Abstract
Background
Chronic stress is a major health concern, often leading to depression, anxiety or when severe enough, post-traumatic stress disorder (PTSD). While many studies demonstrate that the amygdala is hyper-responsive in patients with these disorders, the cellular neurophysiological effects of chronic stress on the systems that underlie psychiatric disorders, such as the amygdala, are relatively unknown.
Methods
In this study, we examined the effects of chronic stress on the activity and excitability of amygdala neurons in vivo in rats. We used in vivo intracellular recordings from single neurons of the lateral amygdala (LAT) to measure neuronal properties, and determine the cellular mechanism for the effects of chronic stress on LAT neurons.
Results
We found a mechanism for the effects of chronic stress on amygdala activity, specifically that chronic stress increased excitability of LAT pyramidal neurons recorded in vivo. This hyperexcitability was caused by a reduction of a regulatory influence during action potential firing, facilitating LAT neuronal activity. The effects of stress on excitability were occluded by agents that block KCa channels, and reversed by pharmacological enhancement of KCa channels.
Conclusions
These data demonstrate a specific channelopathy that occurs in the amygdala after chronic stress. This enhanced excitability of amygdala neurons after chronic stress may explain the observed hyper-responsiveness of the amygdala in patients with PTSD, and may facilitate the emergence of depression or anxiety in other patients.
Keywords: chronic stress, amygdala, neuronal activity, in vivo intracellular electrophysiology, depression, membrane properties
Introduction
Chronic stress can cause a wide range of impairments. Chronic stress increases emotional reactivity of humans (1), as well as the behavioral indices of affect in rodents (2,3). In the extreme, chronic stress induces or exacerbates psychiatric disorders, such as depression, anxiety and post-traumatic stress disorders (4,5). The amygdala is a critical site for some of the effects of stress and stress hormones on affective behaviors (6–9). In particular, stressors can influence amygdala-dependent fear conditioning (10–20), generally increasing cue-specific fear conditioning in adult male rats. Increased emotion output can be driven by increased activity of the amygdala. In particular, the activity of neurons in the lateral nucleus (LAT) of the basolateral amygdala is associated with increased affective responses (21–23). Thus, chronic stress induces abnormally enhanced affective behavior in a manner that may be consistent with increased LAT neuronal activity. While there is some evidence for increased activity of LAT neurons after chronic stress (24,25), or a change of intrinsic properties (26), the mechanism underlying these effects is unknown. This study examines one potential neurophysiological substrate for the effects of chronic stress on emotion.
One fundamental contributor to the activity level of neurons is their responsiveness, or excitability. Numerous ion channels contribute to regulation of membrane excitability of LAT neurons, such as calcium-activated K+ (KCa) channels (27–29). Modulation of these channels is a potent means to regulate neuronal activity (30,31). We hypothesize that chronic stress diminishes the regulatory influence of KCa channels in LAT neurons, leading to hyperexcitability of LAT neurons.
We used in vivo intracellular recordings, a technique to study neuronal properties in the intact brain, to determine whether chronic stress increases LAT neuronal excitability, and if LAT hyperactivity occurs through a reduction of KCa channel activity. By understanding the mechanism for the negative impact of chronic stress we will move closer to the development of novel therapeutic strategies for reversing the effects of stress on mental health.
Methods
All procedures were performed in accordance with the Institutional Animal Care and Use Committee of Rosalind Franklin University of Medicine and Science, and followed the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Male Sprague-Dawley rats (Harlan; age 8–9 weeks at start) were used for this study. Chronic stress rats were placed in a restraint hemi-cylinder for 20 minutes per session, one session per day, for 7 out of 9 consecutive days. This pattern of stress exposure reduces inter-session habituation to restraint, which would otherwise be prominent (32,33). A control group was handled in the same manner as the restraint group for 9 days, except that they remained in a plexiglas transparent cage with bedding, instead of a restraint cylinder. The total amount of handling between groups was equivalent. All further experiments were performed one day after the final restraint session.
Independent measures of stress effectiveness
The behavioral impact of stress was assessed in the elevated plus maze (EPM, Scientific Designs, Pittsburgh, PA; arm 4.25” width × 19.75” length, 15.75” wall height). Rats were placed in the center of the maze and allowed to explore freely for 5 minutes under dim light. Arm entry was logged when all four paws entered the arm. When applicable, drug or vehicle (50% DMSO) was administered 25–30 min before placement in the EPM. Experimenters were blind to drug condition. An anatomical index of endocrine function was determined by removal and weighing of adrenal glands after the conclusion of experiments.
Electrophysiology
In vivo intracellular electrophysiological recordings were obtained from the amygdala of rats (34), specifically, the lateral nucleus of the amygdala (LAT; see Figure S1 in Supplement 1 for recording locations and expanded Methods). Rats were anesthetized with 8% chloral hydrate (all chemicals from Sigma-Aldrich, St. Louis,MO, unless noted otherwise), and supplemented as necessary. Electrodes were filled with 1–2% neurobiotin in 2 M potassium acetate. When indicated, electrodes also included other chemicals, including CsCl (200 mM), BaCl2 (100 mM), CdCl2 (0.5 mM), NiCl2 (0.5 mM), or 4,4′-dinitrostilbene-2,2′-disulfonic acid (0.5 mM DNDS; Tocris Bioscience, Ellisville, MO). These doses ensure blockade of the targeted channels.
Series resistance was compensated using built-in amplifier bridge circuitry (IR-183, Cygnus Technology, Delaware Water Gap, PA). The input resistance and membrane time constant, τ, were measured (see Methods in Supplement 1). Excitability was defined as the number of action potentials evoked by a series of depolarizing current steps (0 – 1000 pA, 800 ms, repeated 4–5 time at each current step). Excitability was measured as the slope of the linear fit to the relationship between the current intensity and the number of action potentials evoked by each depolarizing current step. The fast-, medium-, and slow-afterhyperpolarization potentials (AHP) were measured (see Methods in Supplement 1). In some experiments, rats were injected with drug treatments (1 mg/ kg or 10 mg/ kg 1-EBIO, or 50% DMSO vehicle, i.p.). In these instances, excitability was measured before drug (as above), and repeatedly at approximately 5 minute intervals. Multiple measures of excitability were collected 10–15 minutes after injection, averaged, and used for analysis. At the conclusion of experiments brains were histologically processed (see Methods in Supplement 1). Neurons were excluded from analysis if they were found to lie outside the LAT, if their action potentials did not overshoot 0 m V, they displayed firing characteristics inconsistent with basolateral amygdala pyramidal neurons in vivo (29,34), if their resting membrane potential was more depolarized than −60 m V, or if their morphology was inconsistent with pyramidal neurons (35).
Statistical analysis
When performing planned comparisons between two groups, two-tailed unpaired t-tests were used. All comparisons between more than 2 groups were made with one-way, two-way, or mixed model repeated measures two-way ANOVAs. An alpha level of 0.05 was considered significant. Post-hoc Student’s t-tests with Bonferroni corrections were used to compare individual groups if significant values were obtained in ANOVAs. Data were tested for normality of distribution (Kolmgorov and Smirnov test), and for equality of the standard deviation (Bartlett’s test). If data failed these tests, non-parametric tests were used. Statistical tests were performed using Igor Pro (Wavemetrics, Lake Oswego, OR) or Prism 5 software (GraphPad Software, La Jolla, CA). All values are expressed as the mean ± S.E.M.
Results
Repeated restraint causes behavioral and adrenal gland changes
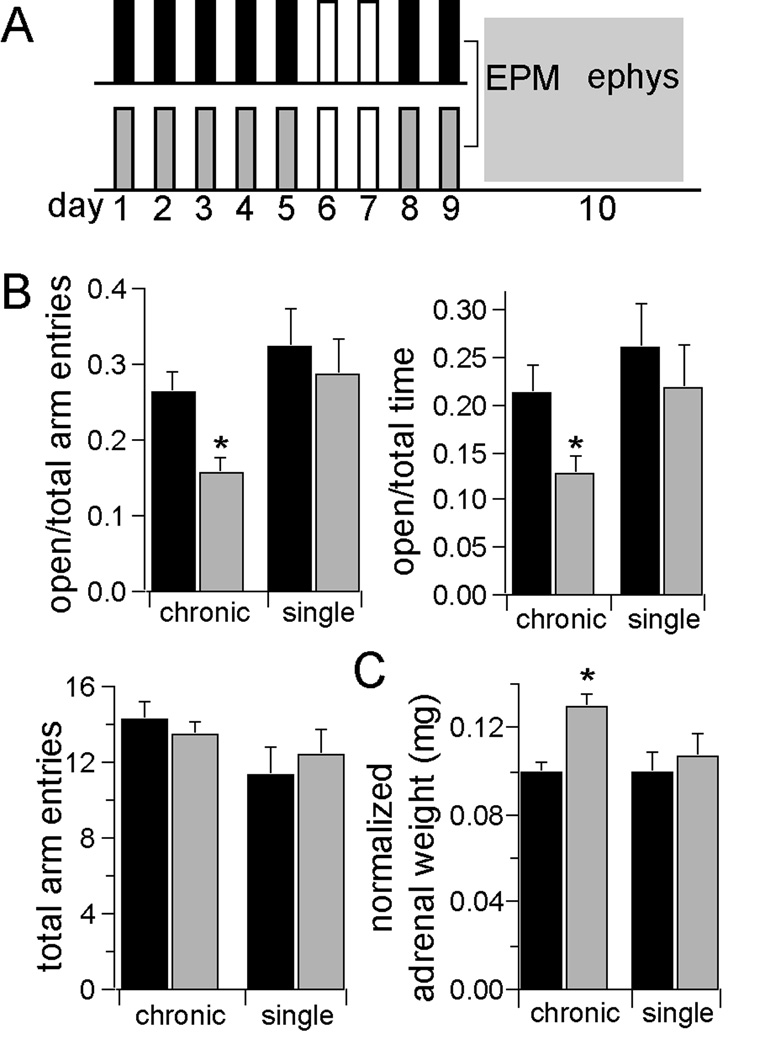
Effectiveness of a stressor, such as the commonly-used repeated restraint, can be measured by its impact on the endocrine system and on behavioral measures of anxiety. We examined aspects of both to ensure effectiveness of the restraint stress.
Repeated restraint resulted in less exploration in the elevated plus maze (EPM; see Fig. 1 and Results in Supplement 1), a measure that is sensitive to chronic stress, (2, 36), and an index of anxiety-like behaviors (37). The total number of arm entries was not significantly different between groups (Fig. 1b). One potential concern is that the observed effects may be caused by the last episode of restraint stress, and do not reflect the chronic nature of the stress. To demonstrate that the group differences are likely caused by the additive nature of the repeated stress, control groups were added that received only one restraint stress, or only one handling session one day prior to testing. Rats that experienced only a single restraint session one day prior to testing displayed smaller differences compared to their controls (Fig. 1 and Supplement 1, Results).
Figure 1. Repeated restraint is an effective chronic stressor.
A) Rats were placed in a restraint chamber for 20 minutes, on 7 days over a 9 day period (gray). Control rats (black) experienced a similar degree of daily handling, but were not restrained. Following the last day of restraint, all rats were tested in the elevated plus maze. Some rats were then prepared for electrophysiology experiments. Remaining rats went through a fear conditioning and testing procedure. B) Repeated restraint stress decreased open arm entries (percent of open arm entries control 25.4 ± 2.7%, n=36, stress 15.6 ± 2.1%, n=34, p=0.0006, two-tailed t-test, t=3.58) and the time spent in open arms (percent of time in open arm, control 21.3 ± 2.9%, n=36, stress 12.8 ± 1.8%, n=34, p=0.016, two-tailed unpaired t-test, t=2.49), indicative of increased anxiety-like state. There was no significant change in the total number of arm entries (control 14.3 ± 0.9 arm entries, stress 13.5 ± 0.7 arm entries, p=0.485, two-tailed t-test, t=0.702). Single restraint did not significantly impact EPM exploration. C) Repeated restraint stress increased the weight of adrenal glands, a prototypical measure of the effectiveness of a stressor measured as raw weight (control 22.7 ± 0.7, n=12, stress 28.1 ± 1.2 mg, n=12, p=0.013, two-tailed unpaired t-test, t=2.92), or normalized to body weight (control 0.10 ± 0.005 mg/ kg, stress 0.13 ± 0.006 mg/ kg, p=0.002, two-tailed unpaired t-test, t=3.94). Single restraint did not significantly increase adrenal gland weight (normalized to body weight, control 0.10 ± 0.009, n=6, stress 0.11 ± 0.01, n=7, p=0.57, two-tailed unpaired t-test, t=0.61). * indicates significance at p<0.05.
In parallel with this behavioral index, rats exposed to repeated restraint stress displayed a significantly greater weight of adrenal glands compared to control groups, when measured as raw weight or normalized to body weight (Fig.1c), an expected effect of chronic activation of the hypothalamic-pituitary-adrenal axis during chronic stress (38). The adrenal weights of rats exposed to a single stress session or handling did not significantly differ from each other (Fig. 1c).
Chronic stress increases in vivo LAT neuronal excitability
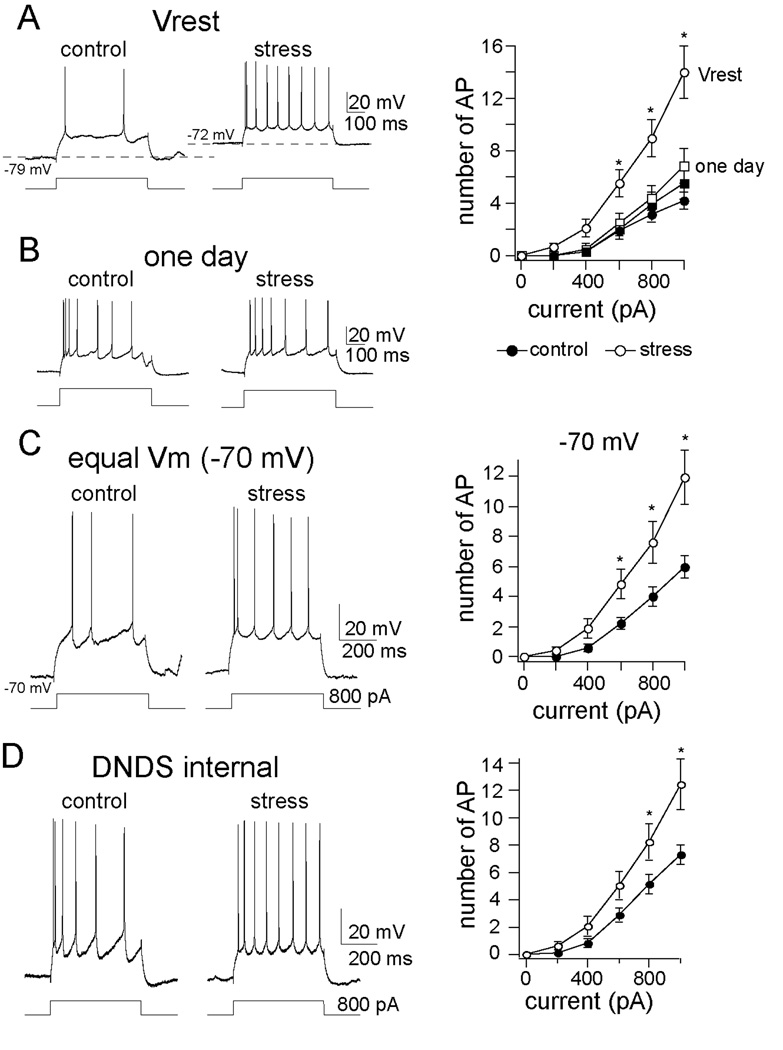
We examined whether chronic stress causes a hyperexcitability of LAT neurons that could underlie the effects of chronic stress on emotion. This was tested using in vivo intracellular recordings of LAT pyramidal neurons (Figure S1 in Supplement 1). In rats that were exposed to chronic stress, LAT neurons displayed a greater basal firing rate than in control rats (control 0.012 ± 0.006 Hz, n=21, stress 0.035 ± 0.008 Hz, n=25, p=0.038, two-tailed t-test, t=2.165). Neuronal excitability contributes to neuronal firing, and was measured to determine if chronic stress increases the responsiveness of LAT neurons. We found that chronic stress increased the excitability of LAT neurons (Fig. 2a; quantified as the slope of the relationship between current injection and action potential firing (slope of excitability), see Methods; control slope of excitability 0.68 ± 0.11 AP/ 100 pA, n=21, stress slope of excitability 1.55 ± 0.19 AP/ 100pA, n=25, p<0.001, two-tailed unpaired t-test, t=6.45). Furthermore, a single restraint stress administered the day before electrophysiological studies was not potent enough to induce an increase in LAT neuronal excitability (Fig. 2b; slope of excitability control 0.69 ± 0.18, n=7, slope of excitability stress 0.77 ± 0.20, n=7, p= 0.771, two-tailed unpaired t-test, t=0.297). This demonstrates that chronic stress increases excitability, and the repeated nature of the stress is an important determinant for the effects on LAT excitability.
Figure 2. Chronic stress increased the excitability of LAT neurons.
A) Repeated restraint stress increased excitability measured from the neuronal resting membrane potential (Vrest; mixed design repeated measures ANOVA of each stimulation intensity, main effect of stimulation intensity F(5,264) = 31.67, p<0.001; main effect of stress F(1, 264) = 47.12, p<0.001; interaction F(5, 264) = 8.19, p<0.001). Shown here are voltage traces at the resting membrane potential of a neuron from the chronic handling control (left, Vrest = −79 m V; black circle in plot) and the chronic stress (right, Vrest = −72 m V, white circle in plot), in response to the same amplitude of current injection. B) The effect of chronic stress on excitability was only observed after repeated restraint, and not after a single restraint session (mixed design repeated measures ANOVA of each stimulation intensity, main effect of stress F(1, 72) = 0.79, p=0.376; main effect of stimulation intensity F(5,72) = 24.02, p<0.001; interaction F(5,72) = 0.20, p=0.96). This is measured as the response of these neurons to a depolarizing steps (squares in plot, one day control, black; one day stress, white). C) Repeated restraint stress caused an increase of LAT neuronal excitability when the membrane potential was held near −70 m V (mixed design repeated measures ANOVA of each stimulation intensity, main effect of stress F(1,264) = 22.19, p<0.001; main effect of stimulation intensity F(5,264) = 34.55, p<0.001; interaction F(5,264) = 3.39, p=0.005, * indicates p<0.05 between control and stress group in post-hoc unpaired t-tests with Bonferroni corrections). D) The effects of chronic stress on excitability were still observed when DNDS was included in the recording pipette (mixed design repeated measures ANOVA, main effect of stress F(1, 126) = 18.93, p<0.001, main effect of stimulation intensity F(5,126) = 47.59, p<0.001; control 0.88 ± 0.09 AP/ 100 pA, n=12; stress 1.67 ± 0.08 AP/ 100 pA, n=11, p<0.001, two-tailed unpaired t-test, t=6.51), demonstrating that the effects of chronic stress on excitability were not caused by a reduction of GABAergic inhibition.
There are several possible underlying causes for increased excitability, including 1) depolarization of the resting membrane potential, 2) a reduction in GABAergic inhibition, 3) increased neuronal responsiveness to subthreshold input, and 4) a change in conductances that dictate the rate of action potential firing.
Depolarization does not underlie the effects of chronic stress on excitability
One potential mechanism for an increase of excitability is a depolarization of the resting membrane potential, bringing the neuron closer to spike threshold. The resting membrane potential was measured in all neurons. There was a small, but significant depolarization of the resting membrane potential in chronic stress rats (control −78.2 ± 0.9 m V, n=21, stress −76.3 ± 1.0 m V, n=25, p=0.035, two-tailed unpaired t-test, t=2.18). To determine if a change of the resting membrane potential is the cause of the increased excitability after chronic stress, we examined action potential initiation and excitability from an equivalent membrane potential. In a subset of neurons, the rheobase current, or current required to evoke a single action potential, was examined. There was a significant difference in rheobase current between control and chronic stress groups when the neuron was at its resting membrane potential, but not when the membrane potential was held equal between groups (−70 m V, see Supplement 1, Results). In addition, no significant difference was observed in the threshold of action potentials between groups (control −52.7 ± 0.9 m V, n=21, stress −54.2 ± 1.0 m V, n=25, p=0.275, two-tailed unpaired t-test, t=1.11). This indicates that the difference in resting membrane potential after chronic stress can contribute to group differences in the initiation of spiking at low currents. In contrast, when excitability was measured at the same membrane potential across groups (−70 m V), there was still significantly greater excitability in chronic stress rats (Fig. 2c; slope of excitability control 0.77 ± 0.18 AP/ 100 pA, n=21, slope of excitability stress 1.44 ± 0.14 AP/ 100pA, n=25, p=0.0047, two-tailed unpaired t-test, t=2.98). Because a difference in excitability still exists, even when the membrane potential is held constant between groups, a change of the resting membrane cannot entirely explain the effects of chronic stress on membrane excitability. For the remaining experiments, measurements were taken while holding the resting membrane potential at −70 m V, to minimize differences in resting membrane potential between groups, and diminish its effect on membrane excitability.
Reduction of GABA ergic inhibition does not underlie the effects of chronic stress on excitability
It has been found that a reduction in basolateral amygdala GABAergic circuits mediates some of the effects of stress on amygdala function (39,40). Therefore, the chloride channel blocker DNDS (0.5 mM) was included in the recording pipette, resulting in single-cell intracellular blockade of GABAA channels (41,42). Intracellular DNDS administration blocked the fast GABAergic components of inhibition (Figure S3A in Supplement 1), indicative of the GABAergic blocking efficacy of DNDS in this preparation. However, even with intracellular DNDS to block GABAergic inputs, there was still greater LAT neuronal excitability after chronic stress (Fig. 2d). Thus, the increase of excitability observed here does not appear to be the result of a reduction of GABAergic inputs.
Increased neuronal subthreshold responsiveness does not underlie the effects of chronic stress on excitability
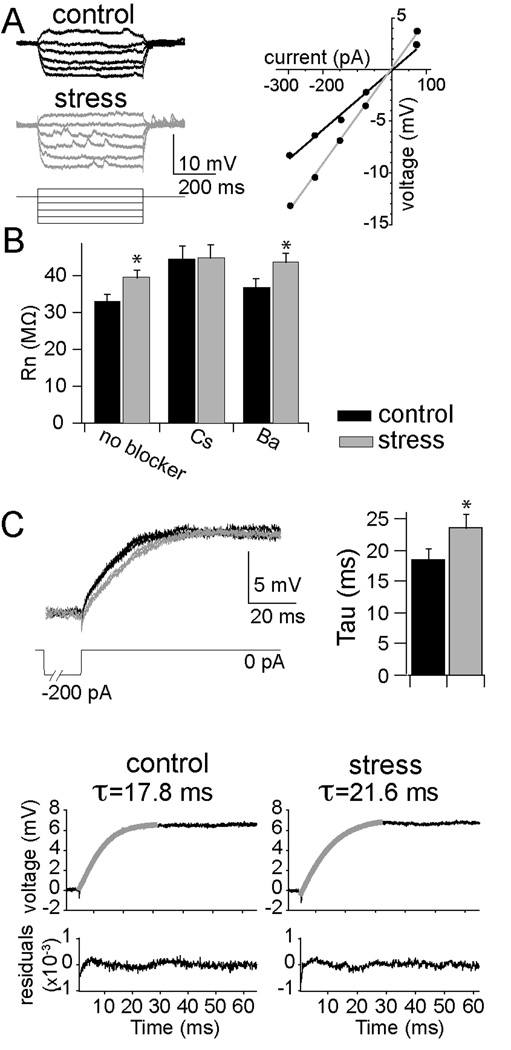
Another factor that contributes to excitability is the neuronal responsiveness to subthreshold stimuli, quantified as input resistance (Rn). Chronic stress caused a small, but significant increase of Rn (Fig. 3a,b; measured from −70 m V), indicating a possible change in somatic conductances that are active near the resting membrane potential. To verify this, in a subset of neurons we also measured the membrane time constant (τ), and found a longer time constant after chronic stress (Fig. 3c). The effects of chronic stress on Rn and τ indicate that a different complement of ion channels are active near rest, reflecting a change in the integrative properties after chronic stress, which may contribute to differences in measures of excitability. However, measurements of Rn and τ in vivo may be dominated by the presence of synaptic activity, even though measurements were taken during quiescent periods. Therefore, we further examined the contribution of resting membrane properties, using single-cell intracellular block of ion channels. Ba2+ (100 mM) or Cs+ (200 mM) were included in the recording pipette to block two primary conductances that are likely to be active near rest: inward rectifier K+ channels and hyperpolarization-activated channels (Ih, which appears to be present in BLA neurons; 43–45). Cs+ mimicked the effects of chronic stress, and blocked the group differences in Rn caused by chronic stress (Fig. 3b), while Ba2+ did not (Fig. 3b), preliminarily consistent with a change of somatic conductances after chronic stress.
Figure 3. Chronic stress altered membrane properties of LAT neurons.
A) Repeated restraint (gray) caused an increase in the responsiveness to input, or input resistance (Rn), of LAT neurons (measured from −70 m V as the slope of the I–V relationship, control 33.3 ± 2.0 MOhms, n=21, stress 39.9 ± 2.1 MOhms, n=25, p=0.028, two-tailed unpaired t-test, t=2.275). B) Intracellular Cs+, but not Ba2+, blocked the effects of chronic stress on the Rn (Cs+ control 44.6 ± 3.3 MOhms, n=10, stress 45.2 ± 3.3 MOhms, n=10, p=0.899, two-tailed unpaired t-test, t=0.129; Ba2+ control 37.1 ± 2.2 MOhms, n=12, stress 43.9 ± 2.3 MOhms, n=11, p=0.041, two-tailed unpaired t-test, t=2.169). C) Chronic stress caused a lengthening of the membrane time constant (τ; control 18.1 ± 1.9 ms, stress 23.6 ± 1.9 ms, p=0.047, two-tailed unpaired t-test, t=1.80), as seen by the overlay of three decaying voltage responses after current injection in an example of a LAT neuron from control (black) and stress (grey) groups, and the time constant of the double exponential fit to this decay. * indicates significance at p<0.05.
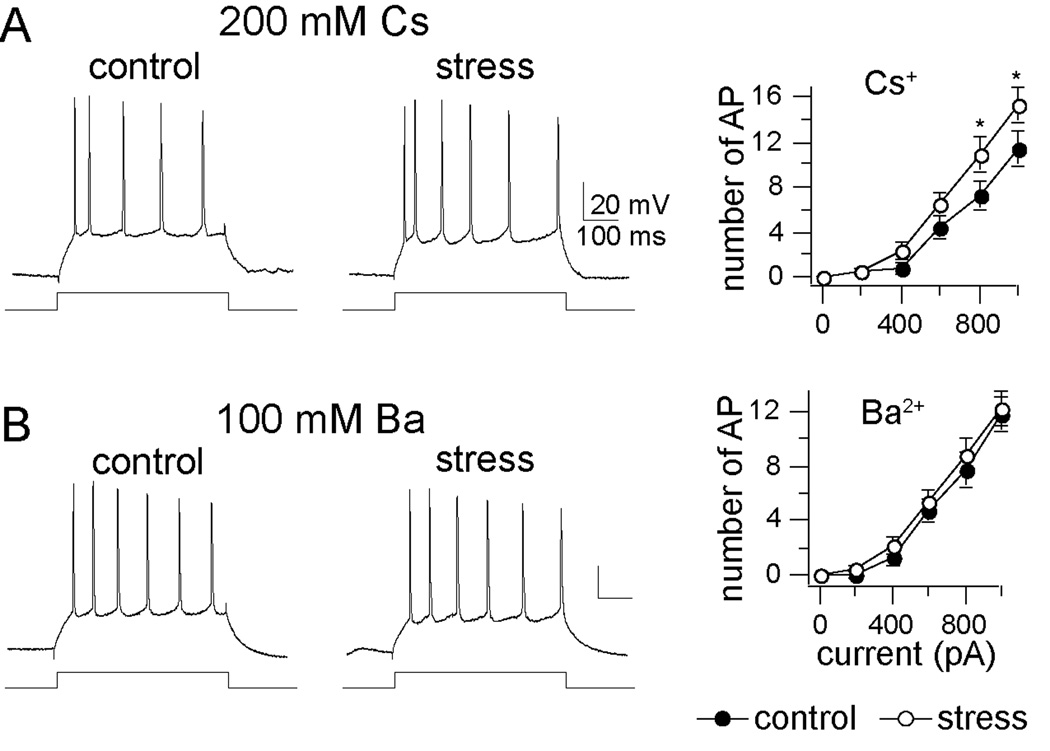
If an increase of Rn and a change of resting conductances contribute to increased excitability after chronic stress, then a treatment that blocks the group differences in Rn should also block the change of excitability. However, the opposite was found: Though intracellular inclusion of either Cs+ or Ba2+ caused a leftward shift in excitability (see Supplement 1, Results), Ba2+, not Cs+, was more effective in occluding the effects of chronic stress on group differences (Fig. 4, and Supplement 1, Results).
Figure 4. Effects of single-cell block of K+ channels on excitability after chronic stress.
A) Cs+ (200 mM; blocker of a variety of K+ channels) did not closely mimic the effects of chronic stress on excitability (mixed design repeated measures ANOVA of each stimulation intensity, main effect of stress F(1,108) = 10.79, p=0.0014; main effect of stimulation intensity F(5,108) = 58.98, p<0.001; interaction F(5,108) = 1.52, p=0.19). B) Ba2+ (100 mM), another K+ channel blocker that also blocks KCa channels, negated the effects of chronic stress on excitability (mixed design repeated measures ANOVA of each stimulation intensity, main effect of stress F(1,126) = 1.36, p=0.247; main effect of stimulation intensity F(5,126) = 64.03, p<0.001; interaction F(5,126) = 0.103, p=0.991). * indicates p<0.05 between control and stress group in post-hoc unpaired t-tests with Bonferroni corrections.
The dissociation between Rn and excitability indicates that an alteration of Cs+-sensitive ion channels that contribute to altered Rn after chronic stress does not account for the effects of chronic stress on excitability. Because Ba2+ mimics the effects of stress on excitability but not Rn, it is likely that Ba2+-sensitive channels that regulate excitability are altered by chronic stress, and not channels that contribute to resting conductances. Both Cs+ and Ba2+ block several channels. Of specific interest would be an ion channel blocked by Ba2+, not blocked by Cs+, that plays a role in regulation of LAT neuronal excitability. One likely candidate group of channels is calcium-activated K+ (KCa) channels.
Chronic stress decreases the in vivo function of KCa channels in LAT neurons
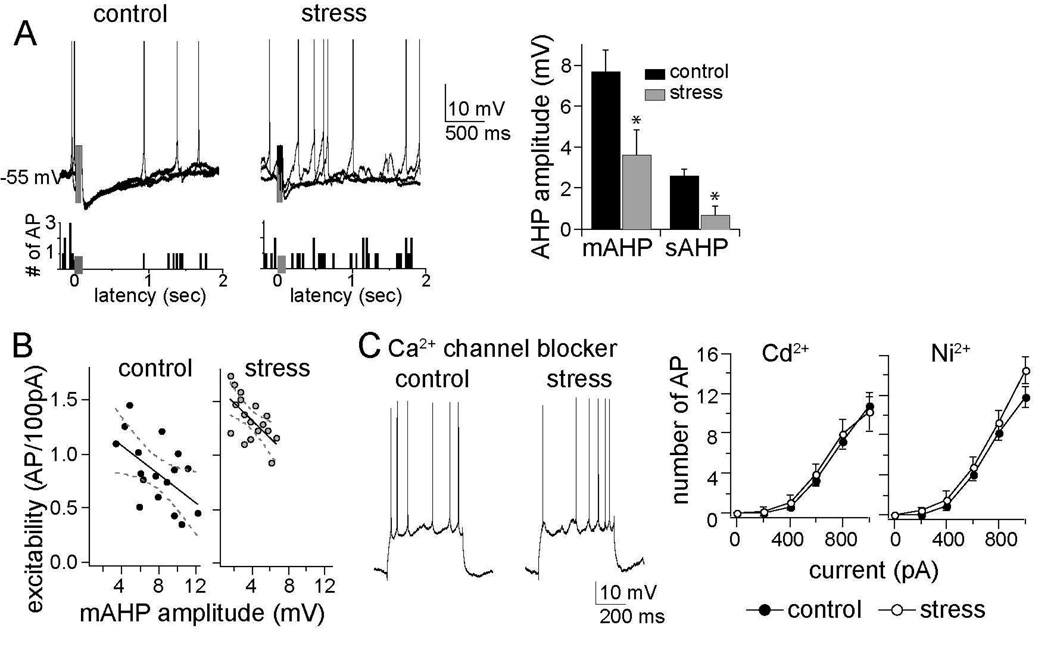
KCa channels play an important role in the regulation of excitability during action potential firing. Their activation by Ca2+ during firing leads to afterhyperpolarization potentials (AHPs), a voltage signature of KCa channel activation. The amplitudes of both the sAHP and mAHP were greatly reduced in chronic stress groups compared to control groups (Fig. 5a), consistent with an inhibiting effect of chronic stress on KCa channel function. Furthermore, there was a significant correlation between the amplitude of the mAHP and the slope of excitability in control (r=−0.56, r2=0.32, p=0.019) and stress groups (Fig. 5b; r=−0.62, r2=0.39, p=0.008, alpha adjusted to 0.025 after Bonferroni correction). Thus, the AHP potently regulates excitability in these neurons and is reduced in amplitude by chronic stress, evidence for a dysfunction of KCa channels underlying hyperexcitability. A single restraint session did not lead to reduction of the AHP amplitudes (control 7.3 ± 1.0 m V, n=7, stress 7.7 ± 1.1 m V, n=7, p= 0.792, two-tailed unpaired t-test, t=0.269).
Figure 5. The AHP is reduced by chronic stress and necessary for the effects of chronic stress.
A) The amplitudes of both the medium- and slow AHP were reduced after chronic stress (sAHP control 2.6 ± 0.3 m V, n=17, stress 0.7 ± 0.4 m V, n=18, p=0.003, two-tailed unpaired t-test, t=3.26; mAHP control 7.7 ± 1.1 m V, n=21, stress 3.6 ± 1.2 m V, n=25, p=0.009, two-tailed unpaired t-test, t=2.72), as seen in the neuronal response to a burst of 5 action potentials evoked by 5 current pulses (presented at grey box in overlay of 3 consecutive voltage traces and firing rate histogram; action potentials are truncated during the burst for clarity, but see Figure S2 in Supplement 1 for details). This reduction of the AHP amplitude was associated with greater spontaneous firing near action potential threshold, demonstrated in a firing rate histogram of 10 consecutive sweeps. B) The amplitude of the mAHP was correlated with neuronal excitability (control, r=−0.56, r2=0.32; stress, r=−0.62, r2=0.39), demonstrating that it is a major factor in regulating LAT neuronal activity. C) Intracellular administration of Ca2+ channel blockers mimicked the effects of chronic stress on neuronal excitability: Cd2+ (mixed design repeated measures ANOVA of each stimulation intensity, main effect of stress F(1,66) = 0.153, p=0.697; main effect of stimulation intensity F(5,66) = 40.72, p<0.001; interaction F(5,66) = 0.124, p=0.987) and Ni2+ (mixed design repeated measures ANOVA of each stimulation intensity, main effect of stress F(1,72) = 3.77, p=0.056; main effect of stimulation intensity F(5,72) = 86.33, p<0.001; interaction F(5,72) = 0.711, p=0.617). Displayed 28 in this panel are traces with intracellular Cd 2+. * indicates p<0.05 between control and stress group in post-hoc unpaired t-tests with Bonferroni corrections.
Activation of KCa channels by Ca2+ is blocked by intracellular Cd2+ or Ni2+. When Cd2+ (0.5 mM) or Ni2+ (0.5 mM) was included in the pipette, the AHPs in LAT neurons were blocked (Figure S4 in Supplement 1). In parallel with blockade of AHPs, both Cd2+ and Ni2+ were able to mimic the effects of chronic stress on excitability and diminished group differences (Fig. 5c; Cd2+ slope of excitability control 1.38 ± 0.29 AP/ 100 pA, n=6; slope of excitability stress 1.33 ± 0.29 AP/ 100 pA, n=7, p=0.849, two-tailed unpaired t-test, t=0.194; Ni2+ slope of excitability control 1.64 ± 0.32 AP/ 100 pA, n=7, stress slope of excitability 1.81 ± 0.34 AP/ 100 pA, n=7, p=0.723, two-tailed unpaired t-test, t=0.364), suggestive of a role for a Ca2+-dependent AHP, such as that produced by KCa channels, in chronic stress.
However, a reduction of the AHP can result from either a reduced function of KCa channels or Ca2+ channels. A decrease of Ca2+ channel function would be expected to reduce BK KCa channel activity that contributes to the fAHP. There was no significant difference in the amplitude of the fAHP evoked after a single action potential between control and chronic stress groups (control 3.6 ± 0.9 m V, n=17, stress 2.8 ± 0.7 m V, n=18, p=0.49, two-tailed unpaired t-test, t=0.70). Both Ba2+ and Cs+ block BK-like channels that underlie the fAHP, while SK channels that likely underlie the s- and mAHP are more sensitive to Ba2+ than Cs+ (46,47). However, Ba2+, but not Cs+, mimicked the effects of chronic stress on excitability.
Activation of KCa channels reverses the amygdala impairments caused by chronic stress
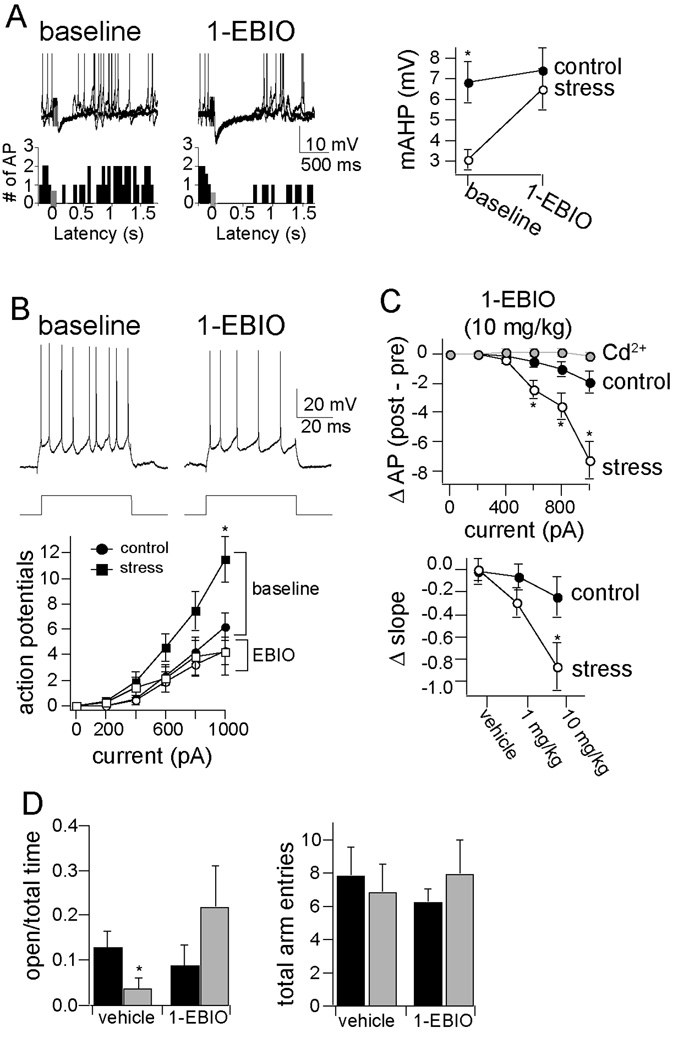
If a dysfunction of KCa channels is fundamentally important for the effects of chronic stress on amygdala neuronal excitability, it is expected that pharmacological activation of these channels should mitigate the effects of chronic stress. Consistent with this, we found that systemic administration of the KCa channels activator, 1-EBIO (doses 1, 10 mg/ kg, i.p., or DMSO control; Fig. 6; and Figure S5 in Supplement 1) caused an increase in the amplitude of the mAHP (Fig. 6a; stress baseline 3.9 ± 0.9 m V, stress + 1-EBIO 7.4 ± 1.5 m V, n=6, p=0.024, two-tailed paired t-test, t=3.21), and significantly reduced the excitability of LAT neurons (Fig. 6b; p<0.001, n=6/ group, two-way ANOVA, main effect of drug, F=30.13), an effect that was greater after chronic stress (Fig. 6c; p=0.0014, two-way ANOVA, significant interaction between drug and stress, F=8.29). Activation of KCa channels in stressed rats with 10 mg/ kg 1-EBIO brought excitability back to near control levels (Fig. 6b). Furthermore, this effect of 1-EBIO (10 mg/ kg) on excitability was blocked by inclusion of Cd2+ (0.5 mM) in the intracellular pipette (intracellular Cd2+ baseline slope of excitability 0.81 ± 0.05 AP/ 100 pA, post-EBIO + intracellular Cd2+ slope of excitability 0.80 ± 0.07 AP/ 100 pA, n=5, p=0.308, paired t -test, t=1.17). This demonstrates that pharmacological enhancement of KCa channel function can reverse the effects of chronic stress. Because the effects of 1-EBIO were blocked by intracellular Cd2+, they may be caused, at least in part, by direct actions of 1-EBIO on LAT neurons.
Figure 6. Pharmacological enhancement of the AHP reversed the effects of chronic stress on excitability and EPM.
A) 1-EBIO is an activator of KCa channels. Administration of 1-EBIO increased the amplitude of the AHP, and augmented the impact of the AHP on spontaneous action potential firing, demonstrated in a firing rate histogram of 10 consecutive sweeps. Displayed here are overlays of three consecutive traces before and after 1-EBIO (10 mg/ kg, i.p.). 1-EBIO brought the amplitude of the mAHP to near-control levels. B) 1-EBIO causes a reduction in the excitability of LAT neurons. C) The effects of 1-EBIO (10 mg/ kg) were greater in chronic stress animals, and returned neuronal excitability to close to control, non-stress levels. These effects were also dose-dependent, and blocked by intracellular application of Cd2+ (0.5 mM; mixed design repeated measure ANOVA, main effect of 1-EBIO F(1,48) = 0.047, p=0.829, main effect of stimulation intensity F(5,48) = 24.46, p<0.001), indicating that its effects on LAT neuronal excitability are caused by direct actions on LAT neurons. D) 1-EBIO (10 mg/ kg) reversed the effects of chronic stress on exploration in the EPM, as demonstrated by increased time spent in the open arms (percent time in open arms, Kruskal-Wallis = 8.22, p=0.042, n=8/ group; vehicle control 12.7 ± 3.7%, vehicle chronic stress 3.8 ± 2.3%, Mann-Whitney U = 10.0, p=0.023; 1-EBIO control 8.6 ± 4.6%, 1-EBIO chronic stress 21.8 ± 8.9%, Mann-Whitney U = 22.0, p=0.33). There was no significant effect on the total number of arm entries (Kruskal-Wallis = 0.198, p = 0.978, n=8/ group; control vehicle 7.8 ± 1.7 entries, stress vehicle 6.8 ± 1.7 entries; control 1-EBIO 6.1 ± 0.8 entries, stress 1-EBIO 7.9 ± 2.4 entries).
To understand whether the effectiveness of 1-EBIO on BLA neuronal physiology after chronic stress may be associated with functional significance, we examined the effects of 1-EBIO on behavior in the EPM. Administration of 1-EBIO (10 mg/ kg, compared to vehicle control; dose effective on BLA neuronal excitability) reversed the effects of chronic stress on exploration in the EPM, measured as the time in open arms (Kruskal-Wallis = 7.89, p=0.04, n=8/ group; vehicle control 37.7 ± 11.2 s compared to vehicle chronic stress 10.1 ± 6.0 s, Mann Whitney = 10, p = 0.023; 1-EBIO control 26.8 ± 13.9 s compared to 1-EBIO chronic stress 65.4 ± 22.7 s, Mann Whitney U = 22.0, p=0.318). Interestingly, there was a trend towards an increase in exploration in the EPM after chronic stress if 1-EBIO is administered. There was no significant difference in the total number of arm entries (Fig. 6d).
Discussion
Chronic stress is a potent contributor to many illnesses, including depression and other affective disorders (5,48). However, the basic effects of chronic stress on the neurons in the amygdala that modulate emotion are unknown. While it has previously been demonstrated that stress can increase LAT-dependent behaviors (3,12,17,49), this study demonstrates for the first time that chronic stress causes a hyperexcitability of LAT pyramidal neuron membrane excitability, which may underlie impairments of affective behavior. Furthermore, this study provides evidence that a KCa channelopathy underlies this abnormality, and provides a pharmacological target for the reversal of these effects of chronic stress.
Plasticity of membrane properties has been observed after prolonged conditions, such as epilepsy, drug addiction and experience (50–52). The effects of chronic stress in the amygdala are unique, and opposite to changes in the hippocampus (53,54), whose function is markedly diminished after chronic stress (55,56). The magnitude of this effect has several contributors, including depolarization of the membrane potential and increased neuronal responsiveness to subthreshold stimuli. However, most important was the increased action potential firing caused by a reduction of the AHP. Because the effects of chronic stress on excitability are sensitive to KCa channel manipulations, and are associated with a decrease of the AHP, our data are consistent with chronic stress increasing excitability through a mechanism that likely involves a reduction in the function or number of KCa channels.
There are several types of KCa channels that contribute to LAT neuronal excitability, and to different AHPs in the LAT (27–29). Furthermore, KCa channels can regulate amygdala-related behaviors (57). This study indicates involvement of the channels that underlie the mAHP and sAHP in the effects of chronic stress, most likely SK channels that produce intermediate or small KCa currents. There are a number of factors and ion channels that contribute to measurements of membrane excitability, as quantified here. The contribution of GABAergic influences on excitability may be minor, as intracellular blockade of Cl− channels had little impact on the effects of chronic stress. However, a change in GABAergic systems may play significant roles in the effects of stress on other aspects of neuronal function (39,40) that were not examined here. Also not tested here is the possible role of norepinephrine, a modulator that decreases activity of KCa channels and the AHP in the BLA(58–60), and whose effectiveness may be altered by chronic stress (61).
Increased excitability is expected to result in greater output of LAT neurons. The greater action potential firing in response to a stimulus allows the LAT to exert a more potent influence over other brain regions, such as the prefrontal cortex, central amygdala and nucleus accumbens, resulting in more affect-driven behavior. The impact of chronic stress on fear conditioning and extinction observed in other studies is consistent with this notion (3,10,12,13,62–68). An inappropriately large contribution of the LAT may produce some of the behavioral abnormalities observed after chronic stress.
1-EBIO, a compound that increases SK channel activity and both the sAHP and mAHP (69), diminished the in vivo excitability of LAT neurons after chronic stress (above). 1-EBIO was administered systemically, an approach that prevents definitive statements about its site of action (however, the effects of 1-EBIO on LAT neurons were blocked when Cd2+ was included in the recording electrode). The effect of 1-EBIO on LAT excitability is not likely due to non-specific actions of 1-EBIO, as it had much weaker effects in control animals. 1-EBIO was also effective at reversing the stress-induced impairments of exploration in the EPM, further supporting a role for KCa channel disruption after chronic stress.
A long-term increase of LAT excitability after chronic stress is expected to lead to heightened emotional lability. This imbalance of LAT activity may exacerbate abnormalities present in individuals with psychiatric illnesses, or introduce a dysregulation in those already predisposed to psychiatric illnesses. This study provides a basic cellular mechanism for the effects of chronic stress on emotion, providing a potential pharmacological intervention for the harmful effects of chronic stress on mental health.
Supplementary Material
Acknowledgements
The authors wish to thank Mitch Beales for significant help with tissue histology, Bijal Shah for assistance with behavioral studies, Jolee Rosenkranz and Jessica McGraw for contributions to experimental design, and Dr. Anthony West and Dr. Kuei Tseng for valuable discussion. Support provided by the Brain Research Foundation (J.A.R.) and NIH (MH084970).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychol Bull. 2008;134:829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- 2.Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. Repeated exposure to stress across the childhood-adolescent period alters rats' anxiety- and depression-like behaviors in adulthood: The importance of stressor type and gender. Behav Neurosci. 2007;121:462–474. doi: 10.1037/0735-7044.121.3.462. [DOI] [PubMed] [Google Scholar]
- 3.Wood GE, Norris EH, Waters E, Stoldt JT, McEwen BS. Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav Neurosci. 2008;122:282–292. doi: 10.1037/0735-7044.122.2.282. [DOI] [PubMed] [Google Scholar]
- 4.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 5.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 6.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 7.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 8.Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8:209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- 9.Tronel S, Alberini CM. Persistent disruption of a traumatic memory by postretrieval inactivation of glucocorticoid receptors in the amygdala. Biol Psychiatry. 2007;62:33–39. doi: 10.1016/j.biopsych.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, et al. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 13.Henningsen K, Andreasen JT, Bouzinova EV, Jayatissa MN, Jensen MS, Redrobe JP, et al. Cognitive deficits in the rat chronic mild stress model for depression: relation to anhedonic-like responses. Behav Brain Res. 2009;198:136–141. doi: 10.1016/j.bbr.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav. 2009;55:248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Rau V, Fanselow MS. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2009;12:125–133. doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- 16.Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 2001;102:329–339. doi: 10.1016/s0306-4522(00)00484-x. [DOI] [PubMed] [Google Scholar]
- 17.Shors TJ. Stressful experience and learning across the lifespan. Annu Rev Psychol. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shors TJ, Servatius RJ. The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning. Neurobiol Learn Mem. 1997;68:92–96. doi: 10.1006/nlme.1997.3763. [DOI] [PubMed] [Google Scholar]
- 19.Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci U S A. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins DR, Pare D. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−) Learn Mem. 2000;7:97–103. doi: 10.1101/lm.7.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 23.Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correll CM, Rosenkranz JA, Grace AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry. 2005;58:382–391. doi: 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, et al. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennur S, Chattarji S. Effects of chronic stress on intrinsic and synaptic plasticity in principal neurons of the basolateral amygdala. Society for Neuroscience Conference; San Diego, CA. Society for Neuroscience; 2004. Program No. 511.2. [Google Scholar]
- 27.Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci. 2005;8:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- 28.Faber ES, Sah P. Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist. 2003;9:181–194. doi: 10.1177/1073858403009003011. [DOI] [PubMed] [Google Scholar]
- 29.Lang EJ, Pare D. Synaptic and synaptically activated intrinsic conductances underlie inhibitory potentials in cat lateral amygdaloid projection neurons in vivo. J Neurophysiol. 1997;77:353–363. doi: 10.1152/jn.1997.77.1.353. [DOI] [PubMed] [Google Scholar]
- 30.McCormick DA, Prince DA. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J Neurophysiol. 1988;59:978–996. doi: 10.1152/jn.1988.59.3.978. [DOI] [PubMed] [Google Scholar]
- 31.Womble MD, Moises HC. Muscarinic modulation of conductances underlying the afterhyperpolarization in neurons of the rat basolateral amygdala. Brain Res. 1993;621:87–96. doi: 10.1016/0006-8993(93)90301-3. [DOI] [PubMed] [Google Scholar]
- 32.Kant GJ, Eggleston T, Landman-Roberts L, Kenion CC, Driver GC, Meyerhoff JL. Habituation to repeated stress is stressor specific. Pharmacol Biochem Behav. 1985;22:631–634. doi: 10.1016/0091-3057(85)90286-2. [DOI] [PubMed] [Google Scholar]
- 33.Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94:1313–1322. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- 34.Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- 35.McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- 36.Vyas A, Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav Neurosci. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- 37.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 38.Selye H. A syndrome produced by diverse nocuous agents. J Neuropsychiatry Clin Neurosci. 1998;10:230–231. doi: 10.1176/jnp.10.2.230a. 1936. [DOI] [PubMed] [Google Scholar]
- 39.Isoardi NA, Bertotto ME, Martijena ID, Molina VA, Carrer HF. Lack of feedback inhibition on rat basolateral amygdala following stress or withdrawal from sedative-hypnotic drugs. Eur J Neurosci. 2007;26:1036–1044. doi: 10.1111/j.1460-9568.2007.05714.x. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J Neurosci. 2005;25:8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bridges RJ, Worrell RT, Frizzell RA, Benos DJ. Stilbene disulfonate blockade of colonic secretory Cl- channels in planar lipid bilayers. Am J Physiol. 1989;256:C902–C912. doi: 10.1152/ajpcell.1989.256.4.C902. [DOI] [PubMed] [Google Scholar]
- 42.Dudek SM, Friedlander MJ. Intracellular blockade of inhibitory synaptic responses in visual cortical layer IV neurons. J Neurophysiol. 1996;75:2167–2173. doi: 10.1152/jn.1996.75.5.2167. [DOI] [PubMed] [Google Scholar]
- 43.Womble MD, Moises HC. Hyperpolarization-activated currents in neurons of the rat basolateral amygdala. J Neurophysiol. 1993;70:2056–2065. doi: 10.1152/jn.1993.70.5.2056. [DOI] [PubMed] [Google Scholar]
- 44.Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci. 1992;12:4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J Neurophysiol. 1993;69:1350–1362. doi: 10.1152/jn.1993.69.4.1350. [DOI] [PubMed] [Google Scholar]
- 46.Cecchi X, Wolff D, Alvarez O, Latorre R. Mechanisms of Cs+ blockade in a Ca2+-activated K+ channel from smooth muscle. Biophys J. 1987;52:707–716. doi: 10.1016/S0006-3495(87)83265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neyton J, Pelleschi M. Multi-ion occupancy alters gating in high-conductance, Ca(2+)-activated K+ channels. J Gen Physiol. 1991;97:641–665. doi: 10.1085/jgp.97.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- 49.Sandi C, Cordero MI, Ugolini A, Varea E, Caberlotto L, Large CH. Chronic stress-induced alterations in amygdala responsiveness and behavior--modulation by trait anxiety and corticotropin-releasing factor systems. Eur J Neurosci. 2008;28:1836–1848. doi: 10.1111/j.1460-9568.2008.06451.x. [DOI] [PubMed] [Google Scholar]
- 50.Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–535. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- 51.Dong Y, Nasif FJ, Tsui JJ, Ju WY, Cooper DC, Hu XT, et al. Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. J Neurosci. 2005;25:936–940. doi: 10.1523/JNEUROSCI.4715-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- 53.Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 57.Mitra R, Ferguson D, Sapolsky RM. SK2 potassium channel overexpression in basolateral amygdala reduces anxiety, stress-induced corticosterone secretion and dendritic arborization. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen JC, Lang EJ. Inhibitory control of rat lateral amygdaloid projection cells. Neuroscience. 2003;121:155–166. doi: 10.1016/s0306-4522(03)00430-5. [DOI] [PubMed] [Google Scholar]
- 59.Faber ES, Sah P. Independent roles of calcium and voltage-dependent potassium currents in controlling spike frequency adaptation in lateral amygdala pyramidal neurons. Eur J Neurosci. 2005;22:1627–1635. doi: 10.1111/j.1460-9568.2005.04357.x. [DOI] [PubMed] [Google Scholar]
- 60.Faber ES, Delaney AJ, Power JM, Sedlak PL, Crane JW, Sah P. Modulation of SK channel trafficking by beta adrenoceptors enhances excitatory synaptic transmission and plasticity in the amygdala. J Neurosci. 2008;28:10803–10813. doi: 10.1523/JNEUROSCI.1796-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buffalari DM, Grace AA. Chronic cold stress increases excitatory effects of norepinephrine on spontaneous and evoked activity of basolateral amygdala neurons. Int J Neuropsychopharmacol. 2009;12:95–107. doi: 10.1017/S1461145708009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conrad CD, Mauldin-Jourdain ML, Hobbs RJ. Metyrapone Reveals That Previous Chronic Stress Differentially Impairs Hippocampal-dependent Memory. Stress. 2001;4:305–318. doi: 10.3109/10253890109014754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cordero MI, Kruyt ND, Sandi C. Modulation of contextual fear conditioning by chronic stress in rats is related to individual differences in behavioral reactivity to novelty. Brain Res. 2003;970:242–245. doi: 10.1016/s0006-8993(03)02352-7. [DOI] [PubMed] [Google Scholar]
- 64.Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 65.Mitra R, Sapolsky RM. Effects of enrichment predominate over those of chronic stress on fear-related behavior in male rats. Stress. 2008;1 doi: 10.1080/10253890802379955. [DOI] [PubMed] [Google Scholar]
- 66.Dagnino-Subiabre A, Terreros G, Carmona-Fontaine C, Zepeda R, Orellana JA, Diaz-Veliz G, et al. Chronic stress impairs acoustic conditioning more than visual conditioning in rats: morphological and behavioural evidence. Neuroscience. 2005;135:1067–1074. doi: 10.1016/j.neuroscience.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 67.Dagnino-Subiabre A, Munoz-Llancao P, Terreros G, Wyneken U, Diaz-Veliz G, Porter B, et al. Chronic stress induces dendritic atrophy in the rat medial geniculate nucleus: effects on auditory conditioning. Behav Brain Res. 2009;203:88–96. doi: 10.1016/j.bbr.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 68.Pego JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OF, Sousa N. Dissociation of the morphological correlates of stress-induced anxiety and fear. Eur J Neurosci. 2008;27:1503–1516. doi: 10.1111/j.1460-9568.2008.06112.x. [DOI] [PubMed] [Google Scholar]
- 69.Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, et al. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem. 2001;276:9762–9769. doi: 10.1074/jbc.M010001200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.