Abstract
BACKGROUND & AIMS
Duchenne muscular dystrophy is a debilitating genetic disorder characterized by severe muscle wasting and early death in afflicted boys. The primary cause of this disease is mutations in the dystrophin gene resulting in massive muscle degeneration and inflammation. The purpose of this study was to determine if dystrophic muscle pathology and inflammation were decreased by pre-natal and early dietary intervention with green tea extract.
METHODS
Mdx breeder mice and pups were fed diets containing 0.25% or 0.5% green tea extract and compared to untreated mdx and C57BL/6J mice. Serum creatine kinase was assessed as a systemic indicator of muscle damage. Quantitative histopathological and immunohistochemical techniques were used to determine muscle pathology, macrophage infiltration, and NF-κB localization.
RESULTS
Early treatment of mdx mice with green tea extract significantly decreased serum creatine kinase by ~85% at age 42 days (P≤0.05). In these mice, the area of normal fiber morphology was increased by as much as ~32% (P≤0.05). The primary histopathological change was a ~21% decrease in the area of regenerating fibers (P≤0.05). NF-κB staining in regenerating muscle fibers was also significantly decreased in green tea extract-treated mdx mice when compared to untreated mdx mice.
CONCLUSION
Early treatment with green tea extract decreases dystrophic muscle pathology potentially by regulating NF-κB activity in regenerating muscle fibers.
Keywords: Duchenne muscular dystrophy, mdx, green tea extract, muscle histopathology, muscle regeneration, NF-κB
Introduction
Duchenne muscular dystrophy (DMD) is a lethal muscle wasting disease affecting approximately one in 3,500 boys.1, 2 Mutations in the dystrophin gene result in the loss of this protein from the sarcolemma of muscle fibers.1, 2 Dystrophin deficiency does not consistently produce muscle degeneration at all life stages, in all muscle phenotypes, or in all animal models.3 In dystrophin-deficient skeletal muscle mechanical injury and proteolysis may be important factors but do not fully explain DMD pathogenesis. Mechanisms such as the immune/inflammatory response to injury appear to contribute substantially to muscle pathophysiology. Observations of activated immune cell infiltrates in dystrophic muscle suggest that the immune/inflammatory response may play a role in exacerbating the disease.3–7 Recently, individual macrophage subpopulations have been reported to influence muscle degeneration and regeneration depending on the proportion of these cells present. M1 macrophages are cytotoxic and pro-inflammatory while M2 macrophages promote muscle regeneration.8 Therefore, a shift in macrophage phenotype may be one mechanism that can regulate the dystrophic disease time course.8
Complementary and alternative medicine (CAM) approaches, including the use of botanicals, are being pursued in the amelioration of DMD. Green tea is a widely consumed beverage believed to elicit anti-oxidant and anti-inflammatory properties.9, 10 Green tea extract (GTE) is the hot water-soluble portion of unfermented Camellia sinensis leaves, which contains high levels of polyphenols. The polyphenols in GTE are mainly composed of the catechins: gallocatechin (GC), epigallocatechin (EGC), epicatechin (EC), and epigallocatechin gallate (EGCG).9, 11 These catechins are strong antioxidants that can quench reactive oxygen species (ROS) such as super oxide radical, singlet oxygen, hydroxyl radical, peroxyl radical, nitric oxide, nitrogen dioxide, and peroxynitrile.11 In GTE, EGCG is the most abundant polyphenol, accounting for 30–50% of total polyphenols, and is believed to provide the majority of the beneficial effects observed with green tea consumption.12 EGCG may lead to decreased inflammation through its antioxidant properties or through other mechanisms. EGCG has been shown to have effects on several signaling pathways including blockade of NF-κB activation by inhibiting IκB kinase (IKK) activity.10, 13–18
The antioxidant potential of GTE may be beneficial in treating dystrophic muscle, because oxidative stress is believed to contribute substantially to muscle pathology.19, 20 Evidence suggest that oxidative stress is involved in early disease stages and occurs before disease onset in mdx mice.21 In one study, diets supplemented with GTE (0.01% or 0.05%) were provided to mdx breeder pairs and their pups prior to and following weaning. At age 28 days, extensor digitorum longus (EDL) muscles of GTE treated mdx pups had significant reductions in areas of necrosis and regeneration.22 In a separate study, mdx mice treated with either GTE (0.05% or 0.25%) or EGCG (0.1%) for one to five weeks after weaning had increased antioxidant capacity, improved contractile properties, and decreased muscle pathology.12 These studies indicate the GTE and EGCG may provide beneficial CAM modalities for reducing oxidative stress and decreasing dystrophic muscle pathology during early disease stages, however; the role of these polyphenols in altering muscle pathology and inflammation has not been fully characterized. This study represents a detailed time-course characterization of pathological and inflammatory markers at which pre-natal and early GTE treatment of mdx mice decreases muscle wasting.
Materials and Methods
Mice
C57BL/6J and mdx mice were obtained from our colony. Breeding mice and offspring were maintained in a supervised laboratory animal facility in polypropylene shoebox cages. Mice were given free access to public tap water via an automatic watering system, and fed a standard pelleted diet ad libitum. Decaffeinated GTE (90DCF- T; Sunphenon) [polyphenols >80%, catechins >80%, (−)-epigallocatechin-3-gallate (EGCG) >45%, caffeine <1%], was a kind gift from Taiyo International (Minneapolis, MN). Mdx breeder pairs were provided standard breeding pelleted diets (7004; Harlan Teklad) with no GTE, 0.25% GTE, or 0.5% GTE. Mdx pups (n = 4–6 for each diet and age group) were weaned at age 21 days and then supplied with a standard maintenance diet (2018; Harlan Teklad) containing the same percentage of GTE provided to the breeder pair from which they came. C57BL/6J breeders were fed the standard breeding diet and weaned pups (n = 3–4 for each age group) were fed the maintenance control diet without GTE. Experimental procedures were approved by the Institutional Animal Care and Use Committee of Virginia Polytechnic Institute and State University and met or exceeded requirements of the Public Health Service/National Institutes of Health and the Animal Welfare Act.
Tissue Collection
Mice of each genotype and treatment were selected at random from available cages. For tissue collection and for humane euthanasia, mice were sacrificed by carbon dioxide narcosis followed by secondary thoracotomy. Blood was collected via cardiac puncture. Tibialis anterior (TA) muscles were removed and were either flash frozen in liquid nitrogen and stored at −80°C or fixed in 10% neutral buffered formalin until prepared for sectioning.
Serum Analysis
Blood collected from mice was placed directly into Microtainer serum separator tubes (Becton Dickinson). Whole blood samples were allowed to clot for 30 minutes at room temperature. The tubes were then centrifuged for 2 minutes at 10,000 × g to separate serum. Serum creatine kinase (CK) assays were performed in the Clinical Pathology Laboratory at Virginia Tech, using an Olympus AU400 chemistry analyzer (Olympus America, Center Valley, PA).
Histopathological Analysis
Formalin fixed TA muscles were prepared for light microscopy by dehydrating using increasing concentrations of ethanol and xylene as transitional solvents. Tissues were infiltrated with paraffin polymer, sectioned at 3 micrometers, and stained with hematoxylin-eosin (HE) on automated staining equipment. A quantitative analysis of muscle histopathology was performed as follows. Degenerating fibers were identified as swollen eosinophilic, hyalinized, and pyknotic muscle fibers. Regenerating muscle fibers were identified by small diameter, centralized nuclei and basophilic cytoplasm. Necrotic fibers were identified as swollen muscle fibers with disrupted cell membranes, invaded by inflammatory cells. Image analysis software (Image-Pro Plus, Media Cubernetics, Inc. Silver Spring, MD) was used to analyze tissue sections for pathological markers in fields selected with battlement technique from the entire cross section of the TA muscle. A grid was superimposed over each selected field and the number of intersections that overlay pathological markers was reported as a percent of the total intersections that overlay muscle tissue.
Frozen serial sections 10 micrometers thick were transferred to positively charged slides for immunohistochemical staining. Sections were fixed in 2% formalin for five minutes and rinsed in PBS. Macrophages were identified by staining for anti-F4/80 (1:500; Serotec). After fixation, sections to be stained for NF-κB were permeabilized in 1% Triton X-100, rinsed in PBS, and covered with anti-NF-κB p65 specific for phosphorylated Serine 536 (1:100; Abcam). Sections were incubated with primary antibody overnight at 4°C, rinsed in PBS, and immunodetection was performed using an anti-rat or anti-rabbit IgG labeled with horseradish peroxidase-diaminobenzidine system (R&D Systems). Additional sections were also incubated without primary antibody to ensure the presence of minimal background staining. The sections were then mounted and cover slipped for evaluation. The presence of macrophages and NF-κB staining was quantified using image analysis software (Image-Pro Plus, Media Cubernetics, Inc. Silver Spring, MD).
Statistics
Data were analyzed in a completely randomized design. Differences in muscle histopathology and serum CK were analyzed to determine the significance of the main effects and interactions. The model was analyzed as a 2 × 5 factorial arrangement for genotype (C57BL/6J and mdx) versus age (14, 21, 28, 35 and 42 d) or a 2 × 3 factorial arrangement for age (28 and 42 days) versus treatment (0%, 0.25%, 0.5% GTE). To determine the significance of the model, two-way analysis of variance (ANOVA) was performed using the general linear model. When the model was significant, the analysis was followed by Fisher’s Least Significant Difference multiple comparisons method (JMP 6.0.2 software. SAS Institute Inc. Cary, NC). Differences in macrophage infiltration and NF-kB staining between GTE treatment groups were analyzed with one-way ANOVA. When significant differences were detected, Tukey’s Honestly Significant Difference post hoc test was used to determine differences between means. Differences for all analyses were considered significant at P≤0.05 and data were presented as the mean ± standard error.
Results
Microscopic muscle lesions in mdx mice
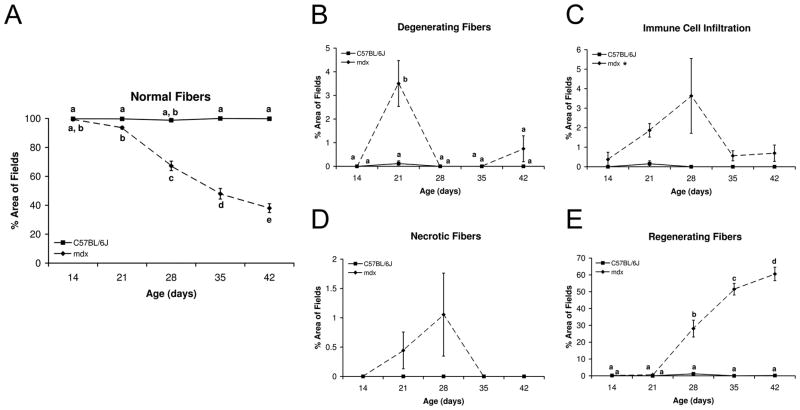
Muscle histopathology was quantified for mdx mice during the initial disease onset stages to further characterize and determine time points that represent early disease progression (Figure 1). Systematically selected fields from TA muscles for both C57BL/6J and mdx mice were quantified (Figure 2). Mdx TA muscles had normal fiber morphology at age 14 days, but by age 21 days the percent area of analyzed fields with normal fiber morphology was significantly decreased compared to C57BL/6J and was further decreased at age 42 days (P≤ 0.05). Degenerating muscle fibers peaked at age 21 days and represented 3.5% of the mdx TA area, but declined to lower levels thereafter. Immune cell infiltration of the muscle tissue and necrotic fibers were seen at age 21 days and peaked at age 28 days. At age 28 days immune cell infiltration and necrotic fibers accounted for 3.6% and 1% of abnormal morphology, respectively. Regenerating muscle fibers were the most abundant histopathological feature accounting for 28% to 61% of the TA area between ages 28 and 42 days (P≤ 0.05). Ages 28 and 42 days were selected as the primary time points for determining the effects of GTE on muscle pathology and inflammation because they represent critical time points in the disease process of the mdx mouse.
Figure 1.
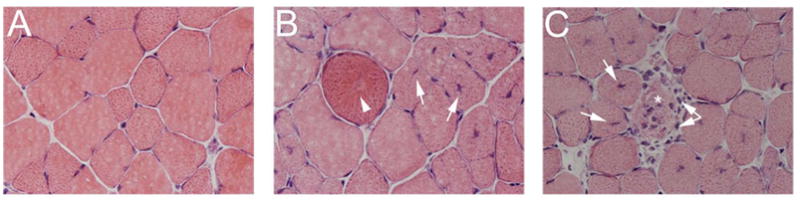
Histopathological features of TA muscles from C57BL/6J and mdx mice age 28 days. (A) Normal muscle morphology was seen in C57BL/6J muscle sections. (B and C) Regenerating fibers (arrow), degenerating fibers (arrowhead), necrotic fibers (asterisk) and immune cell infiltration (double arrow) were apparent in mdx muscle sections. 400X H&E.
Figure 2.
Histopathology time course for C57BL/6J and mdx TA muscles. (A) Fiber morphology appears normal in mdx mice at age 14 days, but by 21 days (B) degenerating fibers and (C) immune cell infiltration were evident, followed by the formation of (D) necrotic fibers and (E) regenerating fibers by age 28 days. Mean values not connected by the same letter were significantly different (P≤0.05).
GTE treatments in mdx mice
Body mass was recorded for treated mdx mice and compared to control mdx mice to determine how GTE supplementation affected overall health. GTE supplementation did not result in a significant change in body mass for either treatment when compared to untreated mdx mice at either age (Average body mass in grams: 28 day: mdx = 13.4, mdx 0.25% GTE = 15.9, mdx 0.5% GTE = 13.8; 42 day: mdx = 21.6, mdx 0.25% GTE = 21.8, mdx 0.5% GTE = 22.6).
Systemic indicator of muscle damage
Serum CK activity was not significantly improved in GTE treated mice at age 28 days (Figure 3). However, at age 42 days there was a significant decrease (~83–85%) in 0.25% (1345.5 U/L) and 0.5% (1194.3 U/L) GTE treated mdx mice when compared to untreated mdx (7896.5 U/L) (P≤ 0.05). There was no significant difference in CK activity for age 28 days when compared to age 42 days. CK activity for GTE treated mdx mice was still considerably elevated when compared to C57BL/6J mice (86.8 U/L).
Figure 3.
Serum CK for mdx and GTE treated mdx mice age 28 and 42 days. Mdx mice treated with 0.25% or 0.5% GTE had significantly reduced serum CK values when compared to untreated mdx mice at age 42 days. *Mean untreated mdx values were significantly different compared to treated mdx mice (P≤0.05).
Muscle lesions in GTE treated mdx mice
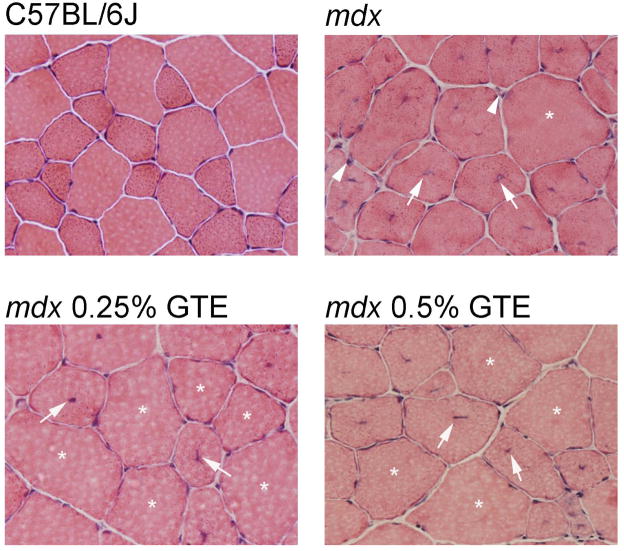
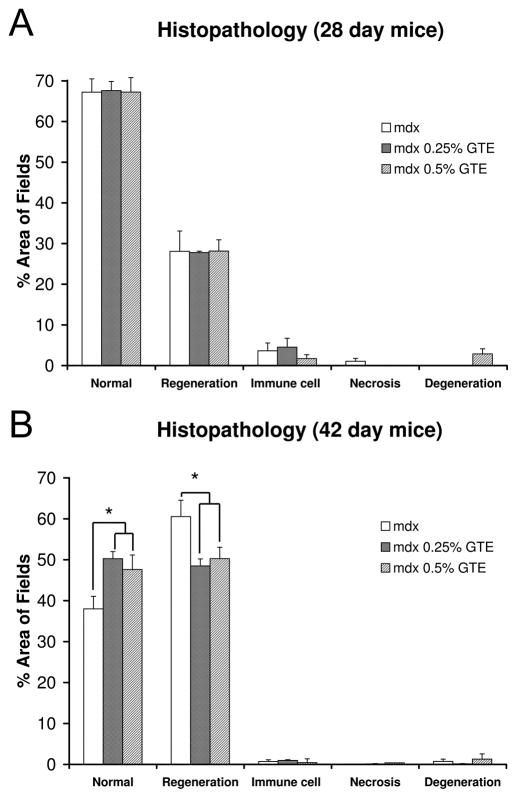
Histopathological features of GTE treated and untreated mdx mice were not significantly different at age 28 days. By age 42 days, 38% of the TA area in untreated mdx mice had normal fiber morphology, while 50% and 48% normal morphology was observed for 0.25% and 0.5% GTE treated mdx mice, respectively (Figure 4 and Figure 5). This corresponds to a significant improvement of 32% (0.25% GTE) and 26% (0.5% GTE) in normal fiber morphology for GTE treated mdx mice (P≤ 0.05). At age 42 days regenerating fibers were the primary histopathological feature that accounted for 61% of the TA area in untreated mdx mice, but only 48% in 0.25%, and 50% in 0.5% GTE treated mdx mice. This is a significant decrease of 21% (0.25% GTE) and 18% (0.5% GTE) in regenerating fibers when compared to untreated mdx mice (P≤ 0.05). There were no significant changes in the percent area of immune cell infiltration, necrosis, and degeneration. These markers do not appear to contribute substantially to the increase in normal fiber morphology observed in GTE treated mdx mice.
Figure 4.
Histopathology of TA muscles for C57BL/6J, mdx and GTE treated mdx mice age 42 days. C57BL/6J muscle sections depicting normal fiber morphology for this age. Untreated mdx sections showing a morphologically normal fiber (asterisks), regenerating fibers (arrow), and immune cell infiltration (arrowhead). Treated mdx sections from 0.25% GTE and 0.5% GTE had altered muscle pathology with an increase in morphologically normal fibers (asterisks) and fewer regenerating fibers (arrow). 400X H&E.
Figure 5.
Quantification of histopathology for mdx and GTE treated mdx TA muscles at ages 28 and 42 days. (A) Although necrotic fibers and fiber degeneration/regeneration were apparent in mdx TA muscles by age 28 days, there was no difference in histopathology in GTE treated mdx mice compared to untreated mdx mice at age 28 days, (B) By age 42 days there was a significant increase in normal fiber morphology and a decrease in regenerating fibers in GTE treated mdx mice. *Mean untreated mdx values were significantly different compared to treated mdx mice (P≤0.05).
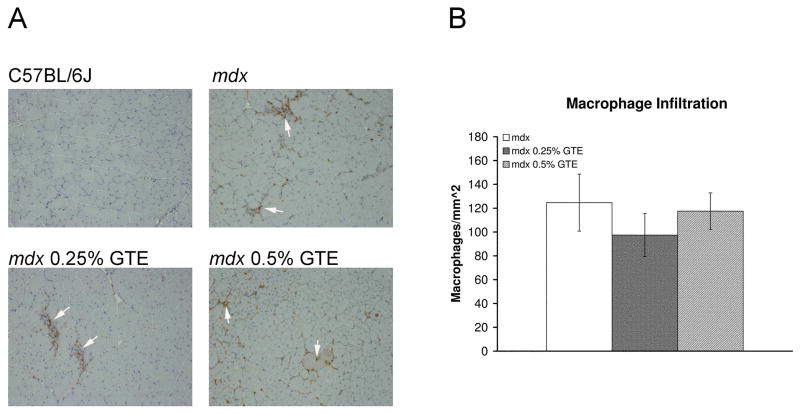
Macrophage infiltration is a major histopathological feature of the dystrophic disease processes (Figure 6). Infiltrating macrophages are found throughout mdx TA muscles with dense foci near necrotic, degenerating and regenerating muscle fibers. GTE is thought to reduce inflammation; however, GTE treatment at 0.25 or 0.5% did not significantly reduce macrophage infiltration in mdx mice age 42 days.
Figure 6.
Macrophage infiltration (F4/80) of TA muscles from mice age 42 days. (A) C57BL/6J muscle sections had very few infiltrating macrophages. Untreated mdx sections had macrophage infiltration (arrow) throughout with dense foci of macrophages. Treated mdx sections from 0.25% GTE and 0.5% GTE had similar macrophage infiltration (arrow) compared to untreated mdx. 100X H&E. (B) Quantification of macrophages revealed there was no difference in the number of infiltrating macrophages for GTE treated and untreated mdx mice.
NF-κB in necrotic and regenerating fibers
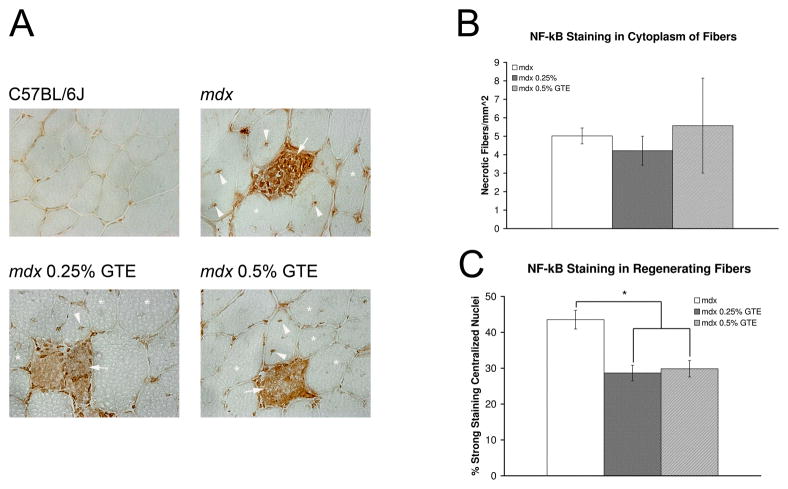
NF-κB is a transcription factor that regulates the expression of many inflammatory genes.23 An antibody specific for the phosphorylated p65 (p-p65) subunit of NF-κB was used to determine if GTE had an effect on activated NF-κB immunostaining (p-NF-κB) (Figure 7). Activated NF-κB is normally sequestered to nuclei; however, in DMD patients necrotic muscle fibers have been reported with cytoplasmic p-NF-kB immunostaining.24 In TA muscles from mdx mice age 42 days, fibers with cytoplasmic staining were also observed. To confirm that cytoplasmic staining was specific to these fibers, negative control sections were stained without primary antibody. All negative control sections showed minimal background staining, no staining of nuclei, and no fibers with cytoplasmic staining were observed (data not shown). In tissues probed with anti-p-NF-κB, fibers with cytoplasmic staining were typically invaded or surrounded by inflammatory cells that also stained positive for p-NF-κB. The presence of inflammatory cells in and around fibers with cytoplasmic NF-κB staining indicates these muscle fibers were undergoing the process of necrosis. There was no difference in the number of fibers with cytoplasmic NF-κB staining for GTE treated and untreated mdx TA muscles at age 42 days (~5 fibers/mm2 of muscle tissue). As expected, the number of mdx fibers with cytoplasmic NF-κB staining was elevated when compared to C57BL/6J control (0.1/mm2 of muscle tissue). After being phosphorylated, NF-κB enters the nucleus to regulate gene expression. NF-κB has been implicated in muscle fiber regeneration and may contribute to the dystrophic disease process.25 Nuclei in C57BL/6J mice had little or no immunostaining. Both GTE treated and untreated mice had strong immunostaining in peripheral and centralized nuclei. Strong staining in regenerating fibers was observed for 44% of centralized nuclei in untreated mdx, while only 29% and 30% were observed in 0.25% and 0.5% GTE treated mdx, respectively. This was a significant decrease of 34% (0.25% GTE) and 31% (0.5% GTE) for GTE treated mdx mice when compared to untreated mdx mice (P≤ 0.05).
Figure 7.
Anit-p-NF-κB (p-p65) staining of TA muscles from mice age 42 days. (A) C57BL/6J muscle sections had no cytoplasmic staining and weak nuclear staining. Untreated mdx sections had staining in peripheral nuclei and the cytoplasm of necrotic fibers infiltrated by inflammatory cells (arrow). Regenerating fibers had centralized nuclei with both strong (arrowhead) and weak (asterisk) immunoreactivity. Treated mdx sections from 0.25% GTE and 0.5% GTE had the same number of infiltrated necrotic fibers with cytoplasmic staining (arrow), but had a decrease in the percent of regenerating fibers with strong immunoreactivity of centralized nuclei compared to untreated mdx. 400X H&E. (B) Quantification of p-NF-κB (p-p65) staining revealed there was no difference in the number of fibers with cytoplasmic staining for GTE treated and untreated mdx mice. Cytoplasmic NF-κB staining in muscle fibers was indicative of necrotic fibers. In regenerating fibers GTE treated mdx mice had a significant decrease in strong centralized nuclear staining. *Mean untreated mdx values were significantly different compared to GTE treated mdx mice (P≤0.05).
Discussion
The objective of this study was to characterize and identify distinct features of dystrophic muscle pathology during the early disease time course and determine what features of dystrophic muscle pathology were reduced by pre-natal and early dietary intervention with GTE. Recent studies have relied on the percent area of centrally nucleated fibers and the area of necrotic muscle tissue to assess changes in muscle pathology and often combine these features to determine overall affected muscle tissue.12, 22, 26–33 However, these studies have not discriminated the percent area of the individual fibers undergoing the histopathologically identifiable processes of degeneration, necrosis or regeneration. Therefore, it is important to note that whole muscle tissue necrosis/degeneration values that are often reported are not the same as the percent area of fiber necrosis or fiber degeneration reported here. One limitation to this approach is that the total area of tissue necrosis may not be fully realized. To compensate for this limitation, areas of immune cell infiltration where there was ongoing tissue necrosis outside of intact fibers were also measured as a separate histopathological marker and quantification of the number of infiltrating macrophages was also reported.
The time course of histopathology shows that the percent area of normal fiber morphology is the same for C57BL/6J and mdx TA muscles at age 14 days, but by age 21 days there is a significant decrease in the percent area of normal fibers for mdx. This time point represents the onset of disease for the mdx mouse. Normal fiber morphology at all other ages analyzed was significantly decreased for the mdx mouse. The percent area of muscle fiber necrosis, degeneration, and immune cell infiltration represent important features of the disease process between ages 21 and 28 days, but overall account for only small percentages of the abnormal fiber morphology (~4.5–6%). The percent area of regenerating fibers represents the largest portion of abnormal fiber morphology at ages 28 (28%) to 42 (61%) days. The large percent area of regenerating fibers compared to other histopathological features indicates that the regenerative process is a substantial contributor to dystrophic muscle pathology. Studies detailing the process of muscle regeneration have shown that in damaged muscle tissue, fibers are capable of returning to normal fiber morphology; however, many regenerated fibers retain centrally located nuclei indefinitely.36–39 The persistence of centrally located nuclei is believed to signify that abnormal processes are occurring in these cells that prevent the nuclei from returning to the periphery of the cell.36–39 From this initial analysis, it was determined that for the GTE treatments muscle pathology should be examined at ages 28 and 42 days because these time points represent the early degenerative and regenerative disease stages.
Serum CK is a systemic indicator of muscle pathology. CK is normally restricted to the cytoplasm of muscle fibers, but when the fibers are damaged it is released into the serum. Serum CK was not different for GTE treated mdx mice compared to untreated mdx at age 28 days, but at age 42 days there was a significant improvement in GTE treated mdx mice. Similarly, there was no difference in normal fiber morphology for GTE treated and untreated mdx mice at age 28 days; however at age 42 days there was a significant improvement for GTE treated mdx mice. There was up to a 32% increase in normal fiber morphology for these mice. The improvement in normal fiber morphology was largely accounted for by up to a ~21% decrease in regenerating fibers. Other histopathological features were unchanged by GTE treatments. Collectively this data indicates that overall muscle pathology was decreased in GTE treated mdx mice.
To further assess how GTE treatments modulate inflammation; macrophages and NF-κB immunostaining were quantified. There was no difference in the number of macrophages infiltrating the TA muscles in GTE treated or untreated mdx mice. Although GTE is believed to have anti-inflammatory properties, it does not appear to decrease the number of infiltrating macrophages in mdx mice at age 42 days. However, a recent study reported that muscle from mdx mice contains two subpopulations of macrophages, M1 and M2.8 M1 macrophages are thought to be cytotoxic and pro-inflammatory while M2 macrophages inhibit the cytotoxicity of M1 macrophages and promote muscle regeneration. In mdx mice there was a shift in the macrophage phenotype to the regenerative subset between age 4 weeks and 12 weeks.8 Although there was no change in the overall number of infiltrating macrophages in GTE treated mdx mice age 42 days, one possibility is that GTE induces a shift in the phenotype of infiltrating macrophages. If GTE treatments did contribute to a shift in macrophage phenotype from M1 to M2 without affecting the total numbers of macrophages, it could result in an improved regenerative process. However, further studies are needed to address this possibility.
A component of GTE, EGCG, has been reported to inhibit IKK activity which results in inhibition of NF-κB.10, 13–15 Acharyya et al. recently reported mdx muscles lacking IKKβ, a subunit of the IKK complex, have an increase in regenerative capacity when compared to control mdx.25 They also found that mdx muscles with macrophages lacking IKKβ had decreased muscle pathology and a decrease in the expression of pro-inflammatory makers.25 Their data suggest a mechanism through which NF-kB signaling in macrophages and regenerating muscle fibers promotes degeneration and represses regeneration.25 Several studies have reported an increase in the number of regenerating fibers after treatments that reduce or block NF-κB activity while others have reported a decrease in regenerating fibers.25, 28, 29, 40, 41 These differing results may stem from the way in which the NF-κB pathway is altered by treatment compounds and the methods used to administer these compounds (i.e. age of treatment initiation and termination, route of administration). Our findings indicate that prenatal and early dietary GTE treatment reduces the amount of regenerating fibers potentially through alterations in NF-κB signaling. Past studies have also shown a decrease in muscle necrosis when NF-κB activity is inhibited.25, 28, 29 Our results indicate that the amount of fiber necrosis is unchanged by GTE treatment. Additionally, the number of fibers with cytoplasmic NF-κB immunostaining was unchanged by the GTE treatment. The presence of inflammatory cells infiltrating fibers with cytoplasmic NF-κB staining signifies these muscle fibers were undergoing necrosis. These findings suggest that while muscle degeneration and necrosis initiates the process of muscle wasting, impaired muscle regeneration plays a key role in the progression of muscular dystrophy.25, 28, 29
GTE has been shown in several studies to decrease serum CK levels and muscle pathology while increasing force output, and endurance capacity in mdx mice.12, 22, 30, 42 The antioxidant properties of GTE are believed to be the primary element responsible for these improvements, other signaling pathways may also be responsible. Previous reports have shown that GTE treatment can result in a significant decrease in muscle pathology,12, 22 which is supported by this study. However, past studies did not discriminate the different pathological markers that were reported here, including the finding that the primary change was in the percent area of regenerating fibers. In this study and previously we reported that serum CK was decreased with pre-natal and early GTE treatment.42 Serum CK is an indicator of the extent of muscle damage and a significant decrease of this marker indicates that GTE is having an important impact on dystrophic muscle pathology. Additionally, no other studies have attempted to determine the effect of dietary GTE on inflammation. Although no significant change in infiltrating macrophages was observed, a decrease in NF-κB immunostaining in regenerating fibers was observed. NF-κB is a known regulator of inflammatory genes and may play an important role in the dystrophic disease process and the recruitment of inflammatory cells. GTE role in inflammation may prove to be an important component of the decreased mdx muscle pathology.
CAM approaches, including the use of anti-inflammatory botanicals in general and GTE in particular, may lead to the amelioration of DMD and provide important insight into disease processes. CAM interventions can be used in conjunction with conventional therapies in a cost-effective manner to improve disease prognosis. GTE treatments of mdx mice resulted in increased normal fiber morphology, decreased regenerating fibers, and decreased NF-κB staining in regenerating fibers. The detailed histopathological analysis revealed that changes in the percent area of degenerating fibers, necrotic fibers, and immune cell infiltration were small in comparison to the change in regenerating fibers. This data indicates that GTE decreases NF-κB activity in regenerating fibers which may result in changes in the regenerative process leading to an increase in normal fiber morphology. Detailed muscle histopathology for pre-natal and early GTE treated mdx mice has not been previously reported and reveals that GTE may be an additional treatment option to decrease early muscle pathology for this incurable disease. In conclusion, GTE decreases muscle pathology potentially by suppressing NF-κB activity in regenerating fibers of mdx mice. While GTE did not decrease overall immune cell or macrophage infiltration, additional studies are needed to examine the role of GTE in modulating the shift of macrophage towards and M2 phenotype and thereby ameliorate the dystrophic disease process by favoring anti-inflammatory responses.
Acknowledgments
This study was supported by National Institutes of Health grant AR049881 to R.W. Grange. Sunphenon 90DCF was a kind gift from Taiyo International (Minneapolis, MN). The contributions of the authors to the manuscript are as follows. N.E.: study design, data collection, data analysis and writing of the manuscript; J.C.: study design and reviewing the manuscript; J.B-R.: study design and reviewing the manuscript; J.R.: data analysis techniques and reviewing the manuscript; R.G.: study design and reviewing the manuscript. All authors read and approved the manuscript.
Footnotes
Conflict of interest statement
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 2.Tidball JG, Wehling-Henricks M. Evolving therapeutic strategies for Duchenne muscular dystrophy: targeting downstream events. Pediatr Res. 2004;56:831–41. doi: 10.1203/01.PDR.0000145578.01985.D0. [DOI] [PubMed] [Google Scholar]
- 3.Porter JD, Guo W, Merriam AP, Khanna S, Cheng G, Zhou X, et al. Persistent over-expression of specific CC class chemokines correlates with macrophage and T-cell recruitment in mdx skeletal muscle. Neuromuscular Disord. 2003;13:223–35. doi: 10.1016/s0960-8966(02)00242-0. [DOI] [PubMed] [Google Scholar]
- 4.Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, et al. A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum Mol Genet. 2002;11:263–72. doi: 10.1093/hmg/11.3.263. [DOI] [PubMed] [Google Scholar]
- 5.Spencer MJ, Tidball JG. Do immune cells promote the pathology of dystrophin-deficient myopathies? Neuromuscular Disord. 2001;11:556–64. doi: 10.1016/s0960-8966(01)00198-5. [DOI] [PubMed] [Google Scholar]
- 6.Spencer MJ, Walsh CM, Dorshkind KA, Rodriguez EM, Tidball JG. Myonuclear Apoptosis in Dystrophic mdx Muscle Occurs by Perforin-mediated Cytotoxicity. J Clin Invest. 1997;99:2745–51. doi: 10.1172/JCI119464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–32. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet. 2009;18:482–96. doi: 10.1093/hmg/ddn376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valcic S, Muders A, Jacobsen NE, Liebler DC, Timmermann BN. Antioxidant chemistry of green tea catechins. Identification of products of the reaction of (−)-epigallocatechin gallate with peroxyl radicals. Chem Res Toxicol. 1999;12:382–6. doi: 10.1021/tx990003t. [DOI] [PubMed] [Google Scholar]
- 10.Chen PC, Wheeler DS, Malhotra V, Odoms K, Denenberg AG, Wong HR. A green tea-derived polyphenol, epigallocatechin-3-gallate, inhibits IkappaB kinase activation and IL-8 gene expression in respiratory epithelium. Inflammation. 2002;26:233–41. doi: 10.1023/A:1019718718977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng WY. Metabolism of green tea catechins: an overview. Curr Drug Metab. 2006;7:755–809. doi: 10.2174/138920006778520552. [DOI] [PubMed] [Google Scholar]
- 12.Dorchies OM, Wagner S, Vuadens O, Waldhauser K, Buetler TM, Kucera P, et al. Green tea extract and its major polyphenol (−)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophy. Am J Physiol Cell Physiol. 2006;290:C616–25. doi: 10.1152/ajpcell.00425.2005. [DOI] [PubMed] [Google Scholar]
- 13.Qin J, Xie LP, Zheng XY, Wang YB, Bai Y, Shen HF, et al. A component of green tea, (−)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochem Biophys Res Commun. 2007;354:852–7. doi: 10.1016/j.bbrc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Sen P, Chakraborty PK, Raha S. Tea polyphenol epigallocatechin 3-gallate impedes the anti-apoptotic effects of low-grade repetitive stress through inhibition of Akt and NFkappaB survival pathways. FEBS Lett. 2006;580:278–84. doi: 10.1016/j.febslet.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Yang F, Oz HS, Barve S, de Villiers WJ, McClain CJ, Varilek GW. The green tea polyphenol (−)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol Pharmacol. 2001;60:528–33. [PubMed] [Google Scholar]
- 16.Liu HS, Chen YH, Hung PF, Kao YH. Inhibitory effect of green tea (−)-epigallocatechin gallate on resistin gene expression in 3T3-L1 adipocytes depends on the ERK pathway. Am J Physiol Endocrinol Metab. 2006;290:E273–81. doi: 10.1152/ajpendo.00325.2005. [DOI] [PubMed] [Google Scholar]
- 17.Sah JF, Balasubramanian S, Eckert RL, Rorke EA. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway. Evidence for direct inhibition of ERK1/2 and AKT kinases. J Biol Chem. 2004;279:12755–62. doi: 10.1074/jbc.M312333200. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Kelly AP, Wang L, French SW, Tang X, Duong HS, et al. Green tea extract and (−)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. J Invest Dermatol. 2006;126:2607–13. doi: 10.1038/sj.jid.5700472. [DOI] [PubMed] [Google Scholar]
- 19.Rando TA. Role of nitric oxide in the pathogenesis of muscular dystrophies: a “two hit” hypothesis of the cause of muscle necrosis. Microsc Res Tech. 2001;55:223–35. doi: 10.1002/jemt.1172. [DOI] [PubMed] [Google Scholar]
- 20.Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol. 2006;33:657–62. doi: 10.1111/j.1440-1681.2006.04394.x. [DOI] [PubMed] [Google Scholar]
- 21.Disatnik MH, Dhawan J, Yu Y, Beal MF, Whirl MM, Franco AA, et al. Evidence of oxidative stress in mdx mouse muscle: studies of the pre-necrotic state. J Neurol Sci. 1998;161:77–84. doi: 10.1016/s0022-510x(98)00258-5. [DOI] [PubMed] [Google Scholar]
- 22.Buetler TM, Renard M, Offord EA, Schneider H, Ruegg UT. Green tea extract decreases muscle necrosis in mdx mice and protects against reactive oxygen species. Am J Clin Nutr. 2002;75:749–53. doi: 10.1093/ajcn/75.4.749. [DOI] [PubMed] [Google Scholar]
- 23.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–66. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 24.Monici MC, Aguennouz M, Mazzeo A, Messina C, Vita G. Activation of nuclear factor-kappaB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology. 2003;60:993–7. doi: 10.1212/01.wnl.0000049913.27181.51. [DOI] [PubMed] [Google Scholar]
- 25.Acharyya S, Villalta S, Bakkar N, Bupha-Intr T, Janssen P, Carathers M, et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grounds MD, Torrisi JO. Anti-TNFalpha (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J. 2004;18:676–82. doi: 10.1096/fj.03-1024com. [DOI] [PubMed] [Google Scholar]
- 27.Hodgetts S, Radley H, Davies M, Grounds MD. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFalpha function with Etanercept in mdx mice. Neuromuscul Disord. 2006;16:591–602. doi: 10.1016/j.nmd.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Messina S, Altavilla D, Aguennouz M, Seminara P, Minutoli L, Monici MC, et al. Lipid peroxidation inhibition blunts nuclear factor-kappaB activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. Am J Pathol. 2006;168:918–26. doi: 10.2353/ajpath.2006.050673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messina S, Bitto A, Aguennouz Mh, Minutoli L, Monici MC, Altavilla D, et al. Nuclear factor kappa-B blockade reduces skeletal muscle degeneration and enhances muscle function in Mdx mice. Exp Neurol. 2006;198:234–41. doi: 10.1016/j.expneurol.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 30.Nakae Y, Hirasaka K, Goto J, Nikawa T, Shono M, Yoshida M, et al. Subcutaneous injection, from birth, of epigallocatechin-3-gallate, a component of green tea, limits the onset of muscular dystrophy in mdx mice: a quantitative histological, immunohistochemical and electrophysiological study. Histochem Cell Biol. 2008;129:489–501. doi: 10.1007/s00418-008-0390-2. [DOI] [PubMed] [Google Scholar]
- 31.Radley HG, Grounds MD. Cromolyn administration (to block mast cell degranulation) reduces necrosis of dystrophic muscle in mdx mice. Neurobiol Dis. 2006;23:387–97. doi: 10.1016/j.nbd.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Stupka N, Gregorevic P, Plant DR, Lynch GS. The calcineurin signal transduction pathway is essential for successful muscle regeneration in mdx dystrophic mice. Acta Neuropathol. 2004;107:299–310. doi: 10.1007/s00401-003-0807-x. [DOI] [PubMed] [Google Scholar]
- 33.Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–32. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carnwath JW, Shotton DM. Muscular dystrophy in the mdx mouse: histopathology of the soleus and extensor digitorum longus muscles. J Neurosci. 1987;80:39–54. doi: 10.1016/0022-510x(87)90219-x. [DOI] [PubMed] [Google Scholar]
- 35.Torres LF, Duchen LW. The mutant mdx: inherited myopathy in the mouse. Morphological studies of nerves, muscles and end-plates. Brain. 1987;110 (Pt 2):269–99. doi: 10.1093/brain/110.2.269. [DOI] [PubMed] [Google Scholar]
- 36.Couteaux R, Mira JC, d’Albis A. Regeneration of muscles after cardiotoxin injury. I. Cytological aspects. Biol Cell. 1988;62:171–82. [PubMed] [Google Scholar]
- 37.Morioka S, Goto K, Kojima A, Naito T, Matsuba Y, Akema T, et al. Functional overloading facilitates the regeneration of injured soleus muscles in mice. J Physiol Sci. 2008;58:397–404. doi: 10.2170/physiolsci.RP004008. [DOI] [PubMed] [Google Scholar]
- 38.Salvini TF, Morini CC, Selistre de Araujo HS, Ownby CL. Long-term regeneration of fast and slow murine skeletal muscles after induced injury by ACL myotoxin isolated from Agkistrodon contortrix laticinctus (broad-banded copperhead) venom. Anat Rec. 1999;254:521–33. doi: 10.1002/(SICI)1097-0185(19990401)254:4<521::AID-AR7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Totsuka T, Watanabe K, Uramoto I, Sakuma K, Mizutani T. Muscular dystrophy: centronucleation may reflect a compensatory activation of defective myonuclei. J Biomed Sci. 1998;5:54–61. doi: 10.1007/BF02253356. [DOI] [PubMed] [Google Scholar]
- 40.Pan Y, Chen C, Shen Y, Zhu CH, Wang G, Wang XC, et al. Curcumin alleviates dystrophic muscle pathology in mdx mice. Mol Cells. 2008;25:531–7. [PubMed] [Google Scholar]
- 41.Whitehead NP, Pham C, Gervasio OL, Allen DG. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol. 2008;586:2003–14. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Call JA, Voelker KA, Wolff AV, McMillan RP, Evans NP, Hulver MW, et al. Endurance capacity in maturing mdx mice is markedly enhanced by combined voluntary wheel running and green tea extract. J Appl Physiol. 2008;105:923–32. doi: 10.1152/japplphysiol.00028.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]