term TNBC is used, with the recognition that TNBC is a histological characterization that is concordant but not completely synonymous with the molecularly defined basal-like breast cancer subgroup. 1, 5 Because the recognition of TNBC as a potentially distinct subtype of breast cancer is relatively recent, much of the data supporting the use of chemotherapy must be inferred from retrospective analyses that sometimes include only hormone receptor status but not HER2. The emerging novel targeted and biologic therapies for TNBC and their combination with chemotherapy will be described elsewhere in this issue.
The case for chemotherapy for TNBC
The benefits of cytotoxic chemotherapy for the treatment of TNBC are now well established with numerous studies demonstrating the effectiveness of chemotherapy in the neoadjuvant, adjuvant and metastatic settings. Many of the earlier studies were conducted before the discovery of HER2 and are therefore limited in their direct relevance to TNBC. Nevertheless, in retrospect the initial observations suggesting that estrogen receptor levels influence chemotherapy response provided an important foundation for modern trials to build upon. One of the earliest studies to suggest a differential benefit of chemotherapy based on ER status was a retrospective study of 70 patients with metastatic breast cancer. 6 Expression of ER in 25 patients correlated with a response rate of only 12% compared to a response rate of 75% among 45 patients without ER expression. However, a conflicting report published the same year suggested the response rate to chemotherapy in the metastatic setting was higher in the ER-rich subset compared to the ER-poor subset.7
The benefit of polychemotherapy in ER-poor breast cancer was evident in the 2005 overview meta-analysis by the Early Breast Cancer Trialist’s Collaborative Group (EBCTCG).8 In over 6,000 women with ER-poor disease enrolled in 46 polychemotherapy trials that began prior to 2000 (but did not include taxanes), a substantial reduction in risk of recurrence and death from breast cancer was seen in younger (10 year HR 0.73 and 0.73, respectively) and older patients (10 year HR 0.82 and 0.86, respectively). This analysis is similarly limited by the lack of data on HER2 status in these older trials, but is consistent with the notion that TNBC derives substantial benefit from chemotherapy. A retrospective analysis of three large CALGB trials including 6,444 patients concluded that ER negative tumors derive substantially greater improvements in outcome from modern intensive and extensive chemotherapy regimens. 9 In comparing the low dose cyclophosphamide, doxorubicin, fluorouracil (CAF) regimen from CALGB8541 to the dose dense regimen of doxorubicin, cyclophosphamide followed by paclitaxel (AC-T) in CALGB9741 10, the relative reduction in risk of recurrence was 55% for ER negative tumors, and 26% in ER positive tumors. The absolute improvement in risk of recurrence at 5 years was 22.8% for ER negative tumors and only 7% for ER positive patients treated with tamoxifen. The concept of dose intensive regimens demonstrating the greatest improvement in outcome in TNBC is supported by a retrospective study evaluating 236 high risk patients in the WSG AM-01 study who received a dose dense regimen of four cycles of epirubicin and cyclophosphamide followed by three cycles of cyclophosphamide, methotrexate, fluorouracil (CMF) compared to high dose chemotherapy with peripheral stem cell support. 11 Although high dose chemotherapy has generally shown no improvement in overall survival, at median follow up of 62 months, TNBC patients who received high dose chemotherapy had an improved overall survival of 76% compared to 61% in the dose dense arm. Together these studies support the benefit of chemotherapy, and particularly dose dense and dose intensive regimens, for TNBC.
Neoadjuvant chemotherapy for TNBC
Several studies have now demonstrated that TNBC has significantly higher pathologic complete response (pCR) rates compared to hormone receptor positive breast cancer when treated with neoadjuvant chemotherapy (Table 1), and pCR correlates well with improved outcomes. One of the largest studies to evaluate the response to neoadjuvant therapy in TNBC compared to non-TNBC included 1,118 patients treated between 1985 and 2004 at the MD Anderson Cancer Center.12 The pCR rate in the 23% of patients with TNBC was double that of the non-TNBC subset (22% versus 11%). The overall 3-year freedom from progression was 63% in the TNBC and 76% in the non-TNBC groups, and the 3 year overall survival between the groups was 74% and 89%, respectively, confirming the relatively poor prognosis in TNBC. However, patients with TNBC who achieved a pCR had similar 3 year overall survival as the non-TNBC (94% and 98%, p=0.24), whereas patients with TNBC who had residual disease after neoadjuvant therapy had a significantly worse 3 year OS (68% vs. 88%, p=.0001). These results demonstrate that TNBC have a higher pCR rate compared to non-TNBC after neoadjuvant chemotherapy. TNBC patients who achieve a pCR have an excellent long term outcome, but those who have less than a pCR are at significantly higher risk of recurrence and death than patients with non-TNBC. A similar retrospective study in an overlapping data set of 1,731 patients from the same institution who received preoperative chemotherapy between 1988 and 2005 found an overall pCR rate of 13%. 13 The 67% of patients who were hormone receptor positive achieved a pCR rate of 8% compared with 24% for the hormone receptor negative patients. Further subgroup analysis identified 317 patients with triple-negative tumors who achieved a pCR rate of 22.4%. There were too few triple-negative patients to correlate overall survival with pCR, but the hormone receptor negative patients who achieved a pCR had a 10 year overall survival of 84% compared with only 59% for those without a pCR. Together, these retrospective studies support the conclusion that achieving pCR is a strong predictor of long term favorable outcome in TNBC.
Table 1.
Representative Neoadjuvant Trials in Triple Negative Breast Cancer
| Title | Regimen/Design | Population | Status | pCR rate (#/total) |
Sponsor | clinicaltrials.gov identifier and/or (Reference) |
|---|---|---|---|---|---|---|
| A Phase II Trial of Cisplatin in Early Stage ER−, PR−, HER-2 Negative Breast Cancer |
Cisplatin 75mg/m2 q3 wk × 4 | TNBC | Complete | 22% (6/28) | Dana-Farber Cancer Institute/Massachusetts General Hospital/Beth Israel Deaconess Medical Center |
NCT00148694, (45, 46) |
| A Phase II Trial of Preoperative Cisplatin and Bevacizumab in ER−, PR−, Her-2 Negative Breast Cancer |
Cisplatin 75 mg/m2 + bevacizumab 15mg/m3 q 3 week × 4 |
TNBC | Complete | 16% (8 /51) 37% Miller- Payne score 4 and 5 |
Massachusetts General Hospital/ Dana-Farber Cancer Institute/ Beth Israel Deaconess Medical Center/Genentech |
NCT00580333, (47) |
| Neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients |
Cisplatin 75mg/m2 q3 wk × 4 | BRCA1 | Complete | 72% (18/25) | Pomeranian Medical University |
(53) |
| Randomized Phase II 2 × 2 Factorial Trial of the Addition of Carboplatin +/− Bevacizumab to Neoadjuvant Weekly Paclitaxel Followed by Dose- Dense AC in Hormone Receptor-Poor/HER2-Negative Resectable Breast Cancer |
Randomized: Pacliataxel weekly +/−Carboplatin →AC, +/− bevacizumab |
TNBC (low HR) |
Ongoing | 362 (planned) | CALGB | NCT00861705 |
| Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer |
Retrospective, various regimens | TNBC, HER2+, ER+ |
Completed | TNBC – 22% (56/255), non-TNBC 11% (95/863) |
MD Anderson Cancer Center |
(12) |
| Breast cancer molecular subtypes respond differently to preoperative chemotherapy |
Retrospectivepaclitaxel weekly × 12→FAC × 4 |
Unselected (basal, luminal A/B, HER2+, normal-like |
Completed | Basal – 45% (10/22), HER2+ − 45% (9/11), Normal-like – 0% (0/10), Luminal 7% (2/28) |
MD Anderson Cancer Center |
(22) |
| Phase II Trial of Neoadjuvant Metronomic Chemotherapy in Triple-Negative Breast Cancer |
Doxorubicin 24mg/m2 IV + cyclophosphamide 60mg/m2 PO weekly × 12→ paclitaxel 80mg/m2 IV+ carboplatin IV AUC 2 weekly × 12 weeks |
TNBC | Ongoing | 28 (planned) | Leo W. Jenkins Cancer Center |
NCT00542191 |
| A Phase II Neo-Adjuvant Study of Cisplatin, Paclitaxel With or Without RAD001 in Patients With Triple-negative Locally Advanced Breast Cancer |
Randomized: cisplatin IV and +/−oral everolimus once weekly in weeks 1-12 and paclitaxel IV weekly in weeks 4-12 |
TNBC | Ongoing | 96 (planned) | Vanderbilt-Ingram Cancer Center |
NCT00930930 |
| Neoadjuvant Treatment of Docetaxel, Anthracycline and Cyclophosphamide (TAC) Versus Docetaxel and Cyclophosphamide (TC) in Triple-Negative or Her2 Positive Breast Cancer (NATT) |
Randomized: Docetaxel 75mg/m2, doxorubicin 50mg/m2 or epirubicin 60mg/m2, cyclophosphamide 500mg/m2 every 3 weeks for six cycles OR Docetaxel 75mg/m2, cyclophosphamide 600mg/m2 every 3 weeks for six cycles |
TNBC or HER2+ |
Ongoing | 600 (planned) | Shanghai Jiao Tong University School of Medicine |
NCT00912444 |
| Open Label Randomized Clinical Trial of Standard Neoadjuvant Chemotherapy (Paclitaxel Followed by FEC) Versus the Combination of Paclitaxel and RAD001 Followed by FEC in Women With Triple Receptor-Negative Breast Cancer (CRAD001C24101) |
Randomized: Paclitaxel +/− RAD001 Followed by FEC |
TNBC | Ongoing | 50 (planned) | M.D. Anderson Cancer Center/ Novartis |
NCT00499603 |
| A Randomized Multicenter Phase II Trial to Evaluate the Effectiveness of Selective Neoadjuvant Treatment According to Immunohistochemical Subtype for HER2 Negative Breast Cancer Patients |
Randomized: EC q 3 wk × 4 →docetaxel q 3 wk × 4 OR carboplatin AUC 6 q 3 wk × 4 |
TNBC, ER+ | Ongoing | 188 (planned) | Spanish Breast Cancer Research Group/AstraZeneca/Pfizer |
NCT00432172 |
| Neoadjuvant Weekly Nab-Paclitaxel (Abraxane®) Plus Carboplatin Followed By Doxorubicin Plus Cyclophosphamide With Bevacizumab Added Concurrently To Chemotherapy For Palpable And Operable Triple Negative Invasive Breast Cancer |
nab-paclitaxel IV on days 1, 8, and 15, Carboplatin IV on day 1, Bevacizumab IV on days 1 and 15, × 4 cycles → Doxorubicin IV, Cyclophosphamide IV every 14 days × 4 cycles + bevacizumab IV every 14 days ×2. |
TNBC | Planned | 60 | University of Tennessee Cancer Institute/ NCCN/ Abraxis/ Genentech |
NCT00777673 |
Additional smaller retrospective studies confirm these findings. In a retrospective study of 151 patients receiving neoadjuvant anthracycline and taxane based therapy, those patients with TNBC (14%) had significantly higher pCR rates compared to non-TNBC (38% vs. 12%). 14 Patients who achieved a pCR had a prolonged DFS, and among patients who did not achieve a pCR the TNBC subgroup had a significantly worse prognosis. A retrospective study of 435 patients who received neoadjuvant therapy for breast cancer between 1985 and 2003 found that ER negative tumors were more likely to achieve a pCR than ER positive (21.6% vs. 8.1%). 15 Overall survival at 5 years was higher in the ER negative subgroup who achieved a pCR compared to those who did not (90% vs. 52%) in agreement with other studies. In 399 patients treated preoperatively between 1994 and 2002, overall 15.7% had a pCR.16 In the 129 hormone receptor negative patients, the pCR rate was 33.3% compared to 7.6% in the HR positive group. In this study, HER2 was evaluated but the combined hormone receptor negative/HER2 negative subgroup was not independently reported. In contrast to other studies, this study reported that patients who achieved a pCR had a slightly worse prognosis than those who did not achieve pCR. The higher proportion of hormone receptor negative patients in the pCR group may account for this finding, reflecting the worse overall prognosis of the hormone receptor negative group. However, in this study pCR was defined as a complete or near complete response in the breast only, not including the nodes. Therefore, it is likely that the less strict definition of pCR also accounts for this finding.
The NSABP B-27 trial randomized 2,411 women to one of three arms to evaluate the response to neoadjuvant therapy and long term outcomes. Patients received either four cycles of standard AC every 3 weeks followed by surgery, four cycles of AC followed by four cycles of docetaxel (D) and then surgery, or four cycles of AC followed by surgery and then four cycles of adjuvant docetaxel. Determination of hormone receptor status was not required for study entry. The addition of preoperative docetaxel nearly doubled the pCR rate from 12.9% and 14.4% in each of the two AC arms, to 26.1% in the AC-D arm. 17 Interestingly, subgroup analysis showed that the pCR rate nearly doubled with the addition of docetaxel for both the ER+ and ER− tumors, from 5.7% to 14.1% and 13.6% to 22.8%, respectively. However, the pCR rate of the ER− subset itself was nearly double that of the ER+ subset in each treatment group (5.7% vs 13.6% for AC, and 14.1% vs. 22.8% for AC-D). Notably, the pCR rate of the ER-“unknown” group was 31.6% and 50.8% for the AC and AC-D cohorts, respectively. However, this is an artifact because the technology to assess receptor status in diagnostic core biopsies was not uniformly present during the time of this study and ER status could not be determined in tumors that had a pCR because no tumor tissue was present at surgery. Retrospective analysis of samples from the NSABP B-27 to confirm hormone receptor and HER2 status is ongoing and may yield further insight into the response to preoperative chemotherapy. Of the 2,411 patients enrolled, over 300 samples are available for analysis and nearly one-third are hormone receptor negative. Surprisingly, the addition of docetaxel did not result in improved DFS or OS in an updated analysis. 18 However, this trial has several significant limitations affecting its interpretation: pCR was defined as no residual tumor in breast but did not consider the lymph nodes; tamoxifen was started concurrently with chemotherapy in all patients; the majority of patients had unknown hormone receptor status due to technical limitations at the time; and the trial was significantly underpowered to detect the difference in overall survival that might have been expected.
The GEPARDUO trial also evaluated the pCR rate in 913 women randomized to receive preoperative doxorubicin and docetaxel for four cycles or doxorubicin and cyclophosphamide for four cycles followed by docetaxel for four cycles. 19 The overall pCR rate was only 10.6% in all patients combined, with an improvement from 7.0% to 14.3% with the three-drug regimen. However, the ER− subgroup was three times more likely to achieve a pCR compared with the ER+ subgroup (22.8% vs. 6.2%). Although HER2 status was not reported in the original study, a recent analysis of samples from the GEPARDUO trial confirmed that the triple-negative subgroup had significantly higher pCR than the hormone receptor positive subgroup. 20 The prospectively designed I-SPY trial of 190 patients who received neoadjuvant anthracycline and taxanes based therapy included 28% patients with TNBC who had a pCR rate of 33%, compared to hormone receptor positive, HER2- patients who had only a 10% response rate.21
A study evaluating preoperative chemotherapy in basal-like breast cancer treated with 12 weeks of weekly paclitaxel followed by 4 cycles of fluorouracil, doxorubicin, cyclophosphamide (FAC) revealed a pCR rate of 45%.22 Of the 22 basal like tumors in that study, 21 (95%) were ER− and 19 (86%) were HER2−. The pCR rates of the Luminal A/B (n=30), normal breast-like (n=10), and HER2+ (n=20) molecular subtypes were 7%, 0% and 45%, respectively. 22 This supports the conclusion that basal-like breast cancer is more highly sensitive to paclitaxel and doxorubicin chemotherapy, and correlates with similar results seen with histological markers defining TNBC. Carey et al. evaluated the response rate to anthracycline-based preoperative therapy in a retrospective study of 107 patients treated between 1998 and 2003. 23 Among the triple-negative tumors that were classified as basal-like, the clinical response rate was 85% and the pCR rate was 27%. The HER2+ subset had similar response rates, but the luminal A and B subsets of the hormone receptor positive tumors had a pCR rate of 0% and 15%, respectively. There were no recurrences among the basal-like cancers that achieved a pCR, but the distant disease-free survival of all patients with basal-like cancer was only about 60%. A similar result was seen in a study of 145 patients with stage 2 or 3 breast cancer who received preoperative doxorubicin and docetaxel for three cycles, followed by surgery and adjuvant chemotherapy with an additional three cycles of the same regimen. In this study, tumors with less than 10% ER staining were considered negative, which is a higher cut-off than most studies use. The overall pCR rate was 8%, but among the one third of patients with TNBC, the pCR rate was 17% compared with only 3% for the non-TNBC group. 24 Overall, the above data clearly indicate that TNBC has a higher response to neoadjuvant chemotherapy resulting in significant improvements in pCR compared to hormone receptor positive tumors. The improvements were seen with a variety of different agents and raise the question of whether specific chemotherapy agents are more active than others.
Platinum Agents: Are they specific to TNBC?
The use of platinum agents for breast cancer has a long history dating to the early 1970’s and includes over 200 clinical trials in breast cancer patients. (25 and unpublished observation) Platinum agents were initially tested in patients with advanced breast cancer, both as a single agent and in combination with other drugs, and were shown to be active when given early in the course of the disease. Platinum agents were not readily adopted, perhaps because of the superior therapeutic index of other drugs under development at the time, notably the taxanes. Small studies demonstrated objective response rates ranging from 42% to 54% with the use of cisplatin as a single agent, but response rates were lower in women who had received prior chemotherapy for metastatic disease.26-28 When cisplatin was given after other chemotherapy, the response rate fell to 0-9%. 29-33 Notably, these studies used cisplatin in patients regardless of ER, PR, and HER2 status. Several combination regimens were also explored, particularly cisplatin combined with taxanes, but there seemed little reason to continue these combinations when the taxanes were found to be highly active and relatively less toxic. 34
Recently there has been renewed interest in cisplatin for the treatment of TNBC, in part because of improved strategies for managing its side effects, and because of additional preclinical data that suggested platinum agents may be particularly active in TNBC and BRCA1 associated breast cancer. Breast tumors arising in BRCA1 mutation carriers share features with the basal-like tumors.35, 36 However, while nearly all BRCA1 tumors are basal-like, not all basal-like tumors have BRCA1 mutations. Several groups have demonstrated that tumor cell lines (human breast and ovary) deficient in BRCA1 are unusually sensitive to the DNA cross-linking agents, including cisplatin and mitomycin, and that this sensitivity is reversed with either BRCA1 upregulation or restoration of BRCA1 function.37-42 In one study, treatment with cisplatin produced a dose-dependent reduction in cell growth in breast cell lines after 48 hours of treatment.41 The BRCA1 defective cell line was 2-3 fold more sensitive to cisplatin compared with BRCA1 competent cell lines. This data suggests that cisplatin may be a good agent for BRCA1-mutated breast cancer.
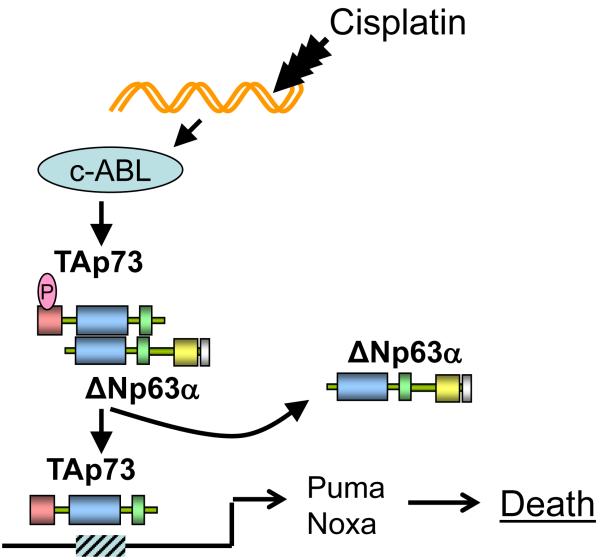
The histological similarities between BRCA1 mutated breast cancer and TNBC raised the possibility that triple negative tumors may also demonstrate relative sensitivity to cisplatin. Recent clinical and preclinical work from our institution and others has identified that platinum agents, such as cisplatin and carboplatin, may be particularly active in a subset of patients with TNBC. Recently a molecular pathway by which cisplatin induces cell death selectively in TNBC has been discovered (Fig. 1).43 Inactivation of this pathway increases the IC50 of breast cancer cells for cisplatin 10 to 100 fold. These findings have led to the discovery of a biomarker present in 30% - 50% of TNBC that may define which tumors will exhibit clinical sensitivity to cisplatin. However, it remains controversial whether platinum agents are particularly beneficial in TNBC compared to other subtypes. For example, in a retrospective study evaluating 802 patients with metastatic breast cancer, 67 were identified as having measurable disease who also received a platinum and paclitaxel based regimen in the first or second line. The ORR among the 67 patients was 38.8%. In the subset with TNBC the ORR was similar, 37.5%, and there was no difference compared to the hormone receptor positive subgroup.44
Figure 1.
Mechanism of p63/p73 mediated platinum sensitivity in triple negative breast cancer. Approximately one-third of triple negative breast cancers express the p53 family members ΔNp63α̣ and TAp73. In proliferating cells ΔNp63α inhibit apoptosis by forming heterodimers with TAp73 and forming homotetramers that bind to the promoters of TAp73 target genes, thereby preventing transcription of proapoptotic genes. Cisplatin treatment induces DNA damage resulting in activation of the c-ABL tyrosine kinase and phosphorylation of TAp73. TAp73 then forms stable homotetramers that bind to TAp73 target genes such as PUMA and NOXA resulting in apoptosis. Expression of -63/p73 in breast cancer cell lines results in 10-100 fold greater sensitivity to platinum chemotherapy.
Preoperative studies
Preoperative therapy with platinum has yielded promising results (Summarized in table 1). Our institution conducted a preoperative phase 2 study evaluating single agent cisplatin (75mg/m2) given for 4 cycles to women with stage 2 or 3 TNBC.45, 46 The pCR rate was 22% (6/28) and 36% had a Miller-Payne score of 4 or 5, which includes complete and near-complete responses. Two patients in this study were BRCA1 carriers and both achieved a pCR. A preliminary biomarker assessment of p63/p73 expression in the 22 available samples demonstrated that a pCR was achieved in 3 of 9 (33%) biomarker positive patients but only 1 of 13 (7%) biomarker negative patients. 46 A follow up study evaluating preoperative cisplatin plus bevacizumab in 51 patients resulted in a pCR rate of 16%, with 37% achieving a Miller-Payne score of 4 or 5.47 Together, both studies suggest single agent cisplatin is active in untreated TNBC.
Platinum agents have also been used in combination with other agents in the neoadjuvant setting. A 2004 phase II study of preoperative paclitaxel and cisplatin demonstrated a 28% complete response rate and a 63% partial response rate in patients enrolled without regard to ER, PR, and HER2 status.48 A study of 88 patients with locally advanced breast cancer who received preoperative cisplatin containing regimens (doxorubicin and paclitaxel for four cycles followed by cisplatin and paclitaxel for four cycles in either order) demonstrated a pCR rate of 35% in the ER negative, HER2 negative subset.
A remarkably high pCR rate of 65% was seen in 74 patients with triple negative breast cancer treated with cisplatin 30mg/m2, epirubicin 50mg/m2 and paclitaxel 120mg/m2 weekly for 8 weeks with GCSF support on days 3-5. 49 Adjuvant therapy with 4 cycles of CMF was administered to all patients, and those with four or more positive nodes after preoperative therapy received an additional 4 cycles. Those patients who achieved a pCR had a 3- and 5-year disease-free survival of 97% and 90%, respectively, compared with 3- and 5-year DFS rates of 61% and 56% in those with residual disease after preoperative therapy. Notably, about equal numbers of patients had T2 and T3 tumors, but the pCR rate was significantly higher in the T2 tumors (74% versus 51%). A similar study of 30 patients treated with preoperative epirubicin, cisplatin and fluorouracil (ECF) × 4 followed by weekly paclitaxel resulted in a pCR rate of 43%.50 In a retrospective study of patients who received platinum-containing regimens, significantly higher clinical responses were seen in the neoadjuvant and metastatic setting in the triple negative subgroup.51
The role of platinum in early stage treatment of TNBC will be addressed in 2 randomized phase 2 studies. The CALGB 40603 study is a 2×2 factorial design evaluating +/−carboplatin and +/−bevacizumab added to preoperative weekly paclitaxel followed by dose dense AC in hormone receptor poor, HER2− breast cancer. The Spanish Breast Cancer Research Group is evaluating preoperative epirubicin, cyclophosphamide (EC) × 4 followed by docetaxel +/−carboplatin in TNBC. These two prospective studies will help address the important question of whether addition of platinum agents improves outcomes in TNBC; because pCR correlates with improved long term outcomes in TNBC, the added benefit of platinum on the pCR rate may predict a benefit on disease-free and overall survival. In addition, these trials will provide a rich opportunity to evaluate predictive biomarkers of platinum sensitivity.
The activity of platinum in the BRCA1 population specifically has recently been demonstrated in several intriguing studies. In a study of 25 BRCA1 patients in Poland in whom 80% were triple-negative (and 3 had incomplete data), a pCR rate of 72% was achieved with preoperative single agent cisplatin (75mg/m2) administered every three weeks for four cycles. 52 In a related retrospective study among 102 consecutive BRCA1 carrier patients treated with a variety of preoperative regimens, cisplatin alone achieved a pCR rate of 83% compared with significantly lower rates with CMF, AC, CAF, or doxorubicin, paclitaxel (AT) (7%, 22%, 21%, 8%, respectively). 53
Metastatic studies
In addition to the evaluation of platinum in the preoperative setting, studies in the metastatic setting further support that platinum may be active in advanced TNBC. The Translational Breast Cancer Research Consortium (TBCRC) 001 study evaluated 100 patients with metastatic TNBC randomized to cetuximab with or without carboplatin (AUC=2) given weekly for 3 weeks every 28 days in up to the third-line setting. 54 Patients crossed over to the combination arm upon progression after single agent cetuximab. A response rate of 18% was observed in the combination arm. The identification of the potential biomarker p63/p73 to predict response to platinum has led to the TBCRC009 trial in 82 patients with TNBC that will address two important questions. 55 First, it will define the single agent response rate to cisplatin (75 mg/m2) or carboplatin (AUC=6) given every three weeks in the first or second line. Second, it will determine prospectively whether p63/p73 expression predicts response to platinum. In a small trial of 15 patients with BRCA1 mutations with metastatic breast cancer treated with single agent cisplatin every 3 weeks, of whom 10 had TNBC, the overall response rate was 72% with 7 patients reported to have clinical complete responses.56
The large randomized phase III Triple Negative Breast Cancer Trial (TNT) with approximately 400 patients in the UK is underway comparing carboplatin with docetaxel for metastatic TNBC. 57 The primary endpoint of the trial is response rate, and a series of secondary endpoints and exploratory studies will be undertaken to evaluate overall survival and identify predictors of response to better define the platinum responsive subgroup of tumors. Patients may receive up to 6 cycles of treatment and will crossover to the other arm either at progression. The TNT study is designed to detect a 15% improvement in response to carboplatin compared to docetaxel. This trial will answer a critical question in the management of TNBC to help define how platinum should be utilized in metastatic disease.
Despite the intriguing data suggesting platinum may be active in TNBC, there is presently no established role for adding platinum to early stage regimens outside of a clinical trial. In the metastatic setting, it is reasonable to consider platinum in the armamentarium of available agents, but there is still insufficient data to recommend its use over standard chemotherapy in early lines. This may change as a result of ongoing studies discussed above. Data in BRCA carriers are particularly exciting, and the results of prospective studies are eagerly awaited.
Taxanes
The benefit of taxanes in adjuvant therapy of TNBC has been realized over the past few years. The first trial that established the benefit of paclitaxel added to AC was CALGB 9344/INT1048. This trial randomized 3,121 patients with node positive operable breast cancer in a 3 × 2 schema to receive 3 different doxorubicin doses followed by further therapy with or without 4 cycles of paclitaxel every three weeks. The addition of paclitaxel resulted in a 17% reduction in the risk of recurrence and 18% reduction in the risk of death, with an improvement in 5 year disease-free and overall survival from 65% to 70%, and 77% to 80%, respectively. 58 The initial study did not evaluate HER2 status, but an unplanned subset analysis of hormone receptor status suggested that the hormone receptor negative subgroup had an improved HR for recurrence of 0.72 (95% CI 0.59-0.86) compared a HR of 0.91 (95% CI 0.78-1.07) for the receptor positive subset. However, this was not considered statistically significant when corrected for multiple comparisons. In an important subsequent analysis of this trial, a subset of 1322 patients was evaluated for the impact of HER2 status on outcomes. 59 Paclitaxel was associated with improvements in DFS in the HER2 positive patients regardless of hormone receptor status, whereas in HER2 negative patients, benefit was only seen in the hormone receptor negative group. Although progesterone receptor was not formally reported in this study, this exploratory analysis suggests that the triple negative subset of breast cancer derives substantial benefit from the addition of paclitaxel in the adjuvant setting, supporting the conclusion that taxanes are important in triple negative breast cancer.
A large trial of 4,950 patients randomized to receive adjuvant doxorubicin and cyclophosphamide followed by docetaxel or paclitaxel given weekly or once every three weeks demonstrated an overall improvement in 5 year DFS and OS of 27% and 32%.60 In the triple negative subgroup the benefit of weekly paclitaxel was 37% over the 3-week regimen. Thus, not only is paclitaxel effective in this setting, but the weekly regimen is more active than the less frequent 3-week regimen. Further support for the benefit of taxanes for ER negative tumors comes from a retrospective analysis of 1,079 patients who received preoperative chemotherapy in clinical trials with or without taxanes. 61 In the ER negative subgroup, pCR was achieved in 15% without a taxanes and 29% with a taxanes. A preoperative study of paclitaxel followed by FAC resulted in a 45% pCR rate among the basal-subgroup of patients 22, further supporting the benefit of taxanes in this subgroup.
However, conflicting data have been reported on the specific benefit of taxanes for adjuvant therapy in TNBC. A subset analysis of the BCIRG001 trial evaluated the benefit of docetaxel versus fluorouracil when added to doxorubicin and cyclophosphamide (TAC vs. FAC) in molecular subgroups. 62, 63 The benefits of docetaxel were independent of hormone receptor status. In addition, the NSABP B28 trial compared doxorubicin and cyclophosphamide with or without four cycles of paclitaxel in 3,060 patients and found no statistically significant difference in the relative risk of recurrence and overall survival based on hormone receptor status. 64 Like the BCIRG001 and the initial analysis of CALGB9344, the HER2 status of tumors in NSABP B28 was not known. However, the majority of the hormone receptor negative subgroup is likely comprised of TNBC.
As with platinum agents, the BRCA1 population may demonstrate distinct patterns of response to taxanes compared to sporadic TNBC. Two retrospective studies from Poland evaluated the response to neoadjuvant therapy with docetaxel regimens. Among 44 BRCA1 carriers identified in a registry of 3,479 patients, only 6 of 15 who received docetaxel and doxorubicin had a complete or partial response, compared to 29 of 29 who received non-taxane, DNA damaging regimens.65 A second retrospective study from Poland of 175 patients with metastatic breast cancer treated with docetaxel-based regimens identified 19 with primary resistance to docetaxel. 66 Mutations in BRCA1 were found in 5 of the 19 (26%), and all 5 were in the TNBC subgroup, suggesting that BRCA1 mutation might confer decreased response to docetaxel. This question will be answered in the UK-based BRCA-trial, which is similar in design to the TNT trial, comparing carboplatin and docetaxel for first line treatment of metastatic breast cancer only in BRCA carriers.
In the metastatic setting, several trials suggest a lack of specific benefit for taxanes for TNBC over other subtypes, and generally support the conclusion that taxanes are effective in all subtypes of breast cancer. In the CALGB9342 trial, which evaluated three different doses of paclitaxel for metastatic breast cancer, there was no statistically significant difference in response rate or time to treatment failure between TNBC and hormone receptor positive tumors. However, the overall survival was significantly worse for the TNBC compared to hormone receptor positive. 67 The ECOG2100 study randomized 722 patients to initial chemotherapy with paclitaxel with or without bevacizumab.68 Over 90% of the patients were HER2 negative, and more than a third were ER and PR negative, suggesting a majority of the hormone receptor negative patients were likely triple negative. All subgroups showed a similar benefit with the addition of bevacizumab. Interestingly, however, the progression free survival was only 4.6 months for the hormone receptor negative subset in the paclitaxel alone arm, compared to 8.0 months in the hormone receptor positive group. As with the CALGB9342, this likely reflects the poor prognosis of hormone receptor negative disease.
Although there does not appear to be a specific benefit of taxanes for TNBC in the metastatic setting, as mentioned above BRCA function may play a role in taxanes sensitivity. Preclinical data demonstrate that intact BRCA1 function contributes to anti-microtubule agent sensitivity. 40 Therefore, if sporadic TNBC also has a functional deficiency of BRCA1, then it follows that TNBC may be more resistant to taxanes. This question will be addressed in the phase III TNT study discussed above, comparing carboplatin and docetaxel. The search for additional biomarkers to predict platinum sensitivity in TNBC may yield additional insights. For example, TNBC was shown to have more frequent expression of Caveolin-1.69 Interestingly, one mechanism of cellular uptake of nanoparticle-albimin bound paclitaxel (nab-paclitaxel) is via Caveolin-1 dependent receptor mediated transcytosis. Therefore, the use of nab-paclitaxel may warrant further testing for TNBC with high Caveolin-1 expression.70
Anthracyclines
The benefit of anthracycline-based therapy is supported by several studies described in the section on preoperative therapy above. However, TNBC may be heterogeneous and it remains unclear with regard to anthracycline sensitivity whether BRCA1 associated TNBC is functionally similar to sporadic TNBC. A provocative study suggests that BRCA1 associated TNBC may be less sensitive to anthracycline-based therapy. 71 Among 55 triple-negative patients who received 6 cycles of FEC100 (fluorouracil/epirubicin 100mg/m2/cyclophosphamide), 12 BRCA1 carriers were identified. The pCR rate for the 12 triple negative BRCA1 carriers was 17% compared with 42% in the 55 sporadic triple-negative non-carriers. However, other studies come to different conclusions and suggest that BRCA1/2 mutation carriers do indeed have high pCR rates to anthracyclines. 72 Although most studies support a benefit for anthracycline based regimens, a recent analysis from the MA5 study comparing adjuvant cyclcophosphamide, epirubicin, fluoruracil (CEF) to CMF showed an improvement in 5-year overall survival in the CMF arm for TNBC (71% vs. 51%), whereas the CEF arm was superior in all other subgroups. 73
Capecitabine
The efficacy of capecitabine has not been prospectively studied in TNBC and there remains relatively scant data on its activity in this group. However, several observations can be made from retrospective subgroup analyses and several trials are underway to evaluate capecitabine in TNBC. In CALGB49907, standard adjuvant chemotherapy (either CMF or AC) was compared to capecitabine in women over age 65 to determine noninferiority.74 After 600 patients were enrolled, the trial found capecitabine was inferior to standard chemotherapy with a HR of 2.09. Importantly, a planned subgroup analysis revealed that the benefit of standard chemotherapy was most pronounced in hormone receptor negative patients compared to hormone receptor positive (HR 3.04 for relapse-free survival, 2.62 for overall survival). The analysis of the TNBC subgroup is ongoing, but due to the small numbers of HER2 positive tumors in this trial, the results are likely to be similar.
In the metastatic setting, 2 randomized phase III trials compared capecitabine plus ixabepilone to capecitabine monotherapy in 1,712 patients treated with prior anthracycline and taxanes therapy.75 In a combined subgroup analysis, 857 total patients received capecitabine alone, of which 208 patients had TNBC. The overall response rate and PFS in the capecitabine monotherapy arm was 25% and 4.2 months in the overall population, but only 15% and 1.7 months in the TNBC subgroup. A single arm phase 2 study of capecitabine with bevacizumab found nearly double the response rate in ER+ patients compared to triple negative patients (47% vs. 27%) with a similar difference in time to progression (8.9 vs. 4.0 months) and overall survival (>16.6 vs. 7.5 months). 4 Results from such studies have caused some to conclude that capecitabine may be less effective in TNBC. However, additional data are needed before concluding that capecitabine has limited activity in TNBC.
Summary
Cytotoxic chemotherapy remains the backbone of current treatment strategies for TNBC because of a lack of known specific therapeutic targets. The benefits of chemotherapy for TNBC have now been clearly demonstrated in multiple studies in the early and advanced stages. Although the specific adjuvant regimens that may be most effective for TNBC are still being determined, there is general consensus that third generation chemotherapy regimens utilizing polychemotherapy administered in a dose dense or metronomic manner are, at the moment, the most effective tools available76 and may preferentially provide greater benefit for TNBC over other subtypes of breast cancer. Neoadjuvant chemotherapy has consistently demonstrated higher response rates for TNBC than non-TNBC, and pCR predicts improved long term outcomes for TNBC. However, the potential selective benefit of specific chemotherapy agents over others warrants careful evaluation in order to select the therapy most likely to provide benefit to an individual patient while minimizing unnecessary toxicity. Platinum agents have seen renewed interest in TNBC based on a growing body of preclinical and clinical data suggesting encouraging activity. Two of the most important ongoing randomized trials are CALGB40603, evaluating the benefit of carboplatin added to paclitaxel and AC, and the Triple Negative Trial, evaluating carboplatin against docetaxel. Each of these trials will help define the role of platinum agents for early and advanced TNBC, and importantly will provide rich resources to identify potential tissue biomarkers to help define subgroups of patients with TNBC most likely to benefit from platinum agents. At the moment, however, there is no established role for adding platinum agents to early stage regimens outside of a trial, and their role in the metastatic setting remains poorly defined but reasonable to consider. Taxanes and anthracyclines are active in TNBC and remain important agents, but have not shown specific benefit over other subgroups. Capecitabine has limited reported data as monotherapy in TNBC, but some reports raise concerns that it may be less active in TNBC compared to hormone receptor positive breast cancer. Although recent efforts have tried to categorize response to specific chemotherapy based on histological subsets of breast cancers, it is also becoming clear the TNBC is itself a heterogeneous group. For example, limited data suggests BRCA1 mutation-associated TNBC may have particular sensitivity to platinum agents and relatively less sensitivity to taxanes. Therefore, the identification of additional molecular biomarkers to predict response to specific treatments is required to further improve our treatment strategies with current chemotherapy options and future combinations with targeted therapies.
Acknowledgements
This work was supported in part by Golfers Against Cancer, Avon Partners for Progress Award NIH/NCI 3P30CA006516-44S2, and the Clinical Investigator Training Program of Harvard HST-MIT. I thank Paula Ryan, MD, PhD and Leif Ellisen MD, PhD for helpful discussions, and Katie Roche for administrative assistance.
Acknowledgments of Support: Golfers Against Cancer, Avon Partners for Progress Award NIH/NCI 3P30CA006516-44S2, and the Clinical Investigator Training Program of Harvard-MIT.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Irvin WJ, Jr., Carey LA. What is triple-negative breast cancer? Eur J Cancer. 2008;44(18):2799–805. doi: 10.1016/j.ejca.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clinical breast cancer. 2009;9(1):29–33. doi: 10.3816/CBC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- 3.Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113(10):2638–45. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sledge G, Miller K, Moisa C, Gradishar W. Safety and efficacy of capecitabine (C) plus bevacizumab (B) as first-line in metastatic breast cancer. J Clin Oncol. 2007 June 20;Vol 25(No. 18S):1013. ASCO Annual Meeting Proceedings Part I. Supplement. [Google Scholar]
- 5.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 6.Lippman ME, Allegra JC, Thompson EB, et al. The relation between estrogen receptors and response rate to cytotoxic chemotherapy in metastatic breast cancer. N Engl J Med. 1978;298(22):1223–8. doi: 10.1056/NEJM197806012982203. [DOI] [PubMed] [Google Scholar]
- 7.Kiang DT, Frenning DH, Goldman AI, Ascensao VF, Kennedy BJ. Estrogen receptors and responses to chemotherapy and hormonal therapy in advanced breast cancer. N Engl J Med. 1978;299(24):1330–4. doi: 10.1056/NEJM197812142992403. [DOI] [PubMed] [Google Scholar]
- 8.Clarke M, Coates AS, Darby SC, et al. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trials. Lancet. 2008;371(9606):29–40. doi: 10.1016/S0140-6736(08)60069-0. [DOI] [PubMed] [Google Scholar]
- 9.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. Jama. 2006;295(14):1658–67. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21(8):1431–9. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 11.Gluz O, Nitz UA, Harbeck N, et al. Triple-negative high-risk breast cancer derives particular benefit from dose intensification of adjuvant chemotherapy: results of WSG AM-01 trial. Ann Oncol. 2008;19(5):861–70. doi: 10.1093/annonc/mdm551. [DOI] [PubMed] [Google Scholar]
- 12.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 13.Guarneri V, Broglio K, Kau SW, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24(7):1037–44. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Yang H, Tong F, et al. Response to neoadjuvant therapy and disease free survival in patients with triple-negative breast cancer. Gan to kagaku ryoho. 2009;36(2):255–8. [PubMed] [Google Scholar]
- 15.Ring AE, Smith IE, Ashley S, Fulford LG, Lakhani SR. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2004;91(12):2012–7. doi: 10.1038/sj.bjc.6602235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colleoni M, Viale G, Zahrieh D, et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res. 2004;10(19):6622–8. doi: 10.1158/1078-0432.CCR-04-0380. [DOI] [PubMed] [Google Scholar]
- 17.Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21(22):4165–74. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 19.von Minckwitz G, Raab G, Caputo A, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol. 2005;23(12):2676–85. doi: 10.1200/JCO.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 20.Darb-Esfahani S, Loibl S, Muller BM, et al. Identification of biology-based breast cancer types with distinct predictive and prognostic features: role of steroid hormone and HER2 receptor expression in patients treated with neoadjuvant anthracycline/taxane-based chemotherapy. Breast Cancer Res. 2009;11(5):R69. doi: 10.1186/bcr2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esserman LJ, Perou C, Cheang M, et al. Breast cancer molecular profiles and tumor response of neoadjuvant doxorubicin and paclitaxel: The I-SPY TRIAL (CALGB 150007/150012, ACRIN 6657) J Clin Oncol. 2009;27(suppl):18s. abstr LBA515. [Google Scholar]
- 22.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11(16):5678–85. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 23.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 24.Keam B, Im SA, Kim HJ, et al. Prognostic impact of clinicopathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: paradoxical features of the triple negative breast cancer. BMC cancer. 2007;7:203. doi: 10.1186/1471-2407-7-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decatris MP, Sundar S, O’Byrne KJ. Platinum-based chemotherapy in metastatic breast cancer: current status. Cancer treatment reviews. 2004;30(1):53–81. doi: 10.1016/S0305-7372(03)00139-7. [DOI] [PubMed] [Google Scholar]
- 26.Kolaric K, Roth A. Phase II clinical trial of cis-dichlorodiammine platinum (cis-DDP) for antitumorigenic activity in previously untreated patients with metastatic breast cancer. Cancer Chemother Pharmacol. 1983;11(2):108–12. doi: 10.1007/BF00254257. [DOI] [PubMed] [Google Scholar]
- 27.Mechl Z, Sopkova B. CAP (cyclophosphamide, adriamycin, cisplatinum) in the treatment of advanced breast cancer. Neoplasma. 1984;31(4):431–5. [PubMed] [Google Scholar]
- 28.Sledge GW, Jr., Loehrer PJ, Sr., Roth BJ, Einhorn LH. Cisplatin as first-line therapy for metastatic breast cancer. J Clin Oncol. 1988;6(12):1811–4. doi: 10.1200/JCO.1988.6.12.1811. [DOI] [PubMed] [Google Scholar]
- 29.Bajorin D, Bosl GJ, Fein R. Phase I trial of escalating doses of cisplatin in hypertonic saline. J Clin Oncol. 1987;5(10):1589–93. doi: 10.1200/JCO.1987.5.10.1589. [DOI] [PubMed] [Google Scholar]
- 30.Forastiere AA, Hakes TB, Wittes JT, Wittes RE. Cisplatin in the treatment of metastatic breast carcinoma: A prospective randomized trial of two dosage schedules. Am J Clin Oncol. 1982;5(3):243–7. doi: 10.1097/00000421-198206000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Martino S, Samal BA, Singhakowinta A, et al. A phase II study of cis-diamminedichloroplatinum II for advanced breast cancer. Two dose schedules. J Cancer Res Clin Oncol. 1984;108(3):354–6. doi: 10.1007/BF00390472. [DOI] [PubMed] [Google Scholar]
- 32.Ostrow S, Egorin M, Aisner J, Bachur N, Wiernik PH. High-dose cis-diamminedichloro-platinum therapy in patients with advanced breast cancer: pharmacokinetics, toxicity, and therapeutic efficacy. Cancer Clin Trials. 1980;3(1):23–7. [PubMed] [Google Scholar]
- 33.Yap HY, Salem P, Hortobagyi GN, et al. Phase II study of cis-dichlorodiammineplatinum(II) in advanced breast cancer. Cancer Treat Rep. 1978;62(3):405–8. [PubMed] [Google Scholar]
- 34.Crown JP. The platinum agents: a role in breast cancer treatment? Semin Oncol. 2001;28(1 Suppl 3):28–37. doi: 10.1016/s0093-7754(01)90190-3. [DOI] [PubMed] [Google Scholar]
- 35.Chappuis PO, Nethercot V, Foulkes WD. Clinico-pathological characteristics of BRCA1- and BRCA2-related breast cancer. Semin Surg Oncol. 2000;18(4):287–95. doi: 10.1002/(sici)1098-2388(200006)18:4<287::aid-ssu3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Foulkes WD, Stefansson IM, Chappuis PO, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95c(19):1482–5. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275(31):23899–903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 38.Husain A, He G, Venkatraman ES, Spriggs DR. BRCA1 up-regulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum(II) Cancer Res. 1998;58(6):1120–3. [PubMed] [Google Scholar]
- 39.Moynahan ME, Cui TY, Jasin M. Homology-directed dna repair, mitomycin-c resistance, and chromosome stability is restored with correction of a Brca1 mutation. Cancer Res. 2001;61(12):4842–50. [PubMed] [Google Scholar]
- 40.Quinn JE, Kennedy RD, Mullan PB, et al. BRCA1 functions as a differential modulator of chemotherapy-induced apoptosis. Cancer Res. 2003;63(19):6221–8. [PubMed] [Google Scholar]
- 41.Tassone P, Tagliaferri P, Perricelli A, et al. BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br J Cancer. 2003;88(8):1285–91. doi: 10.1038/sj.bjc.6600859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lafarge S, Sylvain V, Ferrara M, Bignon YJ. Inhibition of BRCA1 leads to increased chemoresistance to microtubule-interfering agents, an effect that involves the JNK pathway. Oncogene. 2001;20(45):6597–606. doi: 10.1038/sj.onc.1204812. [DOI] [PubMed] [Google Scholar]
- 43.Leong CO, Vidnovic N, Deyoung MP, Sgroi D, Ellisen LW. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. J Clin Invest. 2007 doi: 10.1172/JCI30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uhm JE, Park YH, Yi SY, et al. Treatment outcomes and clinicopathologic characteristics of triple-negative breast cancer patients who received platinum-containing chemotherapy. International journal of cancer. 2009;124(6):1457–62. doi: 10.1002/ijc.24090. [DOI] [PubMed] [Google Scholar]
- 45.Garber JE, Richardson A, Harris LN, et al. Neo-adjuvant cisplatin (CDDP) in triple-negative breast cancer (BC). 2006 San Antonio Breast Cancer Symposium; San Antonio, TX. 2006; 2006. p. Absract # 3074. [Google Scholar]
- 46.Silver DP, Richardson AL, Eklund AC, et al. Efficacy of Neoadjuvant Cisplatin in Triple-Negative Breast. J Clin Oncol. doi: 10.1200/JCO.2009.22.4725. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan PD, Tung NM, Isakoff SJ, et al. Neoadjuvant cisplatin and bevacizumab in triple negative breast cancer (TNBC): Safety and efficacy. J Clin Oncol. 2009;27(suppl):15s. abstr 551. [Google Scholar]
- 48.Ezzat AA, Ibrahim EM, Ajarim DS, et al. Phase II study of neoadjuvant paclitaxel and cisplatin for operable and locally advanced breast cancer: analysis of 126 patients. Br J Cancer. 2004;90(5):968–74. doi: 10.1038/sj.bjc.6601616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frasci G, Comella P, Rinaldo M, et al. Preoperative weekly cisplatin-epirubicinpaclitaxel with G-CSF support in triple-negative large operable breast cancer. Ann Oncol. 2009;20(7):1185–92. doi: 10.1093/annonc/mdn748. [DOI] [PubMed] [Google Scholar]
- 50.Torrisi R, Balduzzi A, Ghisini R, et al. Tailored preoperative treatment of locally advanced triple negative (hormone receptor negative and HER2 negative) breast cancer with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxel. Cancer Chemother Pharmacol. 2008;62(4):667–72. doi: 10.1007/s00280-007-0652-z. [DOI] [PubMed] [Google Scholar]
- 51.Sirohi B, Arnedos M, Popat S, et al. Platinum-based chemotherapy in triple-negative breast cancer. Ann Oncol. 2008;19(11):1847–52. doi: 10.1093/annonc/mdn395. [DOI] [PubMed] [Google Scholar]
- 52.Byrski T, Huzarski T, Dent R, et al. Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2009;115(2):359–63. doi: 10.1007/s10549-008-0128-9. [DOI] [PubMed] [Google Scholar]
- 53.Gronwald J, Byrski T, Huzarski T, et al. Neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. J Clin Oncol. 2009;27(suppl):15s. doi: 10.1007/s10549-008-0128-9. abstr 502. [DOI] [PubMed] [Google Scholar]
- 54.Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: EGFR inhibition with cetuximab added to carboplatin in metastatic triple-negative (basal-like) breast cancer. J Clin Oncol. 2008 May 20;26(15S):1009. Supplement. [Google Scholar]
- 55.Isakoff SJ, Leong C, Vidnovic N, et al. p63/p73 expression mediates cisplatin sensitivity in a subset of triple-negative primary breast cancer: Implications for a new clinical trial. J Clin Oncol. 2007;25(18S):10522. [Google Scholar]
- 56.Byrski T, Foszczynska-Kloda M, Huzarski T, et al. Cisplatin chemotherapy in the treatment of BRCA1-positive metastatic breast cancer (MBC) J Clin Oncol. 2009;27(suppl):15s. abstr 1099. [Google Scholar]
- 57.Kilburn LS. ’Triple negative’ breast cancer: a new area for phase III breast cancer clinical trials. Clinical oncology (Royal College of Radiologists (Great Britain)) 2008;20(1):35–9. doi: 10.1016/j.clon.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 58.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976–83. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 59.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357(15):1496–506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 60.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358(16):1663–71. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazouni C, Kau SW, Frye D, et al. Inclusion of taxanes, particularly weekly paclitaxel, in preoperative chemotherapy improves pathologic complete response rate in estrogen receptor-positive breast cancers. Ann Oncol. 2007;18(5):874–80. doi: 10.1093/annonc/mdm008. [DOI] [PubMed] [Google Scholar]
- 62.Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27(8):1168–76. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352(22):2302–13. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 64.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23(16):3686–96. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 65.Byrski T, Gronwald J, Huzarski T, et al. Response to neo-adjuvant chemotherapy in women with BRCA1-positive breast cancers. Breast Cancer Res Treat. 2008;108(2):289–96. doi: 10.1007/s10549-007-9600-1. [DOI] [PubMed] [Google Scholar]
- 66.Wysocki PJ, Korski K, Lamperska K, Zaluski J, Mackiewicz A. Primary resistance to docetaxel-based chemotherapy in metastatic breast cancer patients correlates with a high frequency of BRCA1 mutations. Med Sci Monit. 2008;14(7):SC7–10. [PubMed] [Google Scholar]
- 67.Harris LN, Broadwater G, Lin NU, et al. Molecular subtypes of breast cancer in relation to paclitaxel response and outcomes in women with metastatic disease: results from CALGB 9342. Breast Cancer Res. 2006;8(6):R66. doi: 10.1186/bcr1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 69.Pinilla SM, Honrado E, Hardisson D, Benitez J, Palacios J. Caveolin-1 expression is associated with a basal-like phenotype in sporadic and hereditary breast cancer. Breast Cancer Res Treat. 2006;99(1):85–90. doi: 10.1007/s10549-006-9184-1. [DOI] [PubMed] [Google Scholar]
- 70.Altundag K, Harputluoglu H, Aksoy S, Gullu IH. Potential chemotherapy options in the triple negative subtype of breast cancer. J Clin Oncol. 2007;25(10):1294–5. doi: 10.1200/JCO.2006.10.0883. author reply 5-6. [DOI] [PubMed] [Google Scholar]
- 71.Petit T, Wilt M, Rodier J, et al. Are BRCA1 mutations a predictive factor for anthracycline-based neoadjuvant chemotherapy response in triple-negative breast cancers? J Clin Oncol. 2007 2007 ASCO Annual Meeting Proceedings Part I 20;June 20;Vol 25(No. 18S):580. Supplement. [Google Scholar]
- 72.Chappuis PO, Goffin J, Wong N, et al. A significant response to neoadjuvant chemotherapy in BRCA1/2 related breast cancer. Journal of medical genetics. 2002;39(8):608–10. doi: 10.1136/jmg.39.8.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheang M, Chia SK, Tu D, et al. Anthracyclines in basal breast cancer: The NCIC-CTG trial MA5 comparing adjuvant CMF to CEF. J Clin Oncol. 2009;27(suppl):15s. abstr 519. [Google Scholar]
- 74.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055–65. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rugo HS, Roche H, Thomas E, et al. Ixabepilone plus capecitabine vs capecitabine in patients with triple negative tumors: a pooled analysis of patients from two large phase III clinical studies. San Antonio Breast Cancer Symposium; San Antonio, TX. 2008; Abstract 3057. [Google Scholar]
- 76.Mehta RS. Dose-dense and/or metronomic schedules of specific chemotherapies consolidate the chemosensitivity of triple-negative breast cancer: a step toward reversing triple-negative paradox. J Clin Oncol. 2008;26(19):3286–8. doi: 10.1200/JCO.2008.17.1116. [DOI] [PubMed] [Google Scholar]