Abstract
The levels of expression of alternatively spliced variants of CTLA-4 and insufficient CTLA-4 signaling have been implicated in type 1 diabetes (T1D). Hence, we hypothesized that increasing CTLA-4 specific ligand strength on autoantigen presenting dendritic cells (DCs) can enhance ligation of CTLA-4 on T cells and lead to modulation of autoreactive T cell response. In this study, we show that DC directed enhanced CTLA-4 engagement upon pancreatic β-cell antigen presentation results in the suppression of autoreactive T cell response in non-obese diabetic (NOD) mice. The T cells from pre-diabetic NOD mice treated with an agonistic anti-CTLA-4-Ab coated DCs showed significantly less proliferative response, and enhanced IL-10 and TGF-β1 production upon exposure to β-cell antigens. Furthermore, these mice showed increased frequency of Foxp3+ and IL-10+ T cells, less severe insulitis, and a significant delay in the onset of hyperglycemia compared to mice treated with a control-Ab coated DCs. Further analyses showed that diabetogenic T cell function was modulated primarily through the induction of Foxp3 and IL-10 expression upon antigen presentation by anti-CTLA-4-Ab coated DCs. The induction of Foxp3 and IL-10 expression appeared to be a consequence of increased TGF-β1 production by T cells activated using anti-CTLA-4 Ab coated DCs and this effect could be enhanced by the addition of exogenous IL-2 or TGF-β1. Collectively, this study demonstrates the potential of DC directed CTLA-4 engagement approach not only in treating autoimmunity in T1D, but also in altering diabetogenic T cell function ex vivo for therapy.
Keywords: Type 1 diabetes, T cells, dendritic cells, co-stimulation, CTLA-4, regulatory T cells, NOD mice
Introduction
Type-1 diabetes (T1D) is an autoimmune disorder resulting from the specific destruction of insulin producing pancreatic β-cells and subsequent hyperglycemia. T cells, specific for β-cell antigens, play a major role in the disease process and they arise and expand likely because of defective immune regulation (1). Immune regulatory defects and disease susceptibility in humans and in experimental animals have been linked to the genetic loci encoding for cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) and major histocompatibility complex (MHC) proteins (2–4).
The CTLA-4 is a critical negative regulator of T-cell activation. This receptor plays a primary role in T cell homeostasis and peripheral tolerance through the inhibition of T cell activation, IL-2 production, and cell cycle progression as evidenced by the lethal lymphoproliferation seen in CTLA-4 deficient mice (5). The mechanisms by which CTLA-4 can mediate negative regulation of T cell responses include its ability to compete with CD28 for ligand binding, negatively regulate TCR signaling, induce release of a tolerogenic enzyme indoleamine 2,3-dioxygenase in APCs, and interfere with the lipid raft formation on the T cell surface and disrupting CD28 localization at the immunological synapse (6–10). These properties suggest the importance of CTLA-4 in maintaining peripheral tolerance and preventing autoimmunity (11–16). These studies together with recently identified abnormalities in the expression levels and signal strength of CTLA-4 in various autoimmune diseases including T1D (17,18) have raised the specter of modulating CTLA-4 signaling to treat autoimmune diseases.
Splice variants of CTLA-4 have been identified as potential risk factors contributing to the development of T1D in both patients and NOD mice (2,4,17). While the disease susceptibility is mapped to an allelic variation in the CTLA-4 gene and correlated with mRNA levels of the soluble CTLA-4 in humans, increased risk for developing the disease has been correlated with differential mRNA levels of a ligand independent form of CTLA-4 (liCTLA-4) in NOD mice (2,17). These reports suggest that inadequate levels of signaling through CTLA-4 could be responsible for inducing and/or promoting T1D, and therefore enhancing CTLA-4 signaling strength on self-antigen presenting APCs could prove effective in preventing autoimmunity.
Recently, using mice that were immunized with specific foreign- and self-antigens, we demonstrated that enhanced engagement of CTLA-4 from antigen presenting dendritic cells (DCs) induces adaptive Tregs that express Foxp3, IL-10 and TGF-β1(16). In a different study, we have shown that preferential ligation of CTLA-4 by CD80 upon pancreatic β-cell antigen presentation results in the generation of TGF-β1 and IL-10 producing adaptive Tregs (19). These observations along with the noted abnormalities of CTLA-4 signaling in T1D prompted us to examine whether enhancing the strength of CTLA-4 signaling during pancreatic β-cell antigen presentation using a DC directed CTLA-4 engagement method could modulate the autoimmune response in T1D. Since autoimmune diseases occur spontaneously in humans, examining the therapeutic potential of this DC directed CTLA-4 engagement approach in a spontaneous autoimmune model is important to realize its clinical applicability. Therefore, we have tested β-cell antigen-pulsed DCs coated with an agonistic anti-CTLA-4-Ab for their ability to suppress autoreactive T cells and T1D in NOD mice. Our results show that this DC directed CTLA-4 engagement approach has the potential to suppress insulitis and produce significantly delayed hyperglycemia in NOD mice. Further, this disease suppressive effect was associated with an increase in the frequencies of Foxp3+, and IL-10 and TGF-β1 producing hypoproliferative T cells.
Materials and Methods
Mice
Wild-type NOD/LtJ and NOD.BDC2.5 TCR-transgenic (TCR-Tg) mice were originally purchased from the Jackson laboratory and breeding colonies were established and maintained in the pathogen free facility of the biological resources laboratory of the University of Illinois at Chicago. Glucose levels in the tail vein blood samples of wild-type mice were monitored with the Ascensia Micro-fill blood glucose test strips and an Ascensia Contour blood glucose meter (Bayer USA). The animal studies were approved by the animal care and use committee of the University of Illinois at Chicago.
Peptide antigens, cell-lines, and antibodies
Immunodominant β-cell antigen peptides (viz., 1. Insulin B(9–23), 2. GAD65(206–220), 3. GAD65(524–543), 4. IA-2beta(755–777) and 5. IGRP(123–145), and BDC2.5 TCR reactive peptide (YVRPLWVRME; referred to as BDC peptide) were custom synthesized (Genescript Inc) as described in the earlier studies (20–26) and used in this study. Peptides 1–5 were pooled at an equal molar ratio and used as β cell Ag for pulsing DCs for in vitro and in vivo experiments as described in our earlier studies (19,27), unless indicated otherwise.
Hamster anti-mouse CTLA-4 hybridoma (UCI0–4-F-I0–11) and anti-mouse CD11c hybridoma (N418) were purchased from ATCC and grown in serum free/protein free medium (BD Biosciences) and the Abs were purified using Protein L or A (Sigma-Aldrich) columns. Anti-CTLA-4-anti-CD11c bispecific-Ab (test BiAb) was prepared by susccinimidyl-3(2)pyridyldithiol propionate (SPDP): succinimidyl-4-(p-meleimidophenyl) butyrate cross-linking approach (SMPB) as described in our earlier studies (11,12,16). Briefly, equal amounts of anti-CD11c and anti-CTLA-4 Abs (in borate buffered saline; pH 8.5) were activated using SPDP and SMPB respectively. The SPDP activated anti-CD11c Ab was treated with dithiothreitol, desalted using a PD-10 column, concentrated by ultra-filtration, mixed with SMPB treated anti-CTLA-4 Ab, and incubated for 4 h. Free active groups of this Ab mixture were blocked with iodoacetamide and purified by gel-filtration chromatography using a sephacryl S200 column. Purified hamster IgG (Fitzgerald International) linked to the anti-CD11c Ab similarly served as a control (control BiAb). Antigen binding efficiencies of BiAbs were tested by FACS using bone marrow (BM) derived DCs (BMDCs) and ELISA using recombinant CTLA-4-Ig (R&D systems) as described earlier (16).
Purified anti-mouse-CD16/CD32 (FC block) Abs; FITC-conjugated anti-mouse CD11c, CD4, CD25, IFN-γ, IL-17, TNF-α and IL-10 Abs; PE-labeled anti-mouse CD80, CD86, CD40, I-Ag7, CD4, CD25, CTLA-4, CD28, CD69, TGF-β1, IL-10 and Foxp3 Abs, and streptavidin; biotin labeled and anti-mouse/human TGF-β1 (clone A-75-3), affinity purified anti-LAP Ab; PEcy5 labeled anti-mouse CD4 and CD62L Abs and streptavidin; PE-TR labeled anti-mouse CD4 Ab were purchased from Invitrogen, BD Pharmingen, eBiosciences, R&D Systems, or Biolegend Laboratories and used in various studies requiring FACS analyses. Magnetic bead-based cell isolation kits were purchased from Miltenyi Biotec. Paired Abs and required cytokine standards for detecting mouse IL-2, IL-4, IL-17, IFN-γ, and IL-10 (eBiosciences), and activated TGF-β1 (R&D systems or BD Pharmingen) were used in ELISA. Multiplex cytokine assay reagents were purchased from Biosource. The lowest detection limits of Ab pairs and reagents for these cytokines were <10 pg/ml. While either multiplex assay or ELISA method was employed for quantifying most cytokines, activated TGF-β1 was quantified by ELISA method.
BM derived DCs and coating with antibodies
DCs were generated in vitro from BM cells and coated with anti-CTLA-4-anti-CD11c or hamster IgG-anti-CD11c BiAb as described in our previous study (16). Briefly, prior to use in some experiments, DCs (1×106/ml) were incubated for 48 h at 37°C in the presence of an equal-molar mixture of immuno-dominant peptides (β cell Ag; 5 µg/ml), or BDC peptide (1µg/ml) and bacterial LPS (5 µg/ml). Cells were washed, incubated with control or test BiAb (10 µg/107 cells/ml) for 30 min on ice, washed further and used as control Ab or anti-CTLA-4 coated DCs for in vitro and/or in vivo experiments. Anti-CTLA Ab and control Ab coated DCs are referred to as anti-CTLA-4 DCs and control Ab DCs respectively. β cell Ag-pulsed anti-CTLA-4 Ab coated DCs and β cell Ag-pulsed control Ab coated DCs are referred to as β cell Ag pulsed anti-CTLA-4-Ab DCs and Ag-pulsed control Ab DCs respectively. Ab coated DCs were tested for bound Ab levels by FACS prior to every experiment after staining with FITC labeled anti-hamster IgG Ab. Maturation status of DCs was also confirmed using fluorochrome labeled Abs against activation markers, CD80, CD86, CD40 and MHCII by FACS as described before (16). The Ab coated DCs were cultured for an additional 36 h, tested for the expression levels of activation markers on the surface by FACS and spontaneously secreted cytokine levels in the culture supernatant by ELISA.
In vitro and ex vivo T cell assays
Ag-pulsed Ab-coated DCs (5× 104 cells/well) were cultured in 96-well round-bottom plates in triplicate along with CFSE labeled or unlabeled purified CD4+ T cells (1 × 105/well) from 8-week old NOD.BDC2.5 TCR-Tg mice. After 5 days of culture, CFSE labeled cells were stained using PE-linked CD4 specific Ab and examined for CFSE dilution by FACS. For some assays, recombinant IL-2 or TGF-β1, or neutralizing Abs against IL-10 and/or TGF-β1, were added to the culture wells. Purified total T cells from diabetic NOD mice were also incubated with β cell Ag-pulsed Ab-coated DCs. After 48 h, cells were pulsed with l µCi/well 3[H]thymidine for 18 h. Thymidine-incorporation was measured as described before (12). T cell proliferation against ex vivo antigenic challenge was tested by CFSE dilution assay using splenic and pancreatic LN (PnLN) cells from Ab-coated DC treated mice as described earlier (19,26). CFSE-dilution was measured by FACS analysis after 5 days of culture.
Treatment of NOD mice with Ab-coated DCs
Female NOD mice of different age groups were injected i.v. with 5×106 Ag-pulsed, or non-pulsed, control or anti-CTLA-4 Ab-coated DCs twice at a 15-day interval and examined for blood glucose levels every week. Two and/or 6 weeks post-injection, mice were sacrificed to test for T cell response to ex vivo challenge with the antigen, T cell phenotype and insulitis. In some experiments, six-week old NOD mice were adoptively transferred (i.v.) with CFSE labeled Abcoated DCs (5×106 cells/mouse). These mice were euthanized 36 h post-injection, and single cell suspensions from spleen, PnLNs, and pancreas were examined for CFSE+ DCs by FACS after staining with PE labeled anti-CD11c Ab.
FACS analysis
Cells were washed (with PBS supplemented with 2% FBS, pH 7.4) and Fc-receptors were blocked using anti-CD16/CD32 Ab or 5% rat serum on ice for 15 min. For surface staining, cells were incubated with FITC-, PE-, and PECy5 or PE-TR-labeled appropriate Abs in different combinations on ice for 30 min. For intracellular staining, surface stained cells were fixed, permeablized using fixation/permeablization kit (Ebiosciences), blocked using 5% normal rat serum, and incubated with fluorochrome labeled isotype control or marker specific Abs at room temperature. For some assays, in vitro stimulated cells were re-stimulated using PMA (50 ng/ml) and ionomycin (500 ng/ml) in the presence of brefeldin A (1 µg/ml) for 4 h before staining for intracellular cytokines. Stained cells were analyzed using FACS-Calibur or LSR analyzer, and the data were analyzed using CellQuest, WinMDI, or Weasel applications. Specific regions were marked, and the gates and quadrants were set based on background staining using isotype control Ab for data analysis.
Cytokine analysis
Cell-free supernatants, collected from 48 h T cell cultures, were tested for cytokines by ELISA or Luminex multiplex assays as per manufacturer’s directions (eBiosciences, BD pharmingen, R&D systems, and/or Biosource). The amount of cytokine was determined using an appropriate cytokine-specific standard curve. Background cytokine levels of effector cell cultures, in the absence of antigen, were subtracted from test values to calculate the actual cytokine response.
Adoptive transfer of T cells
Total T cells and CD4+CD25+ cells were purified using magnetic sorting kits from Miltenyi Biotec, cultured in round bottom 96 well plates for 48 h in the presence of anti-mouse CD3 Ab (2 µg/ml) and anti-mouse CD28 Ab (0.5 µg/ml), washed and injected into 6 week-old NOD-Scid mice individually and/or in combinations. These T cell recipients along with uninjected control mice were tested for blood glucose levels every week. In a separate experiment, purified CD4+ T cells from 6-week old BDC2.5 TCR-Tg mice cultured in the presence of Ab coated- BDC peptide pulsed DCs, as described above, with or without recombinant IL-2 or TGF-β1 for 4 days. T cells isolated from these cultures (1×106) were injected i.v. into 4-week old male NOD mice. Blood glucose levels in these mice were tested every other day. The animals with glucose levels >250 mg/dl for two consecutive bleeds were considered diabetic.
Histochemical analysis of pancreatic tissues
Pancreata were fixed in 10% formaldehyde, 5-µm paraffin sections were made, and stained with hematoxylin and eosin (H&E). Stained sections were analyzed in a blinded fashion, using a grading system, in which 0 = no evidence of infiltration, 1 = peri-islet infiltration, 2 <25%, 3 = 25–50%, and 4 = >50% infiltration of each islet as described in our earlier studies (19, 27,28). Areas that appeared to have complete loss of islets were not included in this grading approach. About 100 islets were examined from each group.
Statistical analysis
Mean, SD and statistical significance (p-value) were calculated for this study. In most cases, values of test group (Ag-pulsed anti-CTLA-4-Ab DCs) were compared with that of control group (Ag-pulsed control-Ab DCs) unless specified otherwise. Since same numbers of test and control values (data points) were compared, paired two tailed t-test was employed unless specified otherwise. Log-rank analysis was performed to compare diabetes free mice in the test group with that in the control group. Fisher’s exact test was employed for comparing the total number of infiltrated islets in the test groups versus the control group. A p-value of ≤0.05 was considered significant.
Results
DC directed CTLA-4 engagement suppresses β-cell antigen specific response of T cells from NOD mice
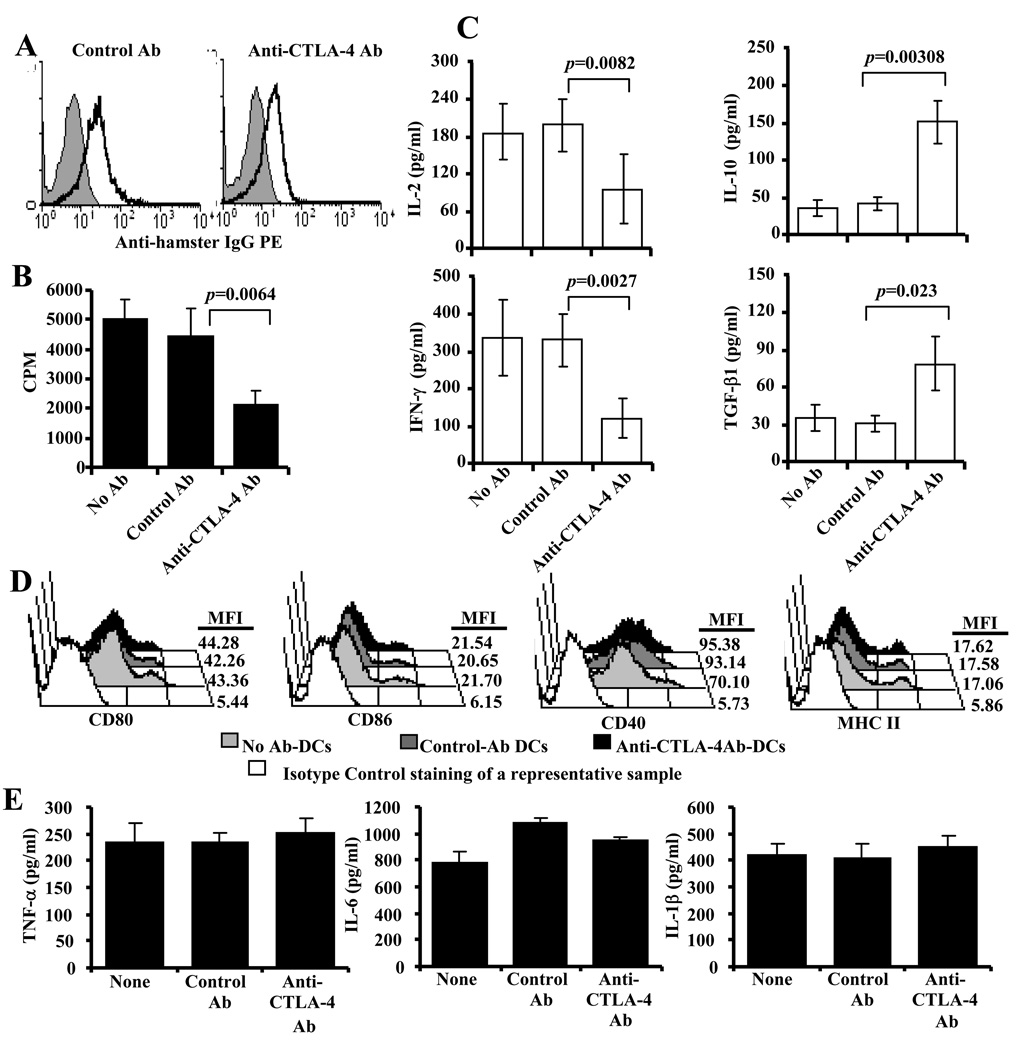
Recently, we reported a method to enhance selective engagement of CTLA-4 on T cells from antigen presenting DCs (16). In this DC directed CTLA-4 engagement approach, we used BiAb (an agonistic anti-CTLA-4-Ab chemically cross-linked to a DC specific anti-mouse CD11c–Ab) coated DCs. Anti-CD11c–Ab linked anti-CTLA-4 and control Abs were tested to confirm their ability to bind to BMDCs as shown in Fig. 1A. The ability of Ag-pulsed anti-CTLA-4-Ab DCs to activate T cells obtained from diabetic NOD mice in comparison with Ag-pulsed control-Ab DCs was tested in vitro. T cell proliferative response was significantly lower in cultures in which Ag-pulsed anti-CTLA-4-Ab DCs were used when compared to Ag-pulsed control-Ab DCs (Fig. 1B). As Observed in Fig. 1C, antigen presentation by Ag-pulsed anti-CTLA-4-Ab DCs resulted in significantly lower IL-2 and IFN-γ responses by T cells from NOD mice. Suppressed proliferative, IL-2, and IFN-γ responses by T cells upon β-cell antigen presentation by Ag-pulsed anti-CTLA-4-Ab DCs correlated with significantly enhanced IL-10 and TGF-β1 response by these T cells. These observations indicated enhanced CTLA-4-mediated negative signaling in T cells from NOD mice during pancreatic β-cell antigen presentation by anti-CTLA-4-Ab coated DCs.
FIGURE 1.
DC directed CTLA-4 engagement suppresses proliferation of T cells from diabetic NOD mice. BM DCs were generated, induced maturation using LPS for 48 h, pulsed with β-cell Ag, and coated with Abs as described in materials and methods. A) Control Ab (hamster IgG) and anti-CTLA-4 Ab coated BMDCs were tested for surface bound hamster IgG Ab by FACS. B) Purified splenic T cells from diabetic NOD mice (glucose: 250–400 mg/dl) were incubated with β cell Ag (a mixture of 5 immuno-dominant peptides; viz., 1. Insulin B(9–23), 2. GAD65(206–220), 3. GAD65(524–543), 4. IA-2beta(755–777) and 5. IGRP(123–145)) pulsed control and anti-CTLA-4 Ab DCs and tested for T cell proliferation. Cells were cultured for 48 h, pulsed with 3[H]-thymidine for an additional 18 h, harvested and tested for thymidine incorporation using a scintillation counter. Cultures in which DCs not pulsed with peptides were included as non-stimulated controls. Control Ab (hamster IgG) and anti-CTLA-4 Ab coated DCs were also used. C) Culture supernatants collected after 72 hours from an assay, similar to that described for A, were tested for cytokines IL-2, IFN-γ, IL-10, and TGF-β1 by ELISA. Background values (non-stimulated control) were subtracted from test values (peptide stimulated). Each bar represents mean±SD of the values of three individual mice tested in triplicate. D) Non-coated (none) and Ab coated DCs were incubated for additional 36 h, tested for surface markers by FACS. Mean fluorescence intensity (MFI) values of representative histograms are shown. E) 36 h supernatants from these cultures were tested for spontaneously (without additional stimulation) released cytokines by ELISA. Mean±SD values from a representative assay carried out in triplicate are shown.
To examine whether the anti-CTLA-4 Ab DC induced modulation of T cell response is the result of enhanced CTLA-4 engagement on T cells or due to some changes in the activation state of DCs upon antibody binding, Ab coated as well as uncoated DCs were cultured for 36 h and examined for surface markers by FACS and secreted cytokines by ELISA. As observed in Fig. 1D and 1E, although both control and anti-CTLA-4 Ab coated DCs expressed relatively higher levels of CD40 and IL-6 compared to uncoated DCs, no difference was observed in the surface activation marker or secreted cytokine levels between control Ab and anti-CTLA-4 Ab coated DCs. Of note, neither Ab coated DCs nor uncoated DCs produced detectable amounts of IL-12, IL-10, TGF-β1 and IL-2 (not shown). These observations indicated that the ability of anti-CTLA-4 Ab coated DCs to modulate T cell response could be primarily due to enhanced CTLA-4 engagement, and not a result of changes in the DC characteristics.
In vivo delivered DCs were detected in lymphoid and pancreatic tissues
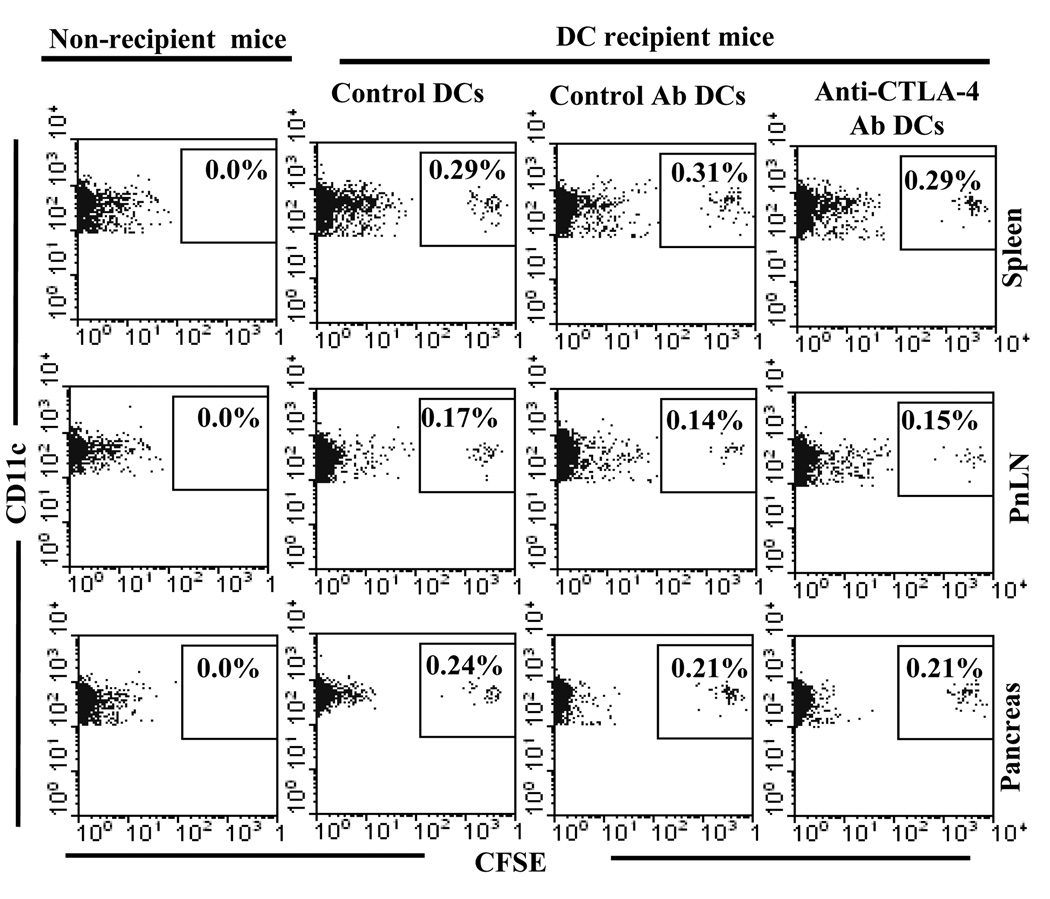
It is believed that pancreatic β-cell antigen specific T cells are primed primarily in the pancreatic lymph nodes (PnLNs), and reside in the pancreatic microenvironment (28–30). Therefore, we tested whether i.v. transferred BMDCs can end up in the pancreatic microenvironment and secondary lymphoid tissues. Eight-week-old female NOD mice were injected with untreated DCs, Ag-pulsed anti-CTLA-4-DCs or Ag-pulsed control-DCs. After 36 h, single cell suspensions from the spleen, PnLNs and pancreatic tissue of these mice were examined for CD11c+CFSE+ cells by FACS after staining with PE labeled anti-CD11c Ab. As shown in Fig. 2, considerable number of CD11c+CFSE+ donor DCs were detected not only in spleen and PnLNs, but also in the pancreas of all three groups of DC recipient mice. These results showed that the DC trafficking is not affected by Ab coating and suggested that anti-CTLA-4-Ab DCs might be able to present β-cell antigen and engage CTLA-4 on T cells in secondary lymphoid organs as well as the target tissue.
FIGURE 2.
Adoptively transferred BMDCs were detected in the lymphoid organs and pancreatic tissue. BMDCs were pulsed with β cell Ag, labeled with CFSE, left uncoated or coated with control or anti-CTLA-4 Ab, and injected i.v. into pre-diabetic female NOD mice (5×106 cell/mouse). One group of mice that did not receive DCs (non-recipient group) were used as background controls. Mice were euthanized after 24 h, single cell suspension of spleen, pancreatic LN and pancreata were examined for CD11c+CFSE+ cells by FACS. Representative scatter plots (gated for CD11c+ population) of three mice/group tested individually are shown. Percentage of CFSE+ population among CD11c+ cells is shown on each scatter plot.
Treatment using Ag-pulsed anti-CTLA-4-Ab DCs delays hyperglycemia
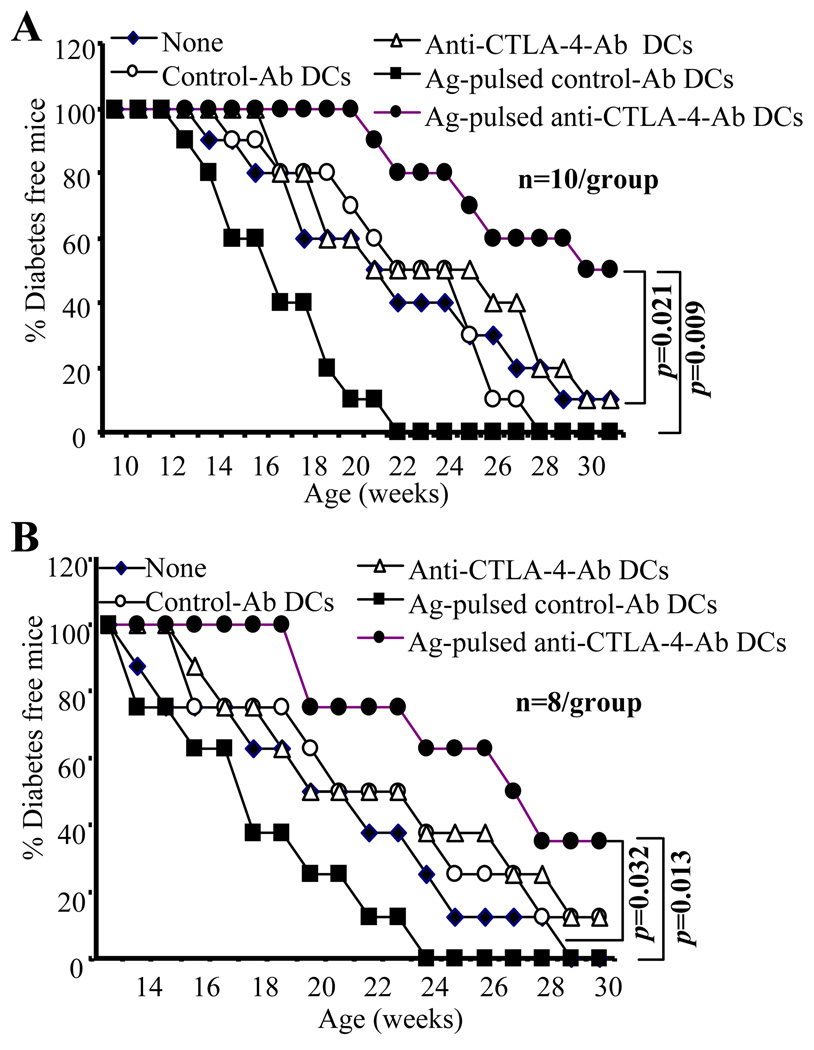
To examine whether DC directed CTLA-4 engagement approach that could suppress autoreactive T cells has an effect on T1D in NOD mice, pre-diabetic 8 or 12 week old female NOD mice were treated, twice at 15 days apart, with anti-CTLA-4 or control Ab coated DCs (5×106 cells/mouse). Ag-pulsed anti-CTLA-4-Ab DCs produced a significant delay in hyperglycemia compared to control mice or Ag-pulsed control-Ab DC recipient mice (Fig. 3A and B). Importantly, mice treated with Ag-pulsed control-Ab DCs developed hyperglycemia more rapidly compared to untreated control mice. Furthermore, anti-CTLA-4 or control Ab coated DCs that were not pulsed with Ag had no significant therapeutic effect even when the treatment was initiated at 8 weeks of age. These results show that while antigen presentation by control mature DCs advances hyperglycemia presumably through induction and/or expansion of auto-reactive T cells, anti-CTLA-4-Ab coated DCs delay hyperglycemia likely through suppression of these T cells. In addition, these results also indicated that the disease suppression observed in Ag-pulsed anti-CTLA-4-Ab DC recipients was most likely achieved through concurrent ligation of TCR and CTLA-4 on antigen specific T cells. Of note, Ag-pulsed anti-CTLA-4-Ab DC treatment had no significant effect on hyperglycemia when the therapy was initiated at an early hyperglycemic or diabetic stage (not shown) perhaps due to irrevocable damage to islets at these later stages of the disease.
FIGURE 3.
DC directed CTLA-4 engagement in pre-diabetic NOD mice delays hyperglycemia. Eight-week (A) and 12-week (B) old euglycemic female NOD mice were left untreated or treated with β-cell Ag -pulsed or non-pulsed control or anti-CTLA-4 Ab coated DCs (5×106 cells/mouse) twice 15-days apart. Mice were bled every week for up to 30 weeks of age to monitor blood glucose levels. Mice that showed glucose levels >250 mg/dl for two consecutive weeks were considered diabetic. Eight to 10 mice were included in each group, and the experiment was repeated with a similar number of mice/group. Values from each group were compared to that of untreated or Ag-pulsed control DC treated group using log-rank test and statistically significant values are shown. Statistically significant values obtained when Ag-pulsed anti-CTLA-4 Ab-DC recipient group was compared with untreated group (p=0.009 of panel A and p=0.013 of panel B) and Ag-pulsed control DC recipient group (p=0.021 of panel A and p=0.032 of panel B) are shown.
Treatment using Ag-pulsed anti-CTLA-4-Ab DCs results in suppressed insulitis in NOD mice
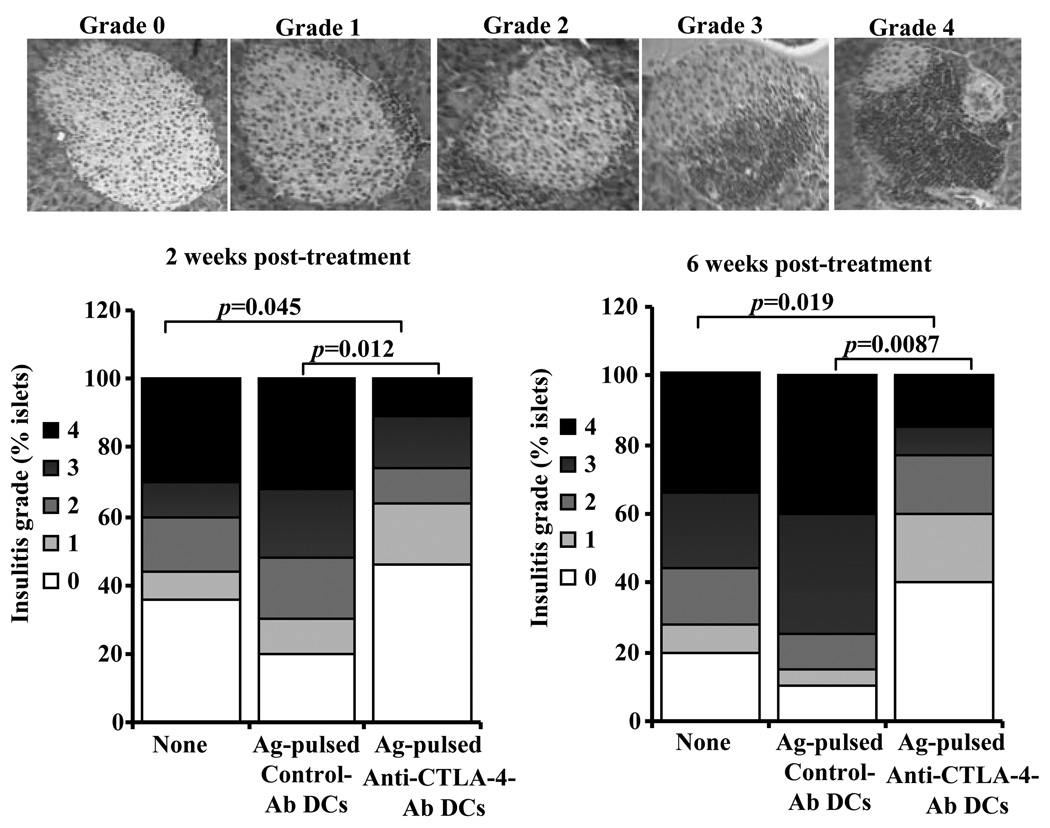
To examine whether treatment with Ag-pulsed anti-CTLA-4-Ab DCs has an effect on insulitis, pancreatic tissues from pre-diabetic NOD mice treated with Ag-pulsed anti-CTLA-4-Ab DCs and Ag-pulsed control-Ab DCs were examined for immune cell infiltration and islet damage. Sets of mice were euthanized 2- and 6-weeks after second injection with DCs to obtain pancreatic tissues. As observed in Fig. 4, mice that received Ag-pulsed anti-CTLA-4-Ab DCs had significantly higher numbers of islets with less severe or no immune cell infiltration compared to control mice or Ag-pulsed control-Ab DC recipient mice. While more than 40% of islets in Ag-pulsed anti-CTLA-4-Ab DC recipients were insulitis free at 2 and 6 weeks post-treatment, less than 20% of islets were immune cell infiltration free in Ag-pulsed control DC recipient mice. On the other hand, while more than 55% of islets in Ag-pulsed control-Ab DC recipient mice showed severe insulitis (grade ≥3), only less than 25% islets had severe immune cell infiltration in Ag-pulsed anti-CTLA-4-Ab DC recipients. In agreement with the disease data observed in Fig. 3, insulitis in Ag-pulsed control-Ab DC recipient mice was relatively more severe than that noted in untreated control mice indicating that treatment with peptide pulsed mature DCs can aggravate the disease process. These results show that dominant selective co-engagement of CTLA-4 along with β-cell antigen presentation by DCs could lead to reduced infiltration and destruction of pancreatic islets by immune cells, and delayed onset of hyperglycemia.
FIGURE 4.
Treatment with Ag-pulsed anti-CTLA-4-Ab DCs suppresses insulitis in pre-diabetic mice. Eight week old euglycemic female NOD mice were left untreated or treated with β cell Ag pulsed control or anti-CTLA-4 Ab coated DCs as described for Fig. 3 and examined for insulitis 2 and 6 weeks after second injection with DCs. H&E-stained pancreatic sections were examined in a blinded fashion and the severity of lymphocyte infiltration was scored as described in materials and methods. Representative sections with different grades of insulitis are shown in the upper panel. Bar diagrams in the lower panel show the percentage of islets in each group with different grades of insulitis. One hundred islets from at least five mice were examined for each group.
DC directed CTLA-4 engagement upon β-cell antigen presentation induces IL-10 and TGF-β1 producing hypo-proliferative T cells in NOD mice
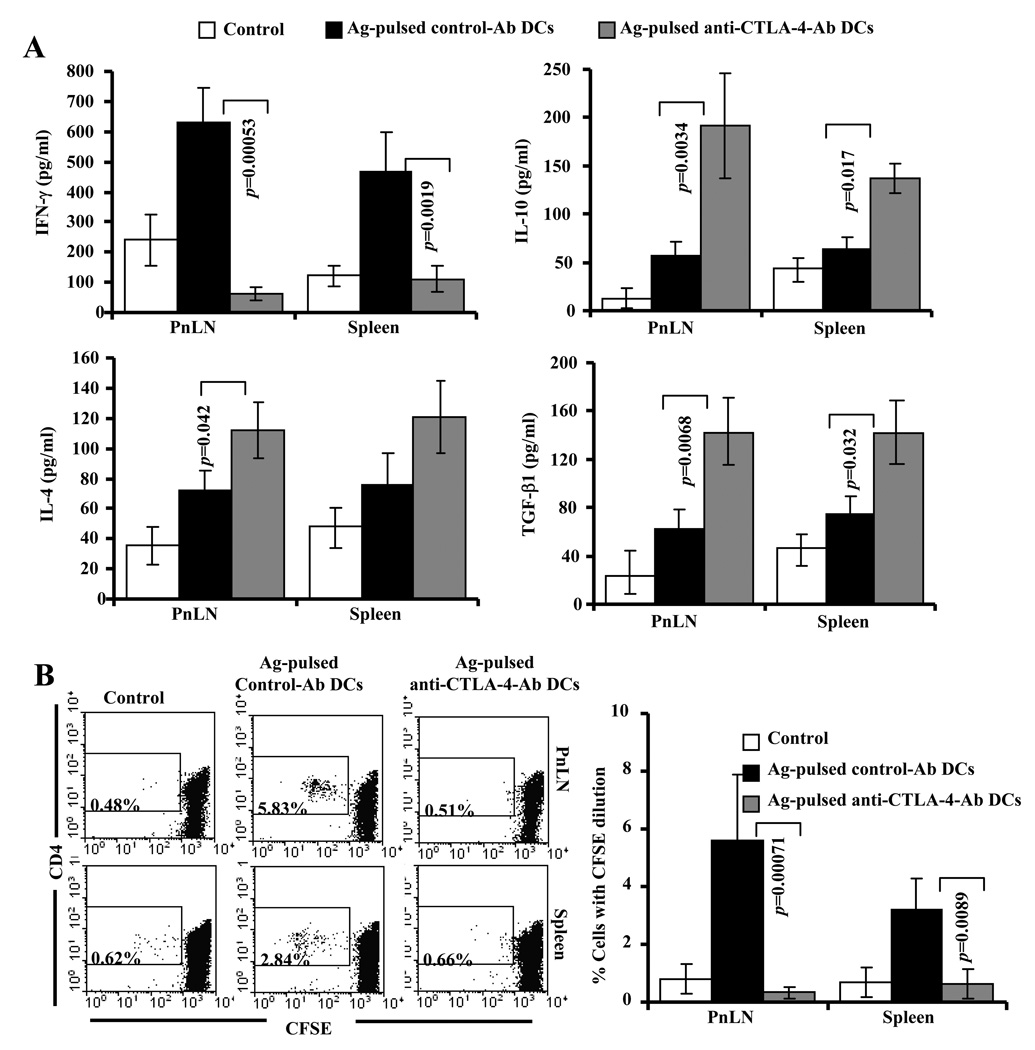
Next, we examined whether Ag-pulsed anti-CTLA-4-Ab DCs can modulate T cells response against β-cell specific antigen in vivo. Fifteen days post 2nd injection with DCs, peptide specific responses of T cells from DC recipients were compared. Ex vivo challenge of spleen and PnLN cells with β-cell antigen peptides resulted in significantly higher TGF-β1 and IL-10 responses by T cells from Ag-pulsed anti-CTLA-4-Ab DC recipients compared to Ag-pulsed control-Ab DC recipients and non-recipient control mice. In contrast, T cells from Ag-pulsed control DC recipients produced significantly higher IFN-γ response when compared to T cells from Ag-pulsed anti-CTLA-4-Ab DCs (Fig. 5A). In addition, PnLN T cells from Ag-pulsed anti-CTLA-4-Ab DC recipients produced relatively higher amounts of IL-4 compared to cells from Ag-pulsed control-Ab DC recipient mice. However, IL-17 response was comparable in both control and anti-CTLA-4 Ab coated DC recipient mice (not shown).
FIGURE 5.
DC directed CTLA-4 engagement induces suppressor cytokine producing hypo-proliferative T cells against β-cell Ag. Eight week old euglycemic female NOD mice were left untreated or treated with antigen-pulsed control or anti-CTLA-4 Ab coated DCs as described for Fig. 3. Cells from untreated (control) and DC recipient mice were examined ex vivo for antigen specific T cell response 15 days after second injection with DCs. A). Spleen and PnLN cells were incubated with β-cell Ag and the spent media collected from 72 h cultures were tested for IFN-γ, IL-4, IL-17, IL-10, and TGF-β1 by multiplex assay or ELISA. B) CFSE labeled spleen and PnLN cells were incubated with β cell Ag for 5 days, CFSE dilution in CD4+ population was examined by FACS after staining using fluorochrome-labeled CD4 specific Ab. Mean±SD values of cells from at least 5 mice tested in triplicate are shown for panels A and B. Representative scatter plots are also shown for panel B.
Examination of T cell proliferative response against β-cell antigen by CFSE dilution method revealed that a significant number of CD4+ T cells from Ag-pulsed control DC recipient mice, but not T cells from Ag-pulsed anti-CTLA-4-Ab DC recipient mice, proliferated in response to ex vivo peptide-challenge (Fig. 5B). Importantly, significantly higher number of T cells from Ag-pulsed control-Ab DC recipient mice proliferated upon peptide stimulation compared to untreated mice. Similar T cell proliferative and cytokine responses were observed with cells obtained from control and test groups of mice 6 weeks post-treatment (not shown). These observations suggested that while β-cell antigen presentation by control Ab coated DCs induced and/or expanded IFN-γ producing autoreactive T cells, dominant engagement of CTLA-4 during antigen presentation by anti-CTLA-4 Ab coated DCs induced IL-10 and TGF-β1 producing hypo-proliferative T cells.
Treatment using Ag-pulsed anti-CTLA-4-Ab DCs results in an increase in the frequencies of T cells with regulatory phenotype
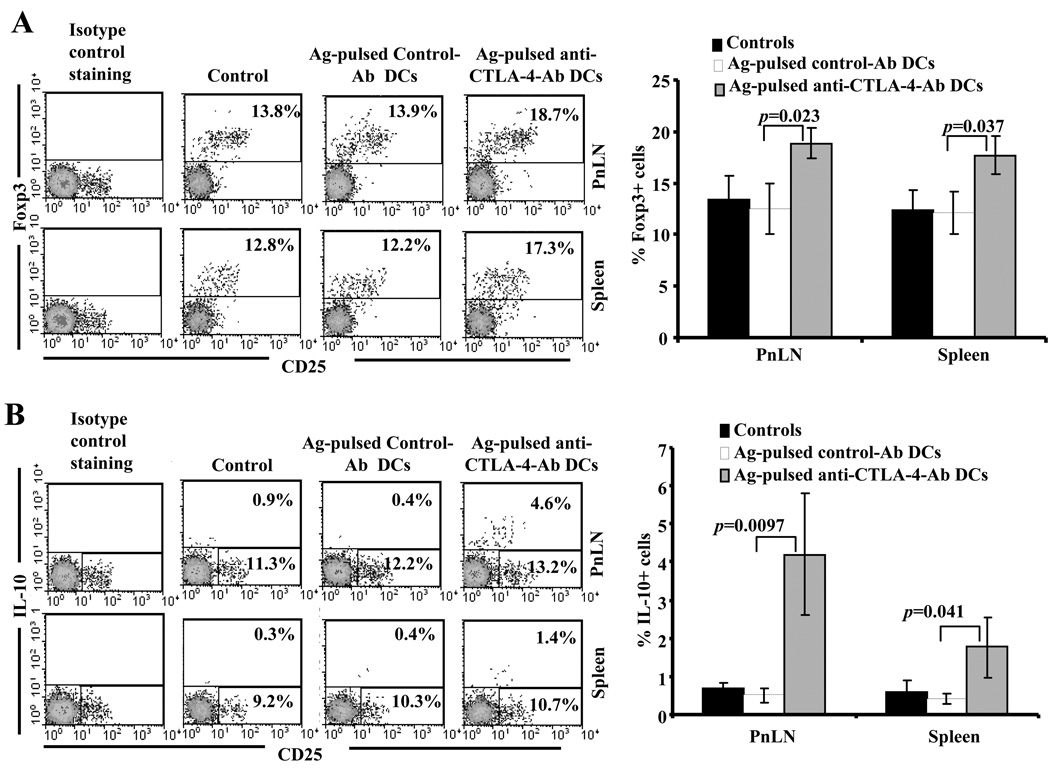
Since T cells from Ag-pulsed anti-CTLA-4-Ab DC treated mice were hypo-proliferative when stimulated with β-cell antigen peptides, we examined whether these T cells exhibit regulatory T cell phenotype. Splenic and PnLN cells were harvested and tested for intracellular Foxp3 and IL-10 without additional peptide challenge ex vivo. As shown in Fig. 6A and B, both splenic and PnLN T cells from Ag-pulsed anti-CTLA-4-Ab DC recipient mice showed significantly higher frequencies of Foxp3+ and IL-10+ T cell as compared to Ag-pulsed control-Ab DC recipients and non-recipient control mice. Since CD4+ T cells from Ag-pulsed anti-CTLA-4-Ab DC recipients secreted significant amounts of TGF-β1 (Fig. 5A), cells from these mice were examined for surface bound active and latent forms of TGF-β1. Unlike our recent study using antigen immunized models (16), NOD mice did not show a significant number T cells with surface bound active or latent forms of TGF-β1 (not shown). These results indicate that suppression of insulitis and delayed onset of hyperglycemia observed in Ag-pulsed anti-CTLA-4-Ab DC recipient NOD mice could be mediated by Foxp3 expressing, IL-10 and TGF-β1 secreting hypo-proliferative T cells.
FIGURE 6.
Treatment of NOD mice with Ag-pulsed anti-CTLA-4-Ab DCs results in the induction of Foxp3+ and IL-10+ T cells. Eight week old euglycemic female NOD mice were left untreated or treated with antigen-pulsed control or anti-CTLA-4 Ab coated DCs as described above for Fig. 3. On day 15 post-treatment, treated and untreated control mice were euthanized and freshly isolated spleen and pancreatic LN cells were stained for surface and intra-cellular markers using fluorochrome labeled Abs and analyzed by FACS. CD4+ population was gated for both panels. Representative scatter plots and percentage values for A) CD4+Foxp3+ and B) CD4+IL-10+ T cells (left panels) and mean±SD of the percentage values obtained using cells from at least 6 mice/group (right panels) are shown.
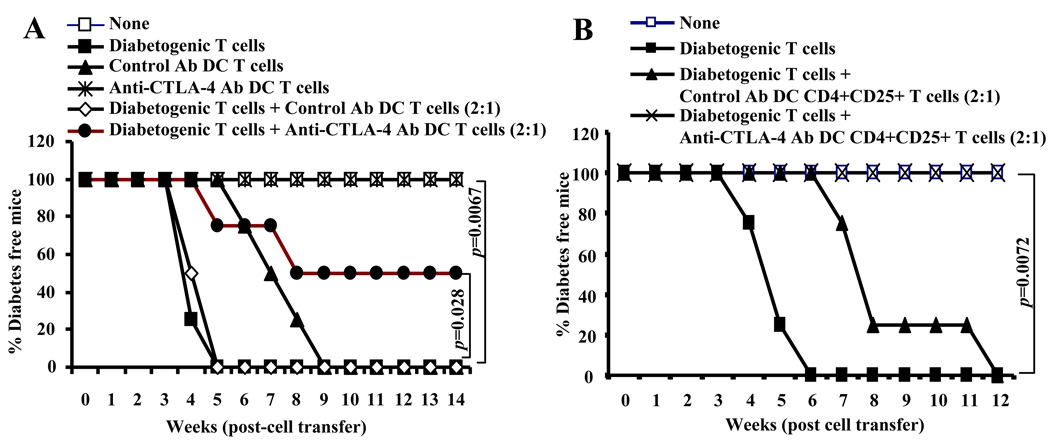
T cells from anti-CTLA-4 Ab DC recipient mice suppress diabetogenic properties of co-adoptively transferred T cells in NOD-Scid mice
Since NOD mice that were treated with anti-CTLA-4 Ab DCs showed delayed hyperglycemia and increased frequencies of Foxp3+ and IL-10+ T cells compared to control Ab DC recipient mice, T cells from these mice were tested for their pathogenic nature, if any, and the ability to suppress diabetogenic cell (i.e. splenic T cells from hyperglycemic mouse) transfer induced hyperglycemia in NOD-Scid mice. Fig. 7A shows that T cells from control Ab DC recipients, but not anti-CTLA-4 Ab DC recipient mice induced hyperglycemia in NOD-Scid mice. More importantly, total T cells from anti-CTLA-4 Ab DC recipient mice, but not control Ab DC recipient mice induced significant protection against diabetogenic T cell induced hyperglycemia. To examine the suppressor properties of Tregs from control and anti-CTLA-4 Ab DC recipient mice, purified CD4+CD25+ T cells were adoptively transferred along with diabetogenic T cells into NOD-Scid mice. As observed in Fig. 7B, although CD4+CD25+ T cells from control Ab DC recipient mice delayed the onset of hyperglycemia, CD4+CD25+ T cells from anti-CTLA-4 Ab DC recipient mice showed superior suppressor ability and prevented hyperglycemia in diabetogenic T cell recipient mice. These observations showed that treatment of NOD mice with β-cell antigen pulsed anti-CTLA-4 Ab DCs results in the induction and/or expansion of T cells with regulatory properties.
FIGURE 7.
T cells from anti-CTLA-4 Ab DC treated mice delay diabetogenic T cell transfer induced hyperglycemia. A) Six week old NOD-Scid mice were left untreated (none) or i.v. injected with purified T cells from hyperglycemic wild-type NOD mice (diabetogenic T cells) (2×106 cells/mouse) and β cell Ag pulsed control or anti-CTLA-4 Ab DC recipient mice (1×106 cells/mouse) separately or in combination. B) In a separate experiment, NOD-Scid mice that received diabetogenic T cells (2×106 cells/mouse) were also injected with purified CD4+CD25+ T cells from β cell Ag pulsed control or anti-CTLA-4- Ab DC treated mice (1×106 cells/mouse). All T cell preparations were stimulated using anti-CD3 Ab (2 µg/ml) and anti-CD28 Ab (0.5 µg/ml) (2×105/well cultured in round bottom pates) for 48 h before injection. Non-recipient and T cell recipient mice were tested for blood glucose levels every week. Mice that showed glucose levels >250 mg/dl for two consecutive bleeds were considered diabetic. Four mice were included in each group. Statistical significance was determined using log-rank test by comparing 1) anti-CTLA-4 Ab-DC T cell recipient group with control Ab-DC T cell recipient (p=0.0067 in panel A); 2) Diabetogenic T cells + Anti-CTLA-4 Ab DC T cell recipient group with diabetogenic T cells + Control Ab DC T cell recipient group (p=0.028 in panel A); 3) diabetogenic T cells + anti-CTLA-4 Ab DC CD4+CD25+ T cell recipient group with Diabetogenic T cells + control Ab DC CD4+CD25+ T cell recipient group (p=0.0072 in panel B).
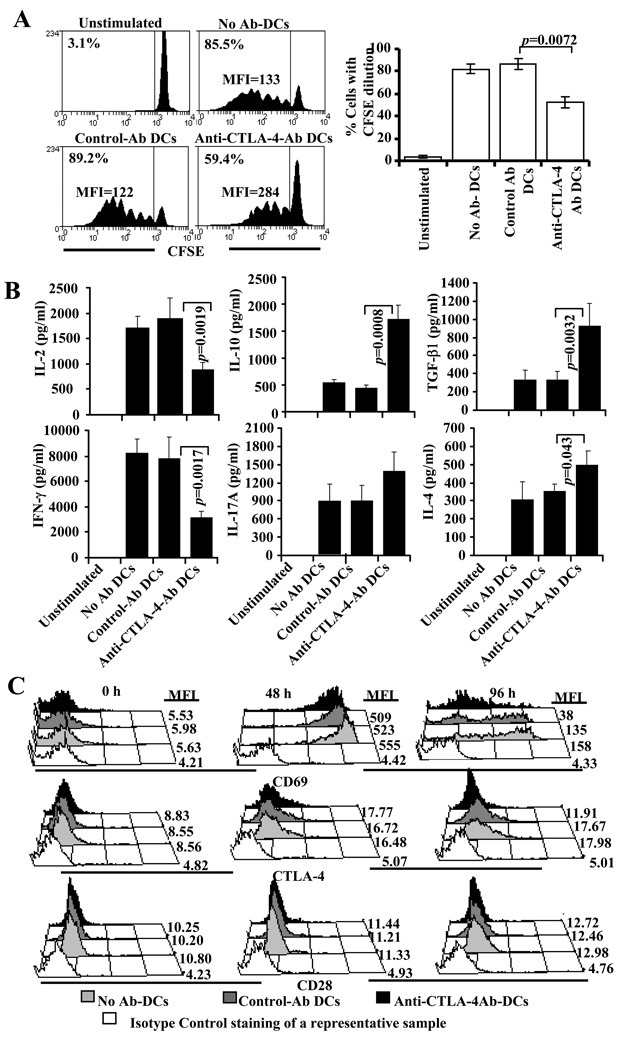
Suppression of T cell activation upon DC directed CTLA-4 engagement is not a result of down regulation of CD28 expression
As observed in Figs 1 and 5, treatment of NOD mice with Ag-pulsed anti-CTLA-4-Ab DCs caused an increase in the frequencies of TGF-β1 and IL-10 producing T cells. Others and we have shown that CTLA-4 engagement induces IL-10 and TGF-β1 production by T cells (12,16,31,32). A recent study showed that CTLA-4 mediated suppression of T cell response results from down-modulation of CD28 expression on CD8+ T cells (33). Therefore, we examined how CD4+ T cell function is modulated using T cells from NOD-BDC2.5 TCR-Tg mice. As anticipated, presentation of BDC peptide by anti-CTLA-4 Ab coated DCs induced relatively lower proliferation of BDC2.5 T cells compared to control Ab coated DCs (Fig. 8A). Further, consistent with the observation of Fig.1, these T cells produced significantly lower IL-2 and IFN-γ, but higher IL-10 and TGF-β1 compared to T cells that were cultured with Ag-pulsed control-Ab DCs (Fig. 8B). We examined the expression levels of co-stimulatory receptor CD28, activation marker CD69, and co-inhibitor CTLA-4 on these T cells at different time-points. As observed in Fig. 8C, no significant difference in the levels of CD28 expression was observed on T cells cultured with anti-CTLA-4- or control-Ab DCs. Although levels of CTLA-4 and CD69 were comparable at early time-points, expression levels of these markers were relatively lower on T cells cultured with Ag-pulsed anti-CTLA-4-Ab DCs at later time-points indicating that these cells are in a less-activated state. This observation indicates that IL-10 and TGF-β1 produced upon enhanced CTLA-4 engagement may be responsible for the suppressed activation state and expression of relatively lower levels of CTLA-4 and CD69 on these T cells. These results also indicated that enhanced CTLA-4 engagement does not significantly affect CD28 expression on CD4+ T cells and suppression of T cell proliferation by anti-CTLA-4 Ab coated DCs may be cytokine dependent.
FIGURE 8.
DC directed enhanced engagement of CTLA-4 does not down-regulate CD28 expression, but promotes regulatory cytokine production leading to suppression of activated T cells. A) Non-pulsed or BDC peptide-pulsed DCs without (none), or with control or anti-CTLA-4 Ab coating were incubated with CFSE labeled purified CD4+ T cells from NOD.BDC2.5 TCR-Tg mice. Cells from these cultures were tested for CFSE dilution by FACS on day 4 after staining with PE-labeled anti-CD4 Ab. CD4+ T cells were gated for this panel. Representative histogram plots and percentage values of CD4+ T cells with CFSE dilution (left panel) and mean±SD of values from two independent assays carried out in triplicate (right panels) are shown. B) Supernatants collected from 72 h parallel cultures were tested for cytokines by ELISA. Mean±SD of values from three separate experiments carried out in triplicate are shown for panel B. C) Purified BDC2.5 T cells were cultured with BDC peptide-pulsed non-coated (control DCs) or Ab coated DCs for different durations, stained with fluorochrome labeled CD4, CD28, CTLA-4, CD69 specific Abs, and analyzed by FACS. The CD4+ population was gated for this panel. Each sub-panel shows a representative sample stained using an isotype control Ab and overlay of samples stained using Ab for a specified marker, and mean fluorescence intensity (MFI) value for each sample. The assay was repeated twice in triplicate with similar results.
IL-10 and TGF-β1 produced by T cells upon enhanced CTLA-4 engagement are important in inducing and/or expanding adaptive Tregs
As observed in Fig. 8, T cells activated using anti-CTLA-4 Ab DCs showed significantly suppressed proliferative, IFN-γ and IL-2 responses compared to those activated using control Ab DCs. On the other hand, antigen presentation by anti-CTLA-4 Ab DCs resulted in IL-10 and TGF-β1 production by T cells. Therefore, we examined the role of IL-10 and TGF-β1 in enhanced CTLA-4 engagement mediated modulation of T cell activation. Addition of IL-10 and TGF-β1 neutralizing Abs individually or in combination resulted in a significantly increased proliferative response by T cells in anti-CTLA-4 Ab DC containing cultures (Fig. 9A). Moreover, IFN-γ and IL-2 levels were significantly increased in these cultures upon neutralization of IL-10 and/or TGF-β1(Fig. 9B). These results suggest that IL-10 and TGF-β1 produced as a result of antigen presentation by anti-CTLA-4 Ab coated DCs are important in suppressed proliferation, and IL-2 and IFN-γ production by T cells.
FIGURE 9.
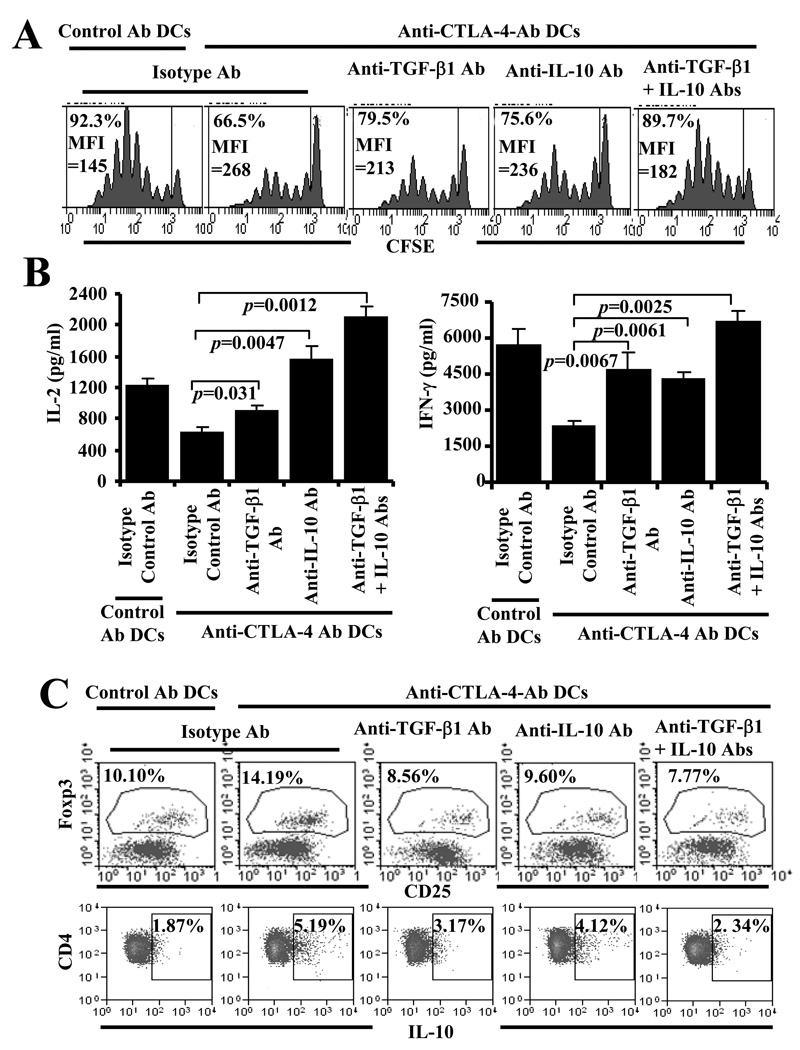
TGF-β1 and IL-10 are the key players of DC directed enhanced CTLA-4 engagement mediated suppression of T cell proliferation and induction of Foxp3 and IL-10. A) BDC peptide-pulsed DCs that were coated with control or anti-CTLA-4 Ab and incubated with CFSE labeled purified CD4+ T cells from NOD.BDC2.5 TCR-Tg mice in the presence of isotype control Ab, or anti-IL-10 and/or anti-TGF-β1 Ab (5 µg individual Ab/ml). Cells from these cultures were tested for CFSE dilution by FACS on day 4 after staining with PE-labeled anti-CD4 Ab. CD4+ T cells were gated for this panel. Representative histogram plots and percentage values of CD4+ T cells with CFSE dilution from two independent assays carried out in triplicate are shown. B) Supernatants collected from 72 h parallel cultures were tested for cytokines by ELISA. Mean±SD of values from 2 separate experiments carried out in triplicate are shown for panel B. C) Unstained BDC2.5 T cells were cultured with Ab coated DCs and different Abs as described for panel A, stained for surface CD4 and CD25, and intracellular Foxp3 or IL-10 and analyzed by FACS. Cells tested for IL-10 were stimulated for 4 h using PMA and ionomycin in the presence of brefeldin A before staining. The CD4+ population was gated for the scatter plots shown and the percentages of Foxp3+ and IL-10+ populations are shown on each scatter plot. Representative scatter plots and percentage values from two independent assays carried out in triplicate are shown. Regions were set based on the background staining using flourchrome labeled isotype control Ab for panels A and C.
Anti-CTLA-4 Ab DCs could induce IL-10 and TF-β1 production by T cells upon antigen presentation and these regulatory cytokines are known to have the potential to promote adaptive Treg generation from effector T cells during activation. Therefore, Foxp3+ and IL-10+ T cell frequencies were examined in cultures in which anti-CTLA-4 Ab DCs were used for antigen presentation. Considerably higher numbers of T cells in anti-CTLA-4 Ab DC containing cultures expressed Foxp3 and IL-10 compared to control Ab DC containing cultures (Fig. 9C). However, addition of TGF-β1 neutralizing Ab alone or along with anti-IL-10 neutralizing Ab negated this increase in Foxp3+ and IL-10+ T cell frequencies in anti-CTLA-4 Ab DC containing cultures. These results indicated that TGF-β1 and IL-10 produced by T cells upon antigen presentation by anti-CTLA-4 Ab DCs can promote adaptive Treg induction and/or expansion.
TGF-β1 produced by T cells activated using anti-CTLA-4 Ab DCs promotes Foxp3+ Treg induction and/or expansion
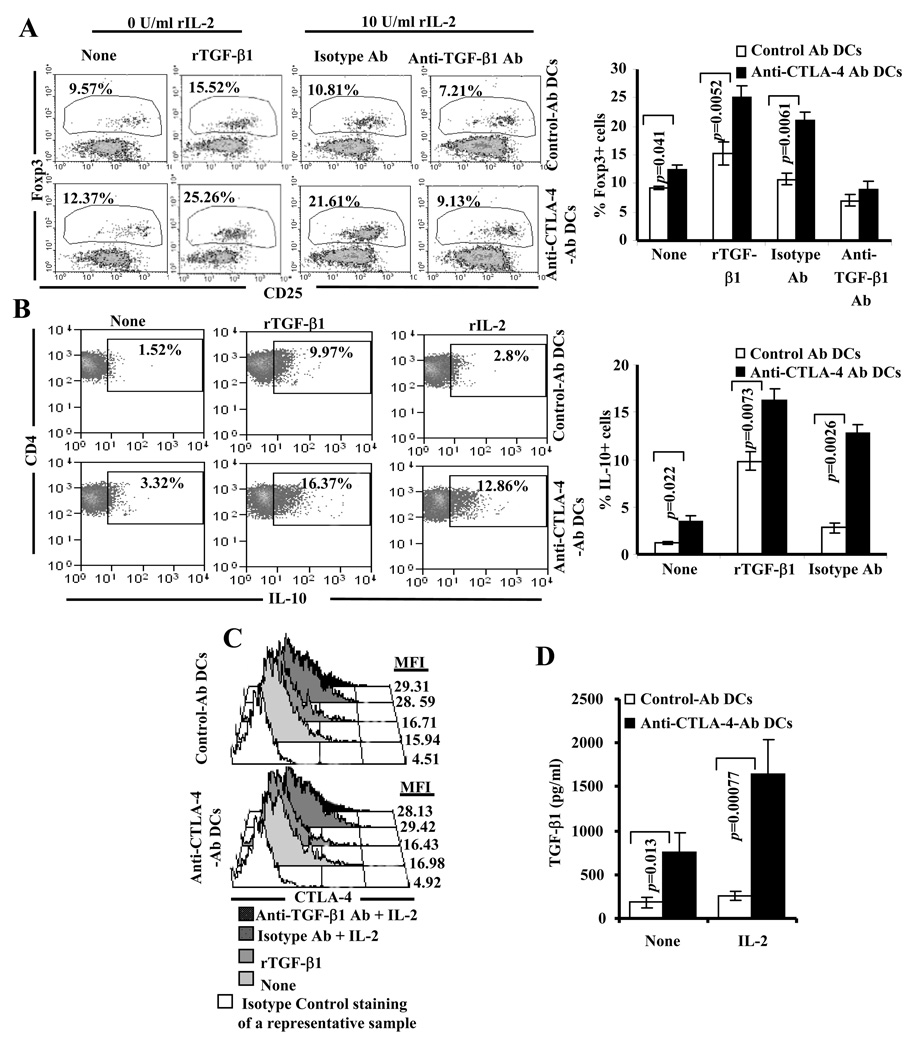
Above described observations and our previous report (16) showed that enhanced engagement of CTLA-4 upon presentation of antigen can result in the induction and/or expansion of T cells that express IL-10, TGF-β1 and Foxp3. Studies have shown that TGF-β1 is the critical cytokine known to promote adaptive Treg induction, and IL-2 supports this activity (34–37). In addition, CTLA-4 plays an important role in TGF-β1 mediated adaptive Treg induction (38). Although enhanced engagement of CTLA-4 on activated T cells induce TGF-β1 production, we and others have shown that CTLA-4 engagement can also suppress IL-2 production by these T cells (31,39). Therefore, whether TGF-β1 produced by T cells when stimulated with anti-CTLA-4 Ab DCs alone is sufficient to induce/expand T cells with regulatory properties was not known. In addition, what effect reduced levels of IL-2 may have on enhanced CTLA-4 engagement associated expression of Foxp3, IL-10 and TGF-β1 by T cells was also not known. To examine these aspects, BDC2.5 TCR-Tg T cells were stimulated using BDC peptide pulsed control- or anti-CTLA-4-Ab DCs with exogenous TGF-β1 or IL-2 and examined for Foxp3 expression. As anticipated, addition of TGF-β1 resulted in an increase in the Foxp3+ T cell frequency in both Ag-pulsed control- and anti-CTLA-4-Ab DC:T cell cultures compared to cultures that are not supplemented with exogenous cytokines (Fig. 10A). However, increase in the Foxp3+ T cell frequency in the presence of exogenous TGF-β1 was profoundly higher in anti-CTLA-4-Ab DC:T cell cultures compared to control Ab DC: T cell cultures. Of note, addition of rIL-10 did not result in an increase in Foxp3+ T cell frequency in these cultures (not shown). Interestingly, addition of rIL-2 also resulted in a significant increase in the frequency of Foxp3+ T cells in cultures where Ag-pulsed anti-CTLA-4-Ab DCs, but not Ag-pulsed control-Ab DCs, were present. Similar to the observation in Fig. 9A, neutralization of TGF-β1 in rIL-2 supplemented cultures also reversed the Foxp3 inducing effect of antigen presentation by anti-CTLA-4 Ab DCs on T cells (Fig. 10A). These observations suggest that although TGF-β1 produced by T cells when the antigen is presented by anti-CTLA-4 Ab coated DCs can promote Foxp3+ induction, reduced IL-2 levels in the microenvironment can limit this property of TGF-β1. Importantly, a significantly higher number of T cells from both rIL-2 and rTGF-β1 supplemented Ag-pulsed anti-CTLA-4-Ab DC:T cell cultures produced IL-10 (Fig. 10B). As observed in Fig. 10C and D addition of IL-2 increased CTLA-4 expression on T cells and elevated TGF-β1 production in the presence of Ag-pulsed anti-CTLA-4-Ab DCs indicating a further enhancement of CTLA-4 mediated signaling in T cells of these cultures. These observations show that IL-2 induced up-regulation of CTLA-4 on T cells likely facilitates selective enhanced ligation of this receptor by DC bound anti-CTLA-4 Ab and triggers larger amounts of TGF-β1 secretion leading to Foxp3 induction in an autocrine/paracrine manner.
FIGURE 10.
Exogenous IL-2 and TGF-β1 enhances DC directed CTLA-4 engagement mediated Foxp3+ Treg induction. Purified CD4+ T cells from BDC2.5 TCR-Tg mice were incubated with BDC peptide pulsed control or anti-CTLA-4 Ab coated DCs in the presence of recombinant IL-2 (10 U/ml) or TGF-β1 (1 ng/ml) for 4 days. A) Cells were stained using flurochrome labeled CD4, CD25, and Foxp3 specific Abs, and analyzed by FACS. In some assay wells, anti-TGF-β1 neutralizing Ab or isotype control Ab (5 µg/ml) was added. The CD4+ population was gated for the scatter plots shown here and the percentages of Foxp3+ population are shown on each scatter plot. B) After 96 h, cells that were cultured without or with exogenous IL-2 and TGF-β1 were stimulated for 4 h using PMA and ionomycin in the presence of brefeldin A, stained using flourochrome labeled anti-IL-10 Ab, and analyzed by FACS. The CD4+ population was gated for the scatter plots shown here and the percentages of IL-10+ population are shown on each scatter plot. Regions were set based on the background staining using flourchrome labeled isotype control Ab for both panel A and B. Representative scatter plots and percentage values of CD4+ T cells with Foxp3 or IL-10 expression (left sub-panels) and mean±SD of values (right sub-panels) from two independent assays carried out in triplicate are shown for both panel A and B. C) The T cells from parallel cultures were examined for surface levels of CTLA-4 by FACS. The CD4+ population was gated for the histograms. D) TGF-β1 levels in supernatants obtained from assay wells in which cells were cultured without rTGF-β1 and TGF-β1 neutralizing Ab were examined by ELISA. Mean±SD of values from two separate experiments carried out in triplicate are shown for this panel.
Ag-pulsed anti-CTLA-4-Ab DCs alter diabetogenic function of BDC2.5-TCR-Tg T cells
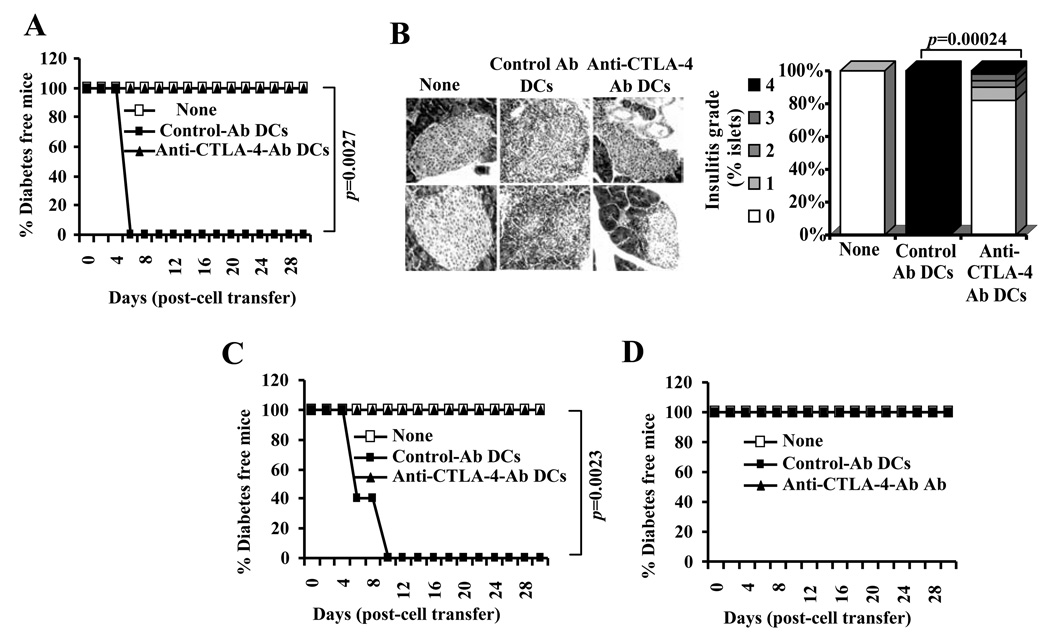
Since a large number of BDC2.5 TCR-Tg T cells cultured with Ag-pulsed anti-CTLA-4-Ab DCs in the presence of rIL-2 or TGF-β1 expressed Foxp3 and IL-10, diabetogenic property of these cells were examined in an adoptive transfer experiment. Many studies, including ours (27,28), have used NOD-Scid mice in adoptive transfer experiments to examine the diabetogenic nature of T cells. However, considering the potential of lymphopenia driven proliferation to affect the function of donor T cells in immune deficient NOD-Scid mice, we used wild-type male NOD mice that are relatively less susceptible to spontaneous T1D compared to females for this study. Four-week-old male NOD mice were injected with BDC2.5 T cells that were cultured in the presence of Ag-pulsed control- or anti-CTLA-4-Ab DCs with or without rIL-2 or rTGF-β1 and monitored for blood glucose levels. As observed in Fig. 11 A, 100% of mice that received T cells stimulated with Ag-pulsed control-Ab DCs developed hyperglycemia within 6 days. In contrast, none of the mice that received T cells stimulated with Ag-pulsed anti-CTLA-4-Ab DCs developed hyperglycemia for at least 30 days. In addition, only the mice that received control Ab DC stimulated T cells, but not T cells from anti-CTLA-4 Ab DC cultures, showed significant level of lymphocyte infiltration in the pancreatic islets (Fig. 11B). Similarly, mice that received T cells activated using control Ab DCs, but not anti-CTLA-4 Ab DCs, in the presence of rIL-2 developed rapid hyperglycemia (Fig. 11C). However, mice that received T cells stimulated using Ag-pulsed control or anti-CTLA-4 Ab DCs in the presence of rTGF-β1 remained non-diabetic (Fig. 11D). These observations show that enhanced CTLA-4 engagement upon antigen presentation alters the diabetogenic property of activated BDC2.5 T cells and suggest that approaches to enhance CTLA-4 signaling could be employed for ex vivo modification of T cell function before their use for therapy.
FIGURE 11.
DC directed enhanced CTLA-4 engagement alters the function of diabetogenic T cells. A) Purified CD4+ T cells from BDC2.5 TCR-Tg mice were incubated with BDC peptide pulsed control or anti-CTLA-4 Ab coated DCs for 4 days. T cells from these cultures were injected i.v. into 4-week-old male NOD mice (1×106/mouse; n=5/group), monitored for blood glucose levels, and the results are plotted as % diabetes free mice at different time-points. B) Pancreatic tissues obtained from an additional set of mice on day 5 post-T cell transfer were examined for insulitis as described under materials and methods. Representative sections with different grades of insulitis (left panel) and bar diagram showing the percentage of islets with different grades of insulitis in each group are shown (right panel). C and D) In parallel experiments, purified CD4+ T cells from BDC2.5 TCR-Tg mice were incubated with BDC peptide pulsed control or anti-CTLA-4 Ab coated DCs in the presence of recombinant IL-2 (10 U/ml) (panel C) or TGF-β1 (1 ng/ml) (panel D) for 4 days, T cells from these cultures were injected i.v. into male NOD mice (n=5/group) and monitored for blood glucose levels as described for panel A, and the results are plotted as % diabetes free mice at different time-points. Statistical significance of disease free status was assessed by log-rank test comparing control Ab coated DC group with anti-CTLA-4 Ab coated DC group.
Discussion
In this study, we examined the potential of enhanced CTLA-4 ligation by β-cell antigen presenting DCs in modulating autoimmunity in a spontaneous model of T1D. We show that treatment using pancreatic β-cell antigen-pulsed DCs coated with agonistic anti-CTLA-4 Ab can significantly delay T1D in NOD mice. This delay in hyperglycemia appears to be dependent on the suppressor function of Tregs induced and/or expanded upon enhanced engagement of CTLA-4 on effector T cells. Our observations demonstrate the potential of approaches to enhance the strength of CTLA-4 specific ligand on professional APCs to induce T cell tolerance and modulation of autoimmunity in T1D.
Susceptibility to T1D and other autoimmune diseases has been linked to CTLA-4 gene polymorphism (2,4,17). In addition, expression levels of soluble and ligand-independent forms of CTLA-4 have been implicated for the lack of peripheral tolerance to self-antigens (17). Previous studies have also suggested that T1D susceptibility is associated with an insufficient T cell regulation through CTLA-4 due to the expression of variants of CTLA-4, ligand strength on APCs and defects in T cell activation (17,18). On the other hand, studies have shown that peripheral and ex vivo generated DCs of T1D patients and NOD mice are defective and, perhaps, deficient in their ability to provide effective help to natural Tregs (40–43). Our studies demonstrating that the natural Treg function can be enhanced in autoimmune models by modulating DC function supported this notion (43–46). Based on these observations, we hypothesized that enhancing the CTLA-4 engagement strength through increasing its selective ligand intensity on pancreatic β-cell antigen presenting APCs would have a modulatory effect on autoreactive T cells. Our recent study using immunization models showing robust adaptive Treg induction upon treatment with antigen pulsed agonistic anti-CTLA-4 Ab coated DCs (16) further supported this notion. Therefore, to realize the clinical applicability of this approach, we examined whether enhancing the CTLA-4 specific ligand strength on pancreatic β-cell antigen presenting DCs using an agonistic Ab can modulate spontaneously occurring autoimmunity and T1D in NOD mice.
In spite of the widely reported abnormalities in DC function and CTLA-4 signaling in NOD mice, our observations show that antigen presentation by anti-CTLA-4 Ab coated NOD mouse DCs can suppress the proliferation of β-cell antigen specific T cells from the spleen cells of diabetic mice. Enhanced CTLA-4 engagement during β-cell antigen presentation not only resulted in the production of significant amounts suppressor cytokines such as of IL-10 and TGF-β1, but also suppressed IL-2 and IFN-γ responses. These findings indicated that the T cells are activated upon antigen recognition, but the cytokine profile is skewed as a result of enhanced CTLA-4 engagement. Importantly, activation of T cells by natural ligands expressed on antigen presenting DCs is critical for providing β-cell antigen specific signal to achieve the primary goal of selectively modulating autoreactive T cell function.
Although several immunodominant self-antigenic peptides are identified in T1D, it is widely believed that autoimmune response in T1D might be directed against a wide array of β-cell associated proteins. Therefore, use of one or a few known antigenic peptides for controlling T1D may have a limited therapeutic value. Nevertheless, the ability of Ag-pulsed anti-CTLA-4 DCs to induce significant amounts of suppressor cytokines and concomitantly suppress effector cytokine production in T cells from diabetic mice in vitro prompted us to examine whether these DCs can delay the onset and/or treat hyperglycemia in pre-diabetic and diabetic NOD mice. A significant delay in the onset of hyperglycemia observed in pre-diabetic mice that received Ag-pulsed anti-CTLA-4-Ab DCs suggests that the autoimmune response is suppressed in these mice, which prolongs the normal functioning of remaining β-cells. However, suppression of autoimmunity using Ag-pulsed anti-CTLA-4-Ab DCs alone does not appear to be sufficient to reverse established hyperglycemia perhaps due to the loss of a majority of the functional islets prior to the initiation of the treatment. Combinational approaches such as therapy to enhance β-cell mass and function along with DC directed CTLA-4 engagement might hold the potential for reversing already established hyperglycemia.
Relentless destruction of β-cells through apoptosis and sustained endogenous antigen presentation in the pancreatic microenvironment leads to continued β-cell destruction under inflammatory conditions and contribute to the progression of T1D (47,48). Therefore, it is possible that β-cell antigens are continuously released from dying or dead cells. If sufficient amounts of β cell antigens are released then treatment with DCs that are not loaded with antigenic peptide might also be able to produce similar protective effect by capturing, processing, and presenting endogenous β-cell antigens to T cells. However, our observations show that β-cell antigen-pulsed DCs, but not DCs that were not pulsed with antigen, modulated the disease outcome (either aggravation or suppression) indicating that mature DCs do not capture sufficient amounts of β-cell antigens in vivo and present to pathogenic T cells. Therefore, loading the DCs with antigenic peptide prior to anti-CTLA-4 Ab coating and injection is critical for achieving a significant therapeutic effect.
T cells from NOD mice treated with Ag-pulsed anti-CTLA-4-Ab DCs produced significant amounts of IL-10 and TGF-β1 suggesting that these cytokines and T cells may be responsible for protecting remaining functional β cells in pre-diabetic mice. These T cells appeared to be hypo-proliferative in nature as indicated by their inability to proliferate significantly upon challenge with β-cell antigenic peptides. While the suppressor cytokine response by T cells exposed to Ag-pulsed anti-CTLA-4-Ab DCs was significantly higher, the effector cytokine (IFN-γ) response was lower when compared to T cells from control-Ab DC recipients. This suggests that enhanced CTLA-4 engagement upon antigen presentation can skew the T cell response from pathogenic towards suppressor type.
The DCs are recognized as the only type of APCs that are capable of activating naïve T cells against a particular antigen. Although upon inoculation Ag-pulsed anti-CTLA-4-Ab DCs may primarily target existing memory T cells, it is possible that they will also activate naïve T cells through β-cell antigen presentation. Therefore, we assume that while Ag-pulsed control-Ab DCs can induce new pathogenic T cells from naïve cells along with expansion of existing memory T cells, Ag-pulsed anti-CTLA-4-Ab DCs may induce and/or expand antigen specific T cells with suppressor phenotype. This may partially explain why significantly higher number of T cells from control DC recipient mice compared to untreated control mice proliferated upon challenge with β-cell antigen. More severe insulitis in Ag-pulsed control-Ab DC recipient mice compared to untreated mice is also indicative of this effect. This is in contrast to the induction of hypo-proliferative T cells with suppressor phenotype that could delay the progression of insulitis upon treatment with Ag-pulsed anti-CTLA-4-Ab DCs.
Our earlier study using animals immunized with foreign- or self-antigen has shown that profound numbers of adaptive Tregs with Foxp3 expression and surface bound TGF-β1 are induced upon treatment using cognate Ag-pulsed anti-CTLA-4-Ab DCs (16). In contrast, current study using spontaneous autoimmune diabetic model showed no significant difference in the frequency of T cells with surface bound TGF-β1 in test and control groups of mice. Further, Treg induced and/or expanded in the spontaneous NOD model does not appear to be as robust as those we have found in the immunization induced disease model. This difference might be attributable to a relatively fewer antigen specific T cells in the former model. Further, in the current study, we used only a limited number of self-antigenic peptides and therefore T cells with specificities towards these peptides, which may represent only a small proportion of the pathogenic T cell repertoire present in this spontaneous disease model, were affected. Yet another explanation for the difference in the frequencies of Tregs in an immunization model versus a spontaneous model upon treatment using Ag-pulsed anti-CTLA-4-Ab DCs could be the difference in the amount of IL-2 and/or TGF-β1 produced in the lymphoid microenvironment during enhanced CTLA-4 engagement. As suggested by our in vitro experiments, both IL-2 and TGF-β1 can promote enhanced CTLA-4 engagement-mediated induction of T cells with regulatory properties. Since the overall levels of IL-2 and TGF-β1 in an immunization model are expected to be higher due to the presence of large numbers of antigen specific T cells compared to a spontaneous model, we believe that these cytokines may be contributing to a robust Treg response in the former model.
Irrespective of the above described difference in the Treg frequencies, current study shows relatively higher frequencies of Foxp3+ and IL-10+ T cells in freshly isolated spleen and PnLN of Ag-pulsed anti-CTLA-4-Ab DC recipient NOD mice compared to Ag-pulsed control-Ab DC recipients. In addition, as observed in our earlier study, T cells from Ag-pulsed anti-CTLA-4-Ab DC recipient mice secreted higher amounts of IL-10 and TGF-β1 upon challenge with antigen. This suggested that enhancing CTLA-4 specific ligand strength on DCs could be an effective way of promoting self-antigen specific tolerance.
Although self-antigen specific T cell tolerance is the key feature of our DC directed CTLA-4 engagement approach, we could not achieve a lasting protection from diabetes in NOD mice using a short-term treatment that targeted a limited repertoire of T cells with known antigen specificity. Failure to achieve long-term benefit may be due to the inherent defects in the NOD immune system, repertoire spreading to include other peptide specificities, regeneration and/or re-activation of autoreative T cells. Targeting a significant portion of autoreactive T cells by pulsing the DCs with a cocktail of full-length pancreatic β-cell antigens instead of selected peptides and/or intermittent injections with these therapeutic DCs may prove effective in achieving a lasting protection from T1D. Importantly, a robust increase in functional Treg frequency that may produce long lasting protection from the disease in anti-CTLA-4 Ab DC treated mice is hindered by enhanced CTLA-4 signaling mediated suppression of IL-2 secretion by T cells. Although enhanced CTLA-4 engagement upon antigen presentation can result in the production of significant amounts of IL-10 and TGF-β1 by T cells, this cytokine milieu alone does not appear to be sufficient to promote a robust Treg response even with a suppressed level of inflammatory cytokines such as IFN-γ. Therefore based on our observations showing the ability of exogenous IL-2 to promote a profound increase in Foxp3+ and IL-10+ T cell frequencies in anti-CTLA-4 Ab DC containing cultures, we believe that therapeutic efficacy of DC directed CTLA-4 engagement approach could be profoundly enhanced by co-administration of recombinant IL-2. Future studies are important and required to address this notion.
Approaches to modulate co-stimulatory and co-inhibitory functions are considered very effective in inducing T cell tolerance for treating autoimmunity and transplant rejection. While blockade of these pathways have shown therapeutic potential in various conditions, methods to enhance signaling through the dominant T cell repressor-receptor concurrent with TCR engagement has excellent therapeutic potential due to the active nature of T cell down-regulation. In fact, enhancing CTLA-4-specific ligand strength on APCs and target cells aimed at down-regulating T cell response has been tested in both allotransplant and autoimmune models (11,16). We have reported that thyroid targeted delivery of anti-CTLA-4 Ab leads to suppression of anti-thyroglobulin immune response and suppression of experimental autoimmune thyroiditis (11). Others and we have also shown that coating allogeneic cells with anti-CTLA-4 Ab or transfecting these cells to express single chain anti-CTLA-4 Ab on the surface could substantially reduce the immune response against that alloantigen in the recipient mice and induce adaptive Tregs with the ability to produce IL-10 and/or TGF-β1 (12–14,16). In addition, transgenic mice expressing anti-CTLA-4 agonistic Ab on B cells can delay T1D in NOD mice (T1D) (15). Similarly, co-administration of vector constructs encoding a CTLA-4 specific ligand, B7.1wa and an islet specific protein in a DNA vaccination study has demonstrated the ability to delay hyperglycemia in NOD mice (49). Our recent study showing that robust adaptive Treg response can be induced using Ag-pulsed anti-CTLA-4-Ab DCs in antigen-primed mice (16) indicated that enhancing CTLA-4 specific ligand strength on APCs, DCs in particular, is an effective strategy for treating autoimmunity. Importantly, defective DC function, CTLA-4 signaling and the spontaneous onset of the disease, as in human T1D patients (17,18,41–43), make NOD mouse model of T1D a unique system to demonstrate the therapeutic efficacy of this DC directed CTLA-4 engagement approach. This study also provides additional insights on the mechanism of enhanced CTLA-4 engagement mediated adaptive Treg induction and/or expansion and suggests methods to enhance the therapeutic efficacy of this approach.
Abbreviations
- BM
Bone marrow
- BMDCs
bone marrow derived dendritic cells
- BiAb
Bispecific Ab
- Tregs
regulatory T cells
- Foxp3
forkhead box p3 transcription factor
- TGF-β1
transforming growth factor 1 beta
- T1D
Type 1 diabetes
- β cell Ag
immuno-dominant β-cell antigen peptide mixture
- anti-CTLA-4-Ab DCs
Anti-CTLA-4 Ab coated dendritic cells
- control-Ab DCs
control Ab coated dendritic cells
- Ag-pulsed anti-CTLA-4-Ab DCs
immuno-dominant peptide pulsed anti-CTLA-4 Ab coated dendritic cells
- Ag-pulsed control-Ab DCs
immuno-dominant peptide pulsed control Ab coated DCs
Footnotes
This work was supported by the National Institutes of Health (NIH) grants R21 AI069848 and R01AI073858, and Juvenile Diabetes Research Foundation regular grants 1-2005-27 and JDRF-32-2008-343 to CV, NIH KO8 AI001821 to MJH, and NIH RO1 AI058190 to BSP.
References
- 1.Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, Ten S, Sanz M, Exley M, Wilson B, Porcelli S, Maclaren N. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 3.Gambelunghe G, Brozzetti A, Ghaderi M, Candeloro P, Tortoioli C, Falorni A. MICA gene polymorphism in the pathogenesis of type 1 diabetes. Ann N Y Acad Sci. 2007;1110:92–98. doi: 10.1196/annals.1423.011. [DOI] [PubMed] [Google Scholar]
- 4.Wicker LS, Clark J, Fraser HI, Garner VE, Gonzalez-Munoz A, Healy B, Howlett S, Hunter K, Rainbow D, Rosa RL, Smink LJ, Todd JA, Peterson LB. Type 1 diabetes genes and pathways shared by humans and NOD mice. J. Autoimmun. 2005;25 Suppl:29–33. doi: 10.1016/j.jaut.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 6.Lee K, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, Straus D, Samelson LE, Thompson CB, Bluestone JA. Molecular Basis of T Cell Inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 7.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 8.Darlington PJ, Baroja ML, Chau TA, Siu E, Ling V, Carreno BM, Madrenas J. Surface Cytotoxic T Lymphocyte-associated Antigen 4 Partitions Within Lipid Rafts and Relocates to the Immunological Synapse under Conditions of Inhibition of T Cell Activation. J. Exp. Med. 2002;195:1337–1347. doi: 10.1084/jem.20011868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 11.Vasu C, Gorla SR, Prabhakar BS, Holterman MJ. Targeted engagement of CTLA-4 prevents autoimmune thyroiditis. Int. Immunol. 2003;15:641–654. doi: 10.1093/intimm/dxg061. [DOI] [PubMed] [Google Scholar]
- 12.Vasu C, Prabhakar BS, Holterman MJ. Targeted CTLA-4 engagement induces CD4+CD25+CTLA-4high T regulatory cells with target (allo)antigen specificity. J. Immunol. 2004;173:2866–2876. doi: 10.4049/jimmunol.173.4.2866. [DOI] [PubMed] [Google Scholar]
- 13.Rao S, Vasu C, Martinez O, Kaithamana S, Prabhakar BS, Holterman MJ. Targeted delivery of anti-CTLA-4 antibody downregulates T cell function in vitro and in vivo. Clin Immunol. 2001;101:136–145. doi: 10.1006/clim.2001.5119. [DOI] [PubMed] [Google Scholar]
- 14.Hwang KW, Sweatt WB, Brown IE, Blank C, Gajewski TF, Bluestone JA, Alegre ML. Targeted ligation of CTLA-4 in vivo by membrane-bound anti-CTLA-4 antibody prevents rejection of allogeneic cells. J. Immunol. 2002;169:633–637. doi: 10.4049/jimmunol.169.2.633. [DOI] [PubMed] [Google Scholar]
- 15.Fife BT, Griffin MD, Abbas AK, Locksley RM, Bluestone JA. Inhibition of T cell activation and autoimmune diabetes using a B cell surface-linked CTLA-4 agonist. J. Clin. Invest. 2006;116:2252–2261. doi: 10.1172/JCI27856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Perez N, Karumuthil-Melethil S, Prabhakar BS, Holterman MJ, Vasu C. Enhanced engagement of CTLA-4 induces antigen-specific CD4+CD25+Foxp3+ and CD4+CD25 TGF-β1+ adaptive regulatory T cells. J. Immunol. 2007;179:5191–5203. doi: 10.4049/jimmunol.179.8.5191. [DOI] [PubMed] [Google Scholar]
- 17.Vijayakrishnan L, Slavik JM, Illés Z, Greenwald RJ, Rainbow D, Greve B, Peterson LB, Hafler DA, Freeman GJ, Sharpe AH, Wicker LS, Kuchroo VK. An autoimmune disease-associated CTLA-4 splice variant lacking the B7 binding domain signals negatively in T cells. Immunity. 2004;20:563–575. doi: 10.1016/s1074-7613(04)00110-4. [DOI] [PubMed] [Google Scholar]
- 18.Dahlén E, Hedlund G, Dawe K. Low CD86 expression in the nonobese diabetic mouse results in the impairment of both T cell activation and CTLA-4 up-regulation. J. Immunol. 2000;164:2444–2456. doi: 10.4049/jimmunol.164.5.2444. [DOI] [PubMed] [Google Scholar]
- 19.Perez N, Karumuthil-Melethil S, Li R, Prabhakar BS, Holterman MJ, Vasu C. Preferential Costimulation by CD80 Results in IL-10-Dependent TGF-{beta}1+-Adaptive Regulatory T Cell Generation. J. Immunol. 2008;180:6566–6576. doi: 10.4049/jimmunol.180.10.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao CC, McDevitt HO. Identification of immunogenic epitopes of GAD 65 presented by Ag7 in non-obese diabetic mice. Immunogenetics. 1997;46:29–34. doi: 10.1007/s002510050238. [DOI] [PubMed] [Google Scholar]
- 22.Kelemen K, Wegmann DR, Huton JC. T-cell epitope analysis on the autoantigen phogrin (IA-2β) in the nonobese diabetic mouse. Diabetes. 2001;50:1729–1734. doi: 10.2337/diabetes.50.8.1729. [DOI] [PubMed] [Google Scholar]
- 23.Quinn A, Sercarz EE. T-cells with multiple fine specificities are used by non-obese diabetic (NOD) mice in the response to GAD(524–543) J. Autoimmun. 1996;9:365–370. doi: 10.1006/jaut.1996.0049. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee R, Wagar D, Stephens TA, Lee-Chan E, Singh B. Identification of CD4+ T-cell-specific epitopes of islet-specific glucose-6-phosphatase catalytic subunit-related protein: a novel beta cell autoantigen in type 1 diabetes. J Immunol. 2005;174:5306–5315. doi: 10.4049/jimmunol.174.9.5306. [DOI] [PubMed] [Google Scholar]
- 25.Wong FS, Karttunen J, Dumont C, Wen L, Visintin I, Pilip IM, Shastri N, Pamer EG, Janeway CA. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat. Med. 1999;5:1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 26.Judkowski V, Pinilla C, Schroder K, Tucker L, Sarvetnik N, Wilson DB. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T-cells from transgenic BDC2.5 nonobese diabetic mice. J.Immunol. 2001;166:908–917. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- 27.Li R, Perez N, Karumuthil-Melethil S, Vasu C. Bone marrow is a preferential homing site for autoreactive T-cells in type 1 diabetes. Diabetes. 2007;56:2251–2259. doi: 10.2337/db07-0502. [DOI] [PubMed] [Google Scholar]
- 28.Karumuthil-Melethil S, Perez N, Li R, Vasu C. Induction of innate immune response through TLR2 and dectin 1 prevents type 1 diabetes. J. Immunol. 2008;181:8323–8334. doi: 10.4049/jimmunol.181.12.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gagnerault C, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of beta cell reactive T-cells in NOD mice. J. Exp. Med. 2002;196:369–377. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabien N, Bergerot I, Maguer-Satta V, Orgiazzi J, Thivolet C. Pancreatic lymph nodes are early targets of T-cells during adoptive transfer of diabetes in NOD mice. J. Autoimmun. 1995;8:323–334. doi: 10.1006/jaut.1994.0025. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4(+) T cells. J. Exp Med. 1998;188:1849–1857. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jovasevic VM, Gorelik L, Bluestone JA, Mokyr MB. Importance of IL-10 for CTLA-4-mediated inhibition of tumor-eradicating immunity. J. Immunol. 2004;172:1449–1454. doi: 10.4049/jimmunol.172.3.1449. [DOI] [PubMed] [Google Scholar]
- 33.Berg M, Zavazava N. Regulation of CD28 expression on CD8+ T cells by CTLA-4. J. Leukocyte Biol. 2008;83:853–863. doi: 10.1189/jlb.0107065. [DOI] [PubMed] [Google Scholar]
- 34.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 35.Pyzik M, Piccirillo CA. TGF-beta1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets. J. Leukoc. Biol. 2007;82:335–346. doi: 10.1189/jlb.1006644. [DOI] [PubMed] [Google Scholar]
- 36.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J. Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 37.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J. Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan TJ, Letterio JJ, van Elsas A, Mamura M, van Amelsfort J, Sharpe S, Metzler B, Chambers CA, Allison JP. Lack of a role for transforming growth factor-beta in cytotoxic T lymphocyte antigen-4-mediated inhibition of T cell activation. Proc. Natl. Acad. Sci.U S A. 2001;98:2587–2592. doi: 10.1073/pnas.051632398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton-Williams EE, Martinez X, Clark J, Howlett S, Hunter KM, Rainbow DB, Wen L, Shlomchik MJ, Katz JD, Beilhack GF, Wicker LS, Sherman LA. Expression of diabetes-associated genes by dendritic cells and CD4 T cells drives the loss of tolerance in nonobese diabetic mice. J. Immunol. 2009;183:1533–1541. doi: 10.4049/jimmunol.0900428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng R, Bathjat K, Li Y, Clare-Salzler MJ. Defective maturation of myeloid dendritic cell (DC) in NOD mice is controlled by IDD10/17/18. Ann. NY Acad Sci. 2003;1005:184–186. doi: 10.1196/annals.1288.023. [DOI] [PubMed] [Google Scholar]
- 42.Marleau AM, Singh B. Myeloid dendritic cells in non-obese diabetic mice have elevated costimulatory and T helper-1-inducing abilities. J. Autoimmun. 2002;19:23–35. doi: 10.1006/jaut.2002.0597. [DOI] [PubMed] [Google Scholar]
- 43.Cheatem D, Ganesh BB, Gangi E, Vasu C, Prabhakar BS. Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function. Clin. Immunol. 2009;131:260–270. doi: 10.1016/j.clim.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasu C, Dogan RN, Holterman MJ, Prabhakar BS. Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J. Immunol. 2003;170:5511–5522. doi: 10.4049/jimmunol.170.11.5511. [DOI] [PubMed] [Google Scholar]
- 45.Gangi E, Vasu C, Cheatem D, Prabhakar BS. IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J. Immunol. 2005;174:7006–7013. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- 46.Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS. GM-CSF-induced CD11c+CD8a--dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int. Immunol. 2009;21:269–282. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaminitz A, Stein J, Yaniv I, Askenasy N. The vicious cycle of apoptotic beta-cell death in type 1 diabetes. Immunol. Cell. Biol. 2007;85:582–589. doi: 10.1038/sj.icb.7100093. [DOI] [PubMed] [Google Scholar]
- 48.Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, Lee MS. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27:321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Glinka Y, Chang Y, Prud’homme GJ. Protective regulatory T cell generation in autoimmune diabetes by DNA covaccination with islet antigens and a selective CTLA-4 ligand. Mol. Ther. 2006;14:578–587. doi: 10.1016/j.ymthe.2006.03.021. [DOI] [PubMed] [Google Scholar]