Abstract
Parkinson’s disease (PD) is a major neuro-degenerative disorder that is usually considered in terms of midbrain and basal ganglia dysfunction. Regarding PD instead as a disconnection syndrome may prove beneficial to understanding aspects of cognition, perception, and other neuropsychological domains in the disease. PD is usually of unilateral onset, providing evidence of intrahemispheric dissociations and an imbalance in the usual relative strengths of the right and left hemispheres. Hence, in order to appreciate the neuropsychology of PD, it is important to apply to this disease our understanding of hemispheric lateralization effects and within-hemisphere circuitry from brainstem to higher-order association cortex. The focus of this review is on the relevance of PD-related disconnections among subcortical and cortical structures to cognition, perception, emotion, and associated brainstem-based domains such as sleep and mood disturbance. Besides providing information on disease characteristics, regarding PD as a disconnection syndrome allows us to more completely understand normal brain-behavior relations in general.
Keywords: Parkinson, Visuospatial, Cognition, Perception, Emotion, Lateralization
Norman Geschwind’s influential recasting of classic behavioral deficits as disconnection syndromes included reevaluations of a number of neurological disorders, including visual agnosia, alexia, and right-hand apraxia (Geschwind 1965a, b). As we are reminded by the recent passing of Edith Kaplan, Geschwind’s important collaborator, a number of researchers of that generation (e.g., Brenda Milner, Roger Sperry, to provide just two prominent examples) were likewise strong proponents of disconnection as a route to understanding normal as well as abnormal brain function. In more recent years, investigators have cited disconnection effects in their efforts to explain perceptual and cognitive deficits in disorders that had not previously been examined from this perspective. For example, in alien-hand syndrome, intermanual conflict and mirror movements of the hands are common, especially in the callosal type (e.g., Fisher 2000; see Scepkowski and Cronin-Golomb 2003 for a review), and are similar to behaviors seen in individuals for whom the corpus callosum and other forebrain commissures were sectioned as treatment for intractable epilepsy (Cronin-Golomb 1986).
The major neurodegenerative disorders are now being scrutinized from this angle as well, with a focus on cortico-cortical disconnection within cerebral hemispheres. In Alzheimer’s disease, investigators have suggested that behavioral deficits arise from interruptions of long cortico-cortical fibers (Hof and Bouras 1991) and from white matter abnormalities in all lobes of the brain and in medial temporal lobe limbic structures (Salat et al. 2009; Stebbins and Murphy 2009), as well as from abnormalities in the corpus callosum (Ukmar et al. 2008). In Parkinson’s disease (PD), breaks in cortico-striatothalamocortical and cortico-cortical circuitry have been invoked to explain a variety of the motor and non-motor symptoms of the disease (Braak and Del Tredici 2004; Braak et al. 2004; Cronin-Golomb and Amick 2001; Cronin-Golomb and Braun 1997) as well as the presymptomatic progression of pathology (Braak et al. 2004). The cell types of PD that are susceptible to pathological inclusion bodies (Lewy neurites and Lewy bodies) include unmyelinated or poorly myelinated projection neurons with axons that are long and thin in relation to the size of their cell bodies (Braak et al. 2004). Subcortical white matter reductions in frontal, parietal, and temporal areas have also been documented (Gattellaro et al. 2009; Karagulle Kendi et al. 2008), even in individuals with early, untreated PD (Martin et al. 2009).
PD is of particular interest in regard to disconnection effects because in this disorder one sees evidence of intrahemispheric dissociations and an imbalance in the usual relative strengths of the right and left hemispheres, depending on the side of onset of the motor symptoms of the disease. In this review, PD is examined as a disconnection syndrome that requires understanding of hemispheric lateralization effects and within-hemisphere circuitry from brainstem to higher-order association cortex. The focus is on the relevance of these disconnections to cognition, perception, emotion, and associated brainstem-based domains such as mood and sleep. Besides providing information on disease characteristics, regarding PD as a disconnection syndrome allows us to more completely understand normal brain-behavior relations with reference to hemispheric lateralization of function.
Parkinson’s Disease: Symptom Asymmetry
PD usually has a unilateral motor onset, and although the disease becomes bilateral, the initial side commonly remains more afflicted than the later-involved side. Because the two hemispheres are specialized for different aspects of perceptual and cognitive function, it is important to contrast PD patients with initial motor symptoms on the right body side (RPD; inferred left-hemisphere pathology) with PD patients with initial symptoms on the left side of the body (LPD; inferred right-hemisphere pathology), as would be true of any disease or disorder affecting one side of the brain more than the other, such as unilateral stroke.
The asymmetrical motor symptoms of PD are associated with asymmetrical depletion of dopamine (DA) in the substantia nigra of the midbrain (Kempster et al. 1989) across the range of disease severity, from never-medicated patients with unilateral symptoms to medicated patients with more severe bilateral involvement (Antonini et al. 1995; Innis et al. 1993; Laulumaa et al. 1993; Leenders et al. 1990; Tissingh et al. 1998). These changes in the substantia nigra lead to asymmetrical dysregulation of the striatum, which may in turn lead to further asymmetrical dysfunction of neural circuits that include the basal ganglia and cortical areas upon which normal cognitive abilities are dependent (Middleton and Strick 2000a, b).
Asymmetrical DA depletion presumably has functional consequences with respect to areas with input to or output from the basal ganglia, including the right posterior parietal regions important for visuospatial cognition (Clower et al. 2005).
Reduced basal ganglia activity correlates with symptom severity in PD (Antonini et al. 1995; Asenbaum et al. 1997; Kim et al. 1999; Torstenson et al. 1997). Studies using single-photon emission computed tomography have found bilateral changes in DA activity in PD patients, with more pronounced changes contralateral to the side of initial motor symptoms (Booij et al. 1997; Innis et al. 1993; Kim et al. 1999; Marek et al. 1996). DA asymmetry as assessed with positron emission tomography has been found in never-medicated patients as well as medicated patients with more advanced disease (Antonini et al. 1995; Laulumaa et al. 1993; Leenders et al. 1990). This asymmetry is maintained long after the disease has progressed from unilateral to bilateral. PD patients with moderate to severe bilateral motor disability still show considerable asymmetry in the putamen and caudate, with relatively reduced DA activity contralateral to the initial motor symptom side (Antonini et al. 1995; Booij et al. 1997). An autopsy study of PD patients with asymmetrical onset of motor disability revealed 25% fewer neurons in the substantia nigra contralateral to the side of the initial motor symptoms than in the ipsilateral substantia nigra (Kempster et al. 1989). In light of these findings, it is important to contrast the behavioral performance of LPD and RPD patients. Failure to distinguish side-of-onset PD subgroups may result in failure to discern deficits characteristic of only one of the subgroups that are of consequence to daily function.
The following sections describe mainly visual and visuospatial dysfunction in PD and the primacy of impairments in LPD relative to RPD patients, which is a focus of our current research. As described above, LPD patients with extensive depletion of DA in the right basal ganglia may be at higher risk than RPD patients for visuospatial deficits. The relevance of applying the perspective of hemispheric specialization and potential disconnection effects of course also holds for the cognitive domains that would be more affected in RPD than LPD, such as in verbally-based tasks of executive function and verbal memory. As an example, RPD patients show poorer verbal memory performance than do those with LPD, who in turn are more impaired on visual memory (Amick et al. 2006a).
Visuospatial Dysfunction in PD
Debate over the existence of visuospatial dysfunction in PD was fueled in the past by concern that poor performance on some spatial tasks was attributable more to the tasks’ motor demands than to genuine visuospatial impairment (Brown et al. 1998; Cooper et al. 1991; Girotti et al. 1988). The current view, by contrast, is that visuospatial deficits are a regular feature of PD (Schapira et al. 2009) and are at least partly independent of motor limitations, as PD patients are impaired on a variety of non-motor spatial tasks such as those assessing size perception (Lee et al. 2001a), visuospatial orienting and attention (Lee et al. 2001b), mental rotation (Amick et al. 2006b), perception of optic flow and egocentric reference (Davidsdottir et al. 2008), visuospatial closure (Cronin-Golomb and Braun 1997), visual form discrimination and face recognition (Pereira et al. 2009), speed of visual object recognition (Meppelink et al. 2009), and hierarchical pattern perception (Schendan et al. 2009) (see Cronin-Golomb and Amick 2001, for a review of other spatial tasks). Many aspects of visuospatial information are thought to be processed by the posterior parietal lobe, especially in the right hemisphere (Cronin-Golomb and Amick 2001; Harris et al. 2000; Ogden et al. 1990). Given the laterality of spatial functions, some discrepancies in the literature on spatial abilities in PD may be due to neglecting the critical factor of body side of motor symptom onset, which has been found to be related to the laterality of brain changes in PD (Rakshi et al. 1999; Thobois et al. 2000) and to relative disturbances on tests of spatial perception (e.g., Blonder et al. 1989; Harris et al. 2003; Lee et al. 2001a, b).
The following is a description of findings from our lab and others of side-of-onset effects on specific measures of visual and visuospatial function in PD. The first set of studies used tests sensitive to hemispheric effects and the second set was further sensitive to side of visual-field presentation, which is a relevant dimension of testing in many disorders with relative unilateral disturbance. The third topic is a description of asymmetries in facial emotion recognition. In all of the studies from our laboratory, the PD participants were non-demented and carefully screened for other neurological disorders and for other conditions that may affect results, such as scores beyond relevant cut-off levels on inventories of depression and anxiety. Most patients had a history of use of anti-parkinsonian medications and were tested during their optimal “on” period. The range of motor severity was mild to moderate as indexed by Hoehn and Yahr stages I to III, with the majority being stage II.
Hemispheric Effects on Visuospatial Function in PD. Hierarchical Pattern Perception and Visual Dependence
Hierarchical Pattern Perception
The unilateral onset of PD may be viewed as a dysregulation of a lateralized corticostriatal network. In regard to visuospatial cognition, the relevant components of the dysfunctional network likely include dorsal posterior cortical areas. Accordingly, we hypothesized that PD would be associated with visuospatial problems that are known to arise from impairments to these areas. We selected hierarchical pattern perception (HPP) and attention for examination (Schendan et al. 2009). Performance on tasks assessing these cognitive abilities has been associated with lateralized areas in parietal cortices implicated in visuospatial transformation and attention (Robertson et al. 1988), but not with the dorsolateral prefrontal cortex (Robertson et al. 1991), a brain region to which some visuospatial deficits in PD have been attributed (Bondi et al. 1991).
The standard HPP task is well known in neuropsychology. Observers view hierarchically arranged stimuli—that is, large letters composed of small letters—and identify letter targets occurring at the global level (large letters) or local level (small letters) (Navon et al. 1977) (Fig. 1). Healthy adults show a right visual field (RVF) (left hemisphere) advantage when the task is to detect local targets and a left visual field (LVF) (right hemisphere) advantage for global targets (Sergent 1982). Neuroimaging studies have identified greater activation of the left posterior temporal-parietal junction (l-TP) during local processing and greater activation of the right posterior temporal-parietal junction (r-TP) during global processing. Further, the pattern of findings suggests that TP may mediate the sustained distribution of attention between and across local and global visuospatial levels by modulating image analysis in more ventral extrastriate regions (Fink et al. 1996, 1997a, b).
Fig. 1.
Hierarchical stimuli. a Small (local) letter “H”s were arranged to form a single large (global) letter “E”. The target is the letter “H” and the foil is the letter “E”. b Small (local) letter “A”s were arranged to form a single large (global) letter “S”. The target is the letter “S” and the foil is the letter “A”. (From Schendan et al. 2009; Behavioral Neuroscience, 123, American Psychological Association)
Besides revealing the processes underlying local and global target detection, performance on HPP can also provide evidence for impaired control of visuospatial attention. Damage to the left rostral inferior parietal lobule (l-IPL) has been shown to negatively affect the ability to appropriately allocate attention to a specific hierarchical level (Robertson et al. 1988). If one manipulates the probability of the level where the target occurs, a reaction time (RT) advantage is demonstrated in healthy adults when targets appear at the more probable (attention-biased) level relative to the less probable level (Robertson and Lamb 1991). It has been shown that, like healthy adults, patients with r-TP or l-TP lesions benefit from this kind of probability information, but patients with l-IPL lesions show a different pattern in that they do not demonstrate a RT cost when the target appears at the less probable level (Robertson et al. 1988). (This type of attentional ability has not been examined in patients with r-IPL lesions). Of relevance, a neuroimaging study with healthy adults has shown activation in both left and right inferior parietal cortex when attention is biased toward one level or the other (Wilkinson et al. 2001).
This evidence for lateralized networks in HPP in healthy adults and in lesion patients led us to investigate the problem in LPD and RPD (Schendan et al. 2009). The hypothesis was that RPD would be impaired at local level processing because l-TP emphasizes processing of local information, and that LPD would show impaired global processing because r-TP emphasizes global processing. In this study, we assessed 20 LPD, 18 RPD, and 22 matched normal control individuals (NC). The participants performed a standard HPP task, identifying targets at the local or global level with and without attention being biased toward the local or global level. LPD and RPD performed differently at the global and local levels, despite normal attentional control between levels. LPD patients showed a single dissociation, demonstrating abnormal global level processing under all conditions. By contrast, RPD patients showed abnormal local level processing mainly when attention was biased toward the local level (Fig. 2).
Fig. 2.
Hierarchical pattern perception results. a Median RTs (ms) for the LPD, RPD, and NC groups in the no-bias condition. b Median RTs (ms) for the LPD, RPD, and NC groups in the biased-attention conditions. The left half of the graph represents median RTs to targets occurring at the global or local levels in the local-biased attention condition. The right half of the graph represent median RTs to targets occurring at the global or local levels in the global-biased attention condition. (From Schendan et al. 2009; Behavioral Neuroscience, 123, American Psychological Association)
These findings in PD provide evidence for disruption of the connections between the basal ganglia and the brain areas at the posterior TP junction that are important for HPP, but not dorsolateral prefrontal cortex areas. That is, dysfunction of basal ganglia-posterior parietal connections, and not of a frontostriatal loop, underlies this visuospatial problem in PD. This view is in accord with our current understanding of closed cortico-striato-thalamocortical circuits, which extend from cortex to striatum and then to the globus pallidus or substantia nigra, output nuclei of the basal ganglia, and back to cortex via the thalamus (Alexander et al. 1986). There are multiple such loops in the brain, denoted by the cortical areas involved. While frontostriatal and temporostriatal loops have been established for some time based on monkey neuroanatomy (Middleton and Strick 2000a, b), evidence for a posterior parietal loop is more recent and not yet definitive in its details. It appears that the anterior intraparietal region (AIP, area 7b, area PF) projects to the ventral putamen and sparse parts of the caudate head and body in the basal ganglia (Yeterian and Pandya 1993). The substantia nigra pars reticulata in turn projects back to area AIP via the thalamus (Clower et al. 2005), possibly forming the output channel for a posterior parietal loop (Middleton and Strick 2000a). This aspect of circuitry has been proposed as an anatomical basis for behavioral observations of visuomotor and visuospatial cognition deficits in PD patients that, as we have described, resemble those seen in patients with direct posterior parietal damage (Clower et al. 2005).
Our HPP findings suggested a link between side of motor symptom onset in PD and hemispheric asymmetry of an inferior parietal-basal ganglia pathway involving the TP and caudate head that is necessary for the visuospatial ability of hierarchical pattern perception. Neuroimaging work will be needed to clarify whether the TP region itself is affected in PD and in a lateralized manner, or whether problems on visuospatial tasks involving TP reflect dysfunctional parieto-basal ganglia interactions emanating from direct basal ganglia dysfunction, which is a hallmark of PD. Such dysfunction may then further disrupt processing along a lateralized parietal-basal ganglia pathway recruited for HPP.
Visual Dependence
While hierarchical pattern perception appears to be associated with parieto-temporal cortex and striatal areas, there are other visuospatial impairments occurring in PD that implicate disordered processing in lower-level visual areas and in more anterior cortex, the latter including the motor areas. We began to explore this issue by asking patients with PD what types of visual and visuospatial problems they were having (Davidsdottir et al. 2005). We received responses to our questionnaire from 81 patients attending PD support group meetings, of whom 70 self-identified as having unilateral onset (35 each of LPD and RPD). While this self-report questionnaire was not designed to probe for side-of-onset effects, it was notable that LPD patients reported more often bumping into the left side of doorways than into the right side, whereas RPD patients reported no such bias. This bias may indicate a mild neglect for the left hemispace in the LPD group, which is consistent with the findings of other groups. For example, on a line bisection task, Lee et al. (2001a) found that a LPD group showed a pattern of performance like mild left hemispatial neglect (bisecting the line to the right of the true midline), whereas RPD and healthy adults demonstrated a mild leftward bias (known as “pseudoneglect” in the healthy group). In an interesting extension of this idea, Lee et al. (2001b) asked PD patients to judge if they could fit through a projected view of a doorway. The LPD group overestimated the amount of space they would require to fit through the doorway (that is, they exhibited perceived spatial compression, as is seen in neglect cases), whereas the RPD group showed mild underestimation.
The idea of spatial neglect is relevant in assessing another characteristic of PD visuospatial dysfunction, which is visual dependence. Visual dependence refers to the extent to which performance on a task is influenced by non-pertinent visual information in the environment. Visual dependence has long been known to be elevated in PD relative to healthy adults (Danta and Hilton 1975), with patients relying on visual guidance or feedback on tasks of simple perception and postural manipulations, and when walking (Azulay et al. 1999, 2002; Bronstein et al. 1990; Cooke et al. 1978; Morris et al. 2005). Whether there is a hemispatial bias to such visual dependence had not been investigated, and we conducted a study to address this question (Davidsdottir et al. 2008).
In a variant of the standard rod-and frame test that has been used in many studies of visual dependence, we used a large-screen presentation of a tilted rod and asked participants to indicate when the rod was horizontal. The participants included 16 LPD, 15 RPD, and 18 matched normal control adults (NC). We found that both the LPD and RPD subgroups were significantly more visually dependent (unable to disregard visual environmental information when attempting to set the line to horizontal) than the control group. In comparing the PD subgroups, we further found that LPD were more visually dependent than RPD. On average, LPD deviated 0.86° from true horizontal compared to 0.43° deviation of RPD and 0.27° deviation of NC. Those who were more visually dependent showed a trend toward more bumping into doorways, as measured with the PD Vision Questionnaire. The findings are in accord with previous evidence that PD patients rely more than healthy adults on visual guidance or feedback when walking and for tasks of perception and postural manipulations as noted above. Our findings describe the extent to which visual dependence varies as a function of laterality of motor symptom onset and hence of affected lateralized brain networks.
In the same study, we assessed the ability of the participants to walk along a corridor while we manipulated visual input. We measured the extent of lateral veering, among other gait-related variables. Visual dependence was correlated with veering under visual input for RPD but not for LPD, with RPD who were more visually dependent veering to a greater extent to their left. The results suggest that in the LPD group there were more salient visual influences on veering, possibly associated with associated shifting of the perceived egocentric midline, as described in the next section.
Side-of-Presentation Effects on Visuospatial Function in PD. Optic Flow and Mental Rotation
Perception of LVF and RVF Optic Flow Speed
Following on from the discussion above, we investigated further irregularities in the perception of the visual world of patients with PD. In particular, we focused on the side of visual field presentation because this paradigm has so often been successful in studies of lateralization of perception and cognition in normal individuals as well as in those with various brain disorders. We expected that for tasks in which hemispheric effects (by side of onset) are ordinarily observed, those effects might be further strengthened with restricted side of visual presentation, and that for other tasks that had not previously been associated with hemispheric effects, such effects might be revealed through restriction to one side of presentation.
Beginning with an aspect of basic visual function, we investigated optic flow perception in PD, contrasting optic flow effects with those seen with a higher-order task of egocentric midline perception (Davidsdottir et al. 2008). Our framework followed from the idea that information about one’s directional heading during navigation may be gained either from optic flow patterns or from the perceived location of a goal (Fajen and Warren 2004; Harris and Carre 2001; Kearns et al. 2002; Turano et al. 2005; Warren et al. 2001). If optic flow speed in the LVF and RVF is perceived as asymmetrical (that is, faster on one side than the other), a bias develops such that heading is perceived to be towards the hemifield in which the flow speed is slower (Dyre and Andersen 1997). In fact, a successful strategy for steering down a corridor is to equalize the speed of optic flow across the left and right hemifields (Duchon and Warren 2002; Srinivasan et al. 1991). As noted, besides optic flow, the perceived location of a goal may influence the path of movement (Harris and Bonas 2002; Rushton et al. 1998). Goal direction, including walking toward a target, is influenced by the egocentric reference point (ECRP) (Hasselbach-Heitzeg and Reuter-Lorenz 2002), which divides space into hemifields with respect to the person’s midline (Karnath et al. 1991). Both midline computation (Vallar et al. 1999) and optic flow perception (Peuskens et al. 2001) have been shown in functional neuroimaging studies to be subserved by the parietal lobes.
Patients with right parietal lesions and neglect experience shifted midline perception (Hasselbach and Butter 1997; Karnath 1994) and shifted allocentric midpoint estimates, such as on line bisection tests (e.g., Rorden et al. 2006). In PD, shifts in allocentric midpoint estimates are consistent with a neglect-like pattern (Lee et al. 2001a), but neither egocentric midline shifting nor optic flow perception had been examined in this disorder. As noted in the section above on hierarchical pattern perception, impairments in these parietal-based functions might be expected in PD because of dysfunction of basal ganglia-thalamocortical circuitry that extends to the parietal lobe (Bartels et al. 2006; Clower et al. 2005; Middleton and Strick 2000a). Evidence supporting the compression of the representation of external space (particularly left hemispace) in LPD (Lee et al. 2001a, b) is consistent with LPD having a shift of midline perception to the right. Because parietal activation during the perception of optic flow patterns is likewise predominantly in the right hemisphere (Peuskens et al. 2001), it seemed possible that optic flow perception as well as egocentric midline alignment might be particularly disrupted in LPD patients.
The aim of our study was to examine navigational veering in PD and the contributions of disturbances in the perception of optic flow and the egocentric midline to biases in this behavior. Optic flow displays were viewed on a projection screen (sitting) and when wearing a virtual reality headset (walking condition). We expected that motion in the non-compressed hemifield would be perceived to be faster than in the compressed hemifield, resulting in a biased path of locomotion. In the case of LPD, perceived compression of the left hemispace would translate into the perception of slowed optic flow speed in the LVF, and hence leftward veering. The opposite prediction would hold in regard to egocentric midline perception: If LPD had a rightward shift of midline, rightward veering should follow. The direction of veering would therefore provide evidence of the preferential use of optic flow perception or midline reference to update position during spatial navigation in PD.
For both the projection-screen and walking-with-headset assessments, the display consisted of two mirror-symmetrical fields composed of sidewalls of random dots (Fig. 3). Under the condition of adjustable optic flow speed using the projection screen, LPD (n=16) tended to perceive LVF speed as slower than RVF speed, consistent with spatial compression, whereas RPD (n=15) and the control participants (n=18) perceived flow in the LVF as faster than in the RVF, consistent with spatial expansion. This perception was demonstrated by their adjustment of flow speed in one hemifield until they perceived that it matched that of the speed-constant hemifield; that is, until they attained the point of subjective equality across hemifields (Fig. 4). On the egocentric midline estimation task, LPD patients overall indicated that their midline was right of center (driven by LPD women; see the comment on relevant gender effects in Section VII below). These results are similar to what is seen in neglect patients, for whom the midline is shifted towards the ipsilesional hemispace (e.g., Chokron and Bartolomeo 1997; Karnath 1997; Karnath et al. 1991; Richard et al. 2004). Our findings suggested that PD affects optic flow perception as well as the ability to estimate the egocentric midline.
Fig. 3.

Optic flow display (as used in Davidsdottir et al., 2008)
Fig. 4.
Optic flow results. When optic flow speeds were equal in the two hemifields, RPD and HC (healthy control) perceived the speed of optic flow in the LVF to be faster than the speed of optic flow in the RVF; that is, they thought the LVF flow speed should be slower in order to reach the point of subjective equality (PSE) with respect to constant flow speed in the RVF. By contrast, LPD tended to perceive the speed in the LVF as slower than the speed in the RVF; that is, they thought the LVF speed should be faster in order to attain the PSE with respect to the constant speed in the RVF. (Results from Davidsdottir et al. 2008)
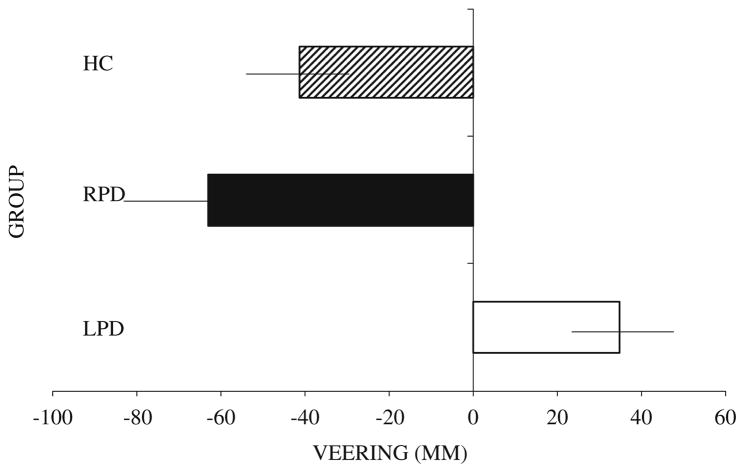
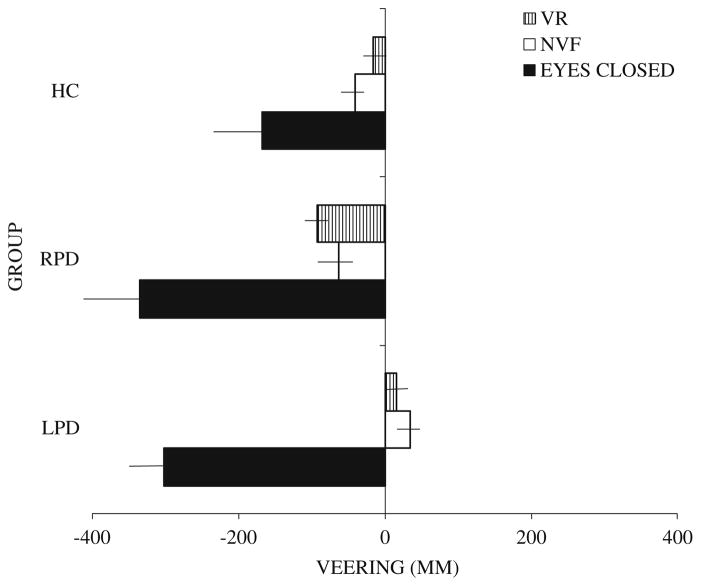
On the walking task with subgroups of the above samples (14 LPD, 8 RPD, 16 NC), all veered leftward during navigation with eyes closed. With eyes open under natural visual feedback or with virtual reality input, LPD deviated to the right of center and differed significantly from the other two groups, whereas the RPD and control participants continued to veer leftward (though less than with eyes closed) and did not differ from each other (Figs. 5 and 6). Navigational veering in LPD and in RPD was correlated with deviation of the perceived egocentric midline and not with perception of optic flow speed asymmetries. Additionally RPD veering was correlated with visual dependence, though in fact LPD were more visually dependent than RPD.
Fig. 5.
Veering during a walking trial with natural visual feedback (eyes open). Healthy control (HC): N = 16, LPD: N = 14, RPD: N = 8. Negative signs represent leftward deviation from the true center. Positive signs represent rightward deviation from the true center. Horizontal lines represent standard error of the mean. (From Davidsdottir et al. 2008; Brain, 131, Oxford University Press)
Fig. 6.
Comparison of veering on three walking conditions: eyes closed, natural visual feedback (NVF) (eyes open), virtual reality (VR). Healthy control (HC): N = 16, LPD: N = 14, RPD: N = 8. Negative signs represent leftward deviation from the true center. Positive signs represent rightward deviation from the true center. Horizontal lines represent standard error of the mean. (From Davidsdottir et al. 2008; Brain, 131, Oxford University Press)
This study provided evidence that parietal-mediated perception of visual space, including optic flow speed and egocentric midline coordinates, is affected in PD, and affected differently according to side of disease onset. The walking assessment showed that visual input affected the direction and extent of lateral veering and that veering corresponded to shifts in the egocentric midline rather than to abnormal perception of hemifield optic flow speed. The gender effects found in this study are beyond the scope of this review, but it should be kept in mind that certain spatial tasks may elicit different performance in men and women. If ignored, these differences and consequent enhanced variability within the PD group as a whole may result in erroneous interpretation of spatial effects in PD and in general.
Mental Rotation
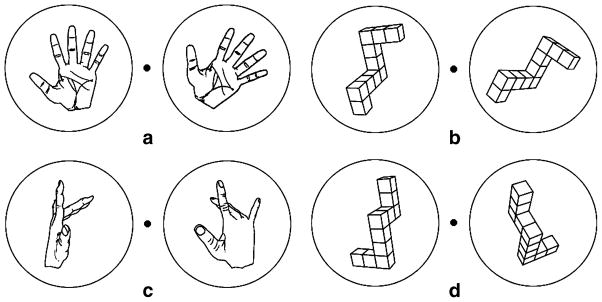
Consistent with the consideration of hemisphere effects, LPD and RPD patients appear to exhibit different mental rotation deficits. RPD but not LPD patients have been shown to make more errors than a control group on a personal orientation task (viewer-centered transformation) (Bowen et al. 1976). RPD patients were also slower at mentally rotating hands (viewer-centered transformation) than a control group, whereas groups did not differ when mentally rotating alphanumeric stimuli (object-centered transformation); LPD patients were not assessed (Dominey et al. 1995). Lee et al. (1998) found that LPD patients were impaired relative to a control group on the mental rotation of objects (object-centered transformation) in three (3D) but not two (2D) dimensions. RPD patients were not assessed. None of these studies directly compared RPD to LPD patients on tasks requiring object- versus viewer-centered transformations, raising the question of what hemisphere effects might emerge through this contrast. We accordingly chose two types of tasks: mental rotation of objects, requiring object-centered transformations, and mental rotation of hands, requiring viewer-centered transformations (Fig. 7). Because mentally rotating hands engages a more extensive region of dysfunctional cortex (primary and association motor and parietal cortices) than rotating objects (parietal), we expected that PD patients in general would perform more poorly on the mental rotation of hands than objects relative to a control group.
Fig. 7.
Mental rotation stimuli. a, b Examples of pairs of hands and objects rotated in 2D space when the correct response is “same”. c, d Examples of pairs of hands and objects rotated in 3D space when the correct response is “different”. (From Amick et al. 2006b; Neuropsychologia, 44, Elsevier)
The hypothesis of interest here was in regard to how side of onset would influence the severity of impairment on mental rotation. Mental rotation of objects is associated with more extensive right-hemisphere processing, suggesting that if there were a PD deficit then LPD patients would exhibit the most severe impairments. By contrast, mental rotation of hands engages largely left parietal and frontal areas, so RPD patients would be expected to be the more impaired subgroup.
The convention in the literature is to design the hands task to elicit maximal involvement of the left hemisphere by always displaying the hand to be mentally rotated in the RVF (e.g., Ganis et al. 2000; Kosslyn et al. 1998). The limitation of this design, however, is that visual field of presentation, instead of stimulus type, could account for poorer performance by the RPD than the LPD group on mental rotation of hands. As information from one visual field is processed first in the contralateral hemisphere, the side of presentation (LVF, RVF) and not the stimulus type (hands, objects) might actually account for any observed lateralized brain activity. In order to examine this possibility, we included a control experiment for a subgroup of participants. In this experiment, the hand to be rotated appeared in the LVF instead of in the RVF. If visual field of presentation determines the hemisphere recruited for hand mental rotation, then in this control experiment the LPD group would be predicted to be the more impaired group.
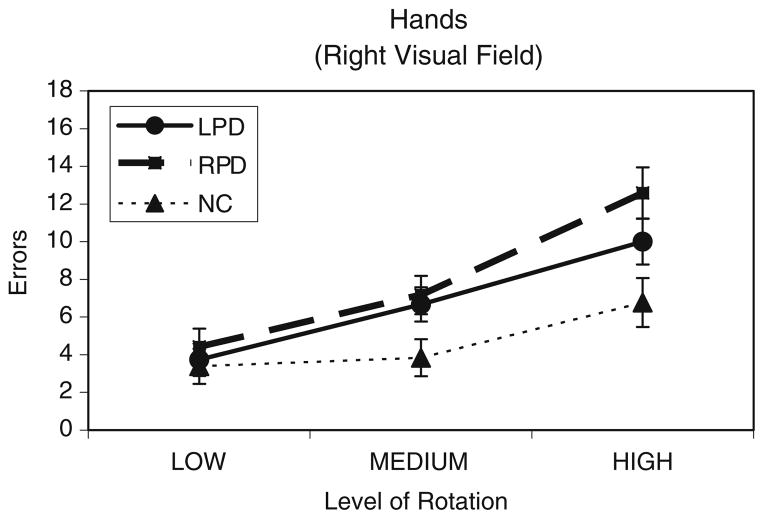
In our study (Amick et al. 2006b), we assessed performance in 15 LPD, 12 RPD, and 13 matched healthy normal control participants (NC) (subgroup of 7 LPD, 6 RPD, 6 NC for the control experiment), who made same/different judgments about pairs of rotated hands or objects. As expected, we found that PD patients, relative to the NC group, were more impaired on the mental rotation of hands than objects. Neither LPD nor RPD performed worse than the control group for objects. Also as expected, side of onset predicted results for hands. In the case of rotating hands with the stimulus to be rotated appearing in the RVF, it was the RPD group, but not LPD, that made more errors than the NC group (Fig. 8). When the stimulus to be rotated appeared instead in the LVF, only LPD made more errors than NC (Fig. 9). Together, these findings suggested a double dissociation between RPD and LPD groups on the hand mental rotation task, depending upon whether the hand to be mentally rotated appeared in the visual field that was ipsilateral to their side of onset. That is, the hemifield location of a to-be-rotated hand stimulus was able to cause the hemispheric frontoparietal networks to be differentially engaged.
Fig. 8.
Mental rotation results. Mean number of errors for the LPD (n= 15), RPD (n=12), and NC (n=13) groups on the mental rotation of hands when the hand to be mentally rotated was presented in the right visual field. Results are collapsed across rotation axis (2-dimensional, 3-dimensional). Nine rotation angles were collapsed into three levels of rotation: Low (20°, 40°, 60°), Medium (80°, 100°, 120°), and High (140°, 160°, 180°). (From Amick et al. 2006b; Neuropsychologia, 44, Elsevier)
Fig. 9.
Mental rotation results. Mean number of errors for the LPD (n= 7), RPD (n=6), and NC (n=6) subgroups on the mental rotation of hands when the hand to be mentally rotated was presented in the left visual field. Results are collapsed across rotation axis (2-dimensional, 3-dimensional). Nine rotation angles were collapsed into three levels of rotation: Low (20°, 40°, 60°), Medium (80°, 100°, 120°), and High (140°, 160°, 180°). (From Amick et al. 2006b; Neuropsychologia, 44, Elsevier)
Further result characteristics were informative. Relative to the NC group, the performance accuracy of the RPD group was described by a steeper mental rotation slope with RVF but not LVF presentation. Because slope results are thought to reflect the mental rotation process itself (Shepard and Cooper 1982), our slope findings provided compelling evidence for a single dissociation of RPD patients on the mental rotation process when hands were shown in the RVF but not LVF. Deficient performance on the mental rotation of hands therefore points to abnormal functioning of cortical regions in RPD, such as frontoparietal circuits, not only the basal ganglia. By contrast, LPD patients did not show the slope effect but rather showed only a higher y-intercept of the mental rotation curve with LVF presentation (and not with RVF presentation). Such y-intercept effects are considered to reflect pre- or post-rotation processes, as opposed to a mental rotation function itself (Shepard and Cooper 1982). In light of the strong visual field dependence experienced by LPD patients, as discussed above, the mental rotation deficit seen in LPD may be related more to an input process preceding mental rotation than to a post-rotation process. For example, the problem for LPD when the hand to be mentally rotated was presented in the LVF may relate to the lack of a right hand representation in the right hemisphere (Parsons et al. 1998). We concluded that LPD and RPD showed a double dissociation regarding errors on hand mental rotation, depending upon the side of visual-field presentation. It is clear from the slope data that the source of the impairment in RPD was the mental rotation process itself. In LPD, the source of impairment may instead have been mainly a pre-rotation input process recruited for the task.
Hemispheric Effects in Facial Emotion Recognition
There has been debate concerning the integrity of the ability to recognize emotional facial expressions in PD. While several studies have shown impairments in this domain (Dujardin et al. 2004; Jacobs et al. 1995; Kan et al. 2002; Lawrence et al. 2007; Sprengelmeyer et al. 2003), there has been no consensus as to the cause. One problem is that the methods of assessing facial emotion recognition have been inherently limited. Our review of the literature suggested that emotion recognition may have been confounded by cognitive task requirements owing to the lack of a control condition using non-emotional stimuli. This confound exists for facial emotion recognition studies in general, and is not limited to studies of PD.
In order to determine whether performance on a standard facial emotion recognition task was impaired in PD, we included a carefully designed control condition of landscape recognition (Clark et al. 2008). The landscape images were judged by independent observers to be as recognizable as the facial images, and the task requirements for the two types of stimuli were identical. Using this method, we assessed the recognition of facial emotions in 11 LPD, 9 RPD, and 23 matched healthy normal control participants (NC). The task was to view the face or landscape and indicate its type from a seven-choice array. Faces corresponded to the six emotions (anger, disgust, fear, happy, sad, surprise) plus neutral, and landscapes included environmental types (canyon, city, forest, mountain, shore, town, tropical) (Fig. 10). We found that PD patients performed normally on the landscape task but were impaired on the recognition of facial emotion. The normal performance on the landscape task, which was matched to the facial emotion task for recognizability and for processing and response requirements, strengthens the interpretation of the specificity of the facial emotion findings. Moreover, the PD patients performed normally on a task of emotional prosody recognition, indicating that their emotion identification difficulty was tied to visual processing of faces rather than to a more general impairment in emotion assessment.
Fig. 10.
Examples of stimuli from the Facial Emotion Recognition task and the Landscape Categorization task. (From Clark et al. 2008; Neuropsychologia, 46, Elsevier)
Side of disease onset played a role in the PD groups’ deficiencies in facial emotion recognition, specifically for anger (driven by LPD) and surprise (driven by RPD). The LPD patients displayed impairments in the recognition of facial emotion (anger) and landscapes, whereas the RPD group displayed a specific impairment in the recognition of facial emotion only (surprise) (Fig. 11). Our finding that LPD patients were less accurate than the control group at identifying angry facial expressions suggests the relative importance of right over the left hemisphere in the recognition of angry expressions. This result is consistent with studies of groups besides PD that implicate a greater right than left hemisphere contribution to the perception of negative emotions in general (e.g., Adolphs et al. 2001), and observations of greater activation of limbic structures in the right than left hemisphere (i.e., right orbitofrontal cortex, cingulate gyrus, and amygdala) when viewing angry faces (Blair et al. 1999; Hariri et al. 2002; Sprengelmeyer et al. 1998; Yang et al. 2002). By contrast, the left hemisphere appears to be more important than the right in mediating positive emotions (Ahern and Schwartz 1979; Davidson and Fox 1982; Natale et al. 1983; for a review see Reuter-Lorenz and Davidson 1981; Silberman and Weingartner 1986).
Fig. 11.
Results on facial emotion recognition and landscape categorization. Mean (+ standard error of the mean) percentage of matched Anger and City images, and matched Surprise and Shore images that were correctly identified by each group. HC = Healthy control. Asterisks indicate that the groups’ means are significantly different at the p<.05 level (*) or p<.01 level (**). (From Clark et al. 2008; Neuropsychologia, 46, Elsevier)
In a second study with a subset of the participants described above (9 LPD, 7 RPD, 20 NC), we explored whether the PD deficit in identifying facial emotions may have arisen from visual scanning abnormalities (Clark et al. 2010). We recorded eye movements while the participants engaged in tasks assessing prosaccades (looking toward a stimulus), antisaccades (looking in the direction opposite a stimulus), and category identification using facial and landscape images similar to those included in the first study. LPD but not RPD showed abnormal antisaccades, consistent with findings indicating greater involvement of the prefrontal regions in the right than left hemisphere in antisaccade generation (Ettinger et al. 2005; Sweeney et al. 1996). Antisaccade performance did not correlate with performance on the categorization tasks, however. For the landscape task, PD and NC performed similarly in regard to accuracy and scanning patterns.
For the facial emotion identification task, there were LPD-RPD differences in scanning patterns. As noted above for the first study, RPD but not LPD showed impaired accuracy in recognizing the expression of surprise. In the LPD group specifically, there was a positive correlation between recognition accuracy of surprise facial expressions and the number and duration of fixations in the top regions of the face. The result suggests that LPD participants who attended more to salient emotional information present in the top regions of the face (i.e., eyes) were better able to recognize the emotion. Because LPD and RPD participants did not differ on top-bottom fixation measures, it is possible that RPD participants, who were impaired in identifying this emotion, did not appropriately use this salient information. These findings suggest a modest but specific relation between facial emotion categorization impairments and visual scanning of facial expressions in hemi-PD.
Hemispheric Effects on Sleep and Mood in PD
Sleep problems occur in at least 75% of PD patients over the course of the disease (Factor et al. 1990; Goetz et al. 2005; Lees et al. 1988), including sleep fragmentation, sleep-related breathing disorders, restless legs/periodic leg movements, REM sleep behavior disorder, sleep-related psychosis (nocturnal hallucinations), and altered sleep-wake cycle (Askenasy 2001; Fahn 2003; Gunn et al. 2010). Relatedly, patients with PD experience disturbances of arousal, including excessive daytime sleepiness (Askenasy 1993; Fahn 2003; Gunn et al. 2010). It is now recognized that the pathology of PD probably begins in the brainstem, with an ascending progression of Lewy body and Lewy neurite pathology from the medulla to cortical areas (Braak et al. 2004). This pathology, associated with neuronal loss in the cholinergic, dopaminergic, and serotonergic systems, is likely to affect the pons and reticular activating system, which are important in sleep and wake regulation as well as in dream formation (Hobson et al. 1998).
Observed lateralization of some aspects of sleep has raised the question of its potential relevance to PD. Right-hemisphere neural networks are implicated in arousal and vigilance in healthy adults (Bearden et al. 2004; Liotti and Tucker 1992; Posner and Dehaene 1994), and these right-hemisphere vigilance functions are particularly sensitive to sleep deprivation (Johnsen et al. 2002). There is also evidence for a functional deterioration of the fronto-temporo-parietal network in the right hemisphere of patients with narcolepsy (Saletu et al. 2004). We undertook an initial study with a focus on PD side of onset, using a sleep questionnaire (Parkinson’s Disease Sleep Scale) in 14 LPD, 17 RPD, and 17 age-matched control participants with chronic health conditions (Stavitsky et al. 2008). It should be noted that unlike in our other studies, the PD group here was almost exclusively male, being a sample of veterans. While both PD subgroups endorsed more nighttime motor symptoms than the control group, only the LPD patients reported more nocturnal hallucinations and daytime dozing (Fig. 12). The RPD patients had higher scores on a scale of depression, anxiety, and stress, and we accordingly controlled for these variables. The analysis of covariance confirmed the propensity of the LPD group for nocturnal hallucinations and daytime dozing and further revealed more distressing and vivid dreams in this group relative to the RPD and control groups.
Fig. 12.
Mean frequency of vivid dreaming, nocturnal hallucinations, and daytime dozing in Control (CO), RPD and LPD groups. * Indicates differences between LPD and CO groups after controlling for mood; ** indicates differences between LPD and RPD after controlling for mood. No differences were found between RPD and CO on these variables. In the standard version of the questionnaire, higher numbers indicate lower frequency of symptoms. For greater clarity of interpretation, we modified the scale so that higher numbers indicate higher frequency of symptoms. (From Stavitsky et al. 2008; Cognitive and Behavioral Neurology, 21, Lippincott Williams & Wilkins)
Increased dreaming, hallucinations, and daytime sleepiness in LPD may be related to changes in right-hemisphere neural networks implicated in the generation and control of visual images, arousal and vigilance. More specifically, there may be alterations in right-hemispheric cortical activation via asymmetrical basal ganglia functioning in LPD. As noted earlier in this review, the basal ganglia are part of the cortico-striato-thalamocortical circuits projecting to cortical areas involved in higher-order visual processing (Middleton and Strick 2000b). Supporting this view, imaging studies of patients with Dementia with Lewy Bodies and of patients with PD have shown selective activation of right-hemisphere brain regions (i.e., right parietal and temporal areas) (Imamura et al. 1999; Oishi et al. 2005) in patients with visual hallucinations. In normal adults, there is activation of the right parietal operculum despite the general deactivation of the parietal and frontal cortices during REM sleep dreaming (Maquet et al. 1996). In LPD, there may be increased thalamic input to right temporoparietal areas that is caused by dysfunction of the corticostriatal circuitry (Middleton and Strick 1996), leading to abnormal ponto-geniculo-occipital activity (Diederich et al. 2005). These alterations in right-hemispheric cortical activation may result in disturbed visual processing and more frequent dreaming in patients with LPD.
In all three groups of participants in our study, levels of stress, anxiety and depression were related to their reported sleep disturbances. RPD patients had higher scores than the LPD or control groups on these symptoms. Those individuals who reported more mood symptoms also reported more frequent sleep problems. In younger and older adults without PD as well as in patients with PD, those expressing a greater degree of sleep problems also endorsed more mood symptoms such as depression and anxiety (Gunn et al. 2010; Ohayon 2005). Adjusting for mood symptoms strengthened our finding of LPD-RPD differences on dreaming, hallucinations and daytime dozing, thereby highlighting sleep disturbances that were intrinsic to the disease rather than secondary to other disease-related symptoms such as depression and anxiety (Friedman and Chou 2004). The results suggest that mood disturbances, like sleep disturbances, may be mediated by lateralized brain regions. These findings complement the report of an association between mood and pain perception in LPD but not in RPD, reflecting the differential contribution of right-hemispheric neural networks in processing of mood and pain states (McNamara et al. 2009). Mood disorders are quite common in PD, with depression and anxiety each occurring in roughly 40% of patients (Aarsland et al. 2009; Farabaugh et al. 2009; Pontone et al. 2009; Richard et al. 1996). This observation underscores the importance of considering mood in studies of PD as well as of healthy adults in which outcome measures (cognitive, perceptual, emotional) may be affected by mood.
A summary of some of the differences that our laboratory has found in the performance of LPD and RPD patients on tasks of visuospatial function, facial emotion recognition and scanning, sleep and mood is provided in Table 1.
Table 1.
Lateralization effects in studies of visuospatial function, facial emotion recognition and scanning, and sleep and mood
| Testing domain | Sample sizes | LPD finding | RPD finding |
|---|---|---|---|
| Visuospatial | |||
| Hierarchical pattern perception (Schendan et al. 2009) | 20 LPD, 18 RPD, 22 NC | Impaired global-level processing | Impaired local-level processing |
| Visual dependence (Davidsdottir et al. 2008) | 16 LPD, 15 RPD, 18 NC | More visually dependent than RPD or NC | More visually dependent than NC |
| Optic flow: point of subjective equality of hemifield optic flow speed (Davidsdottir et al. 2008) | Sample as above | Perceive optic flow speed in LVF as slower than speed in RVF (trend) | Perceive optic flow speed in RVF as slower than speed in LVF; same as for NC |
| Egocentric reference point (Davidsdottir et al. 2008) | Sample as above (LPD, RPD only) | Point right of center; driven by women | None |
| Veering while walking (Davidsdottir et al. 2008) | (Subset of above) 14 LPD, 8 RPD, 16 NC | Veer right of center | Veer left of center; same as for NC |
| Mental rotation (hands) (Amick et al. 2006a) | 15 LPD, 12 RPD, 13 NC (RVF conventional experiment); 7 LPD, 6 RPD, 6 NC ( LVF control experiment) | Worse than NC when stimulus to be rotated was in LVF (control experiment); problem of pre- or post-rotational processing | Worse than NC when stimulus to be rotated was in RVF (conventional experiment); problem of mental rotation process itself |
| Facial emotion recognition and scanning | |||
| Facial emotion identification (Clark et al. 2008) | 11 LPD, 9 RPD, 23 NC | Impaired at anger identification relative to NC | Impaired at surprise identification relative to LPD and NC |
| Visual scanning (Clark et al. 2010) | (Subset of above) 9 LPD, 7 RPD, 20 NC | Impaired antisaccades | Viewing top half of face does not increase accuracy for surprise faces |
| Sleep and mood (Stavitsky et al. 2008) | 14 LPD, 17 RPD, 17 chronic- disease control participants | More vivid and distressing dreams, noctural hallucinations, daytime sleepiness | More depression/anxiety/stress |
LPD left-onset Parkinson’s disease; RPD right-onset Parkinson’s disease; NC healthy normal control participants; LVF left visual field; RVF right visual field. All PD and control participants were non-demented. There were approximately equal numbers of men and women in all studies except Stavitsky et al. (2008), which was a mostly male veteran sample
The Relation of Gender to Hemispheric Effects in PD
PD affects more men than women (Dluzen and McDermott 2000; Van Den Eeden et al. 2003) and results in different clinical symptom profiles (Arabia et al. 2002; Baba et al. 2005; Haaxma et al. 2007; Scott et al. 2000). The basis of this gender specificity may be differential vulnerability to neurotoxins affecting the nigrostriatal dopaminergic system, with estrogen possibly inhibiting neurotoxin uptake through the dopamine transporter (Disshon and Dluzen 1999). Review of the effects of estrogen in the brain (Liu and Dluzen 2007) suggests a neuroprotective effect of estrogen against dopamine neurodegeneration in the nigrostriatal regions, based on animal and human studies. Estrogen effects may be especially salient for spatial ability in light of robust gender differences on such tasks as mental rotation (For a review see Cronin-Golomb and Amick 2001).
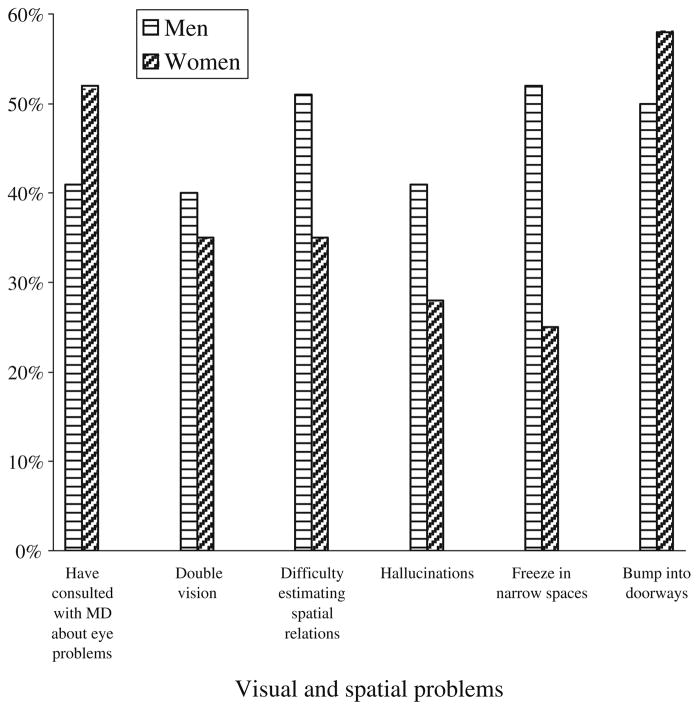
In our questionnaire study of PD, women and men endorsed items about visual and spatial difficulties to a different extent (Davidsdottir et al. 2005) (Fig. 13). In our experimental studies, we have detected a complex pattern of biases in the perception of spatial relations in PD, which in some instances was influenced by interactions between side of motor symptom onset, hemifield presentation, and gender (Davidsdottir et al. 2008). The interactions may at least partly explain the variable patterns of visuospatial impairment that have emerged from previous studies. In our study of facial emotion recognition, we reported that men but not women with PD, and women but not men in the control group, displayed relative weakness in the recognition of specific emotional expressions (Fear) (Fig. 14) (Clark et al. 2008). Our associated study of visual scanning patterns (Clark et al. 2010) found that control women spent less time fixating on fearful expressions than control men did. We found no male–female difference in fixation duration for the PD group, raising the intriguing possibility that for some perceptual or cognitive functions, PD may be associated with the disappearance of normal gender-based differences.
Fig. 13.
Percentage of men and women with PD who endorsed visual or visuospatial problems. N=81 (56 men, 25 women) except for two items (difficulty estimating spatial relations and double vision) where N=55 (35 men, 20 women). (From Davidsdottir et al. 2005; Vision Research, 45, Elsevier)
Fig. 14.
Gender effects in facial emotion recognition. Mean (+ standard error of the mean) percentage of Fearful facial expressions that were correctly identified by men and women in each group. HC = Healthy control. Asterisks indicate that the groups’ means are significantly different at the p<.05 (*) and p<.01 (**) level. (From Clark et al. 2008; Neuropsychologia, 46, Elsevier)
Studies of PD have often recruited only or mostly male participants and have failed to analyze data by side of disease onset, making generalizations from the results problematic. Revisiting data from previously published studies that could be examined by side of onset and gender, together with designing future studies with these comparisons in mind, would undoubtedly enhance our understanding of the relation of brain changes to cognition, emotion, mood, sleep, and behavior in PD.
Relevance of PD Disconnection Findings to Normal Brain Organization and Function
To the extent that it may be regarded as a disconnection syndrome, PD provides information on normal brain circuitry within hemispheres and on interhemispheric relations. Results from the studies described above may be applied to this purpose. Perhaps the clearest implications emerge from the studies of mental rotation and hierarchical pattern perception.
Our findings from the study of mental rotation provided evidence that in PD, the visuospatial and motor-related neural networks supporting mental rotation of body parts within each hemisphere can be differentially recruited depending upon the visual-field location of the to-be-rotated limb. The findings raise the broader question of whether different hemispheric networks are invoked by LVF and RVF presentation of similar material in the normal brain as well. Moreover, they indicate that frontostriatal motor systems and the parietal lobes play a necessary role during the mental rotation of hands, which requires integrating visuospatial cognition with motor imagery.
The results from hierarchical pattern perception link the side of motor symptom onset to visuospatial abilities that are supported by contralateral temporal-parietal junction (TP) in PD and thereby inform us of cognitive abilities in this disease. Further, they provide evidence that an inferior parietal-basal ganglia pathway involving the caudate head and hemispherically asymmetrical TP region is necessary for the visuospatial ability of hierarchical pattern perception. As discussed earlier, prior neuropsychological work has identified TP but not the dorsolateral prefrontal cortex as necessary for hierarchical pattern perception. Because we found abnormal performance on this type of pattern perception in PD, it follows that visuospatial dysfunction in PD can reflect dysfunction primarily in the posterior parietal-basal ganglia network rather than in the fronto-striatal loop. On the basis of this evidence, we propose that hierarchical pattern perception tasks require the recruitment of a lateralized TP-basal ganglia network, possibly a parieto-striatal loop (Clower et al. 2005; Middleton and Strick 2000a, b).
The findings discussed here add to the growing list of visuospatial abilities that depend upon cortical-basal ganglia connections and are important for characterizing the range of visuospatial impairments in PD as well as for theoretical understanding of the role these interconnections play in cognition.
In summary, there is convincing evidence in PD for dysfunction of subcortical and cortical structures and for the repercussions of this dysfunction on cognition, perception, emotion, sleep, and mood. Regarding PD as a disconnection syndrome not only provides information on the characteristics of the disease, but it also—in the spirit of the approach advanced by Norman Geschwind and other influential investigators of his generation—equips us with an effective means for exploring normal brain-behavior relations.
Acknowledgments
I am grateful to my colleagues who collaborated on the experiments described here and who discussed and critiqued earlier versions of this review, including Melissa Amick, Sanford Auerbach, Justin Centi, Uraina Clark, Sigurros Davidsdottir, Mirella Diaz-Santos, Raymon Durso, Georgio Ganis, Erica Harris, Tom Laudate, Alison Lee, Patrick McNamara, Ivy Miller, Sandy Neargarder, Haline Schendan, Daniel Seichepine, Karina Stavitsky, Robert Wagenaar, and Daniel Young. Chelsea Toner provided technical assistance in the preparation of the manuscript. This work was conducted with the support of the National Institute of Neurological Disorders and Stroke (1 R01 NS050446-01).
Footnotes
Disclosures The author declares that no conflicts of interest are associated with the preparation of this article.
References
- Aarsland D, Bronnick K, Alves G, Tysnes OB, Pedersen KF, Ehrt U, et al. The spectrum of neuropsychiatric symptoms in patients with early untreated Parkinson’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 2009;80(8):928–930. doi: 10.1136/jnnp.2008.166959. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Jansari A, Tranel D. Hemispheric perception of emotional valence from facial expressions. Neuropsychology. 2001;15(4):516–524. [PubMed] [Google Scholar]
- Ahern GL, Schwartz GE. Differential lateralization for positive versus negative emotion. Neuropsychologia. 1979;17(6):693–698. doi: 10.1016/0028-3932(79)90045-9. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amick MM, Grace J, Chou KL. Body side of motor symptom onset in Parkinson’s disease is associated with memory performance. Journal of the International Neuropsychological Society. 2006a;12(5):736–740. doi: 10.1017/S1355617706060875. [DOI] [PubMed] [Google Scholar]
- Amick MM, Schendan HE, Ganis G, Cronin-Golomb A. Frontostriatal circuits are necessary for visuomotor transformation: mental rotation in Parkinson’s disease. Neuropsychologia. 2006b;44:339–349. doi: 10.1016/j.neuropsychologia.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Antonini A, Vontobel P, Psylla M, Gunther I, Maguire PR, Missimer J, et al. Complementary positron emission tomographic studies of the striatal dopaminergic system in Parkinson’s disease. Archives of Neurology. 1995;52(12):1183–1190. doi: 10.1001/archneur.1995.00540360061017. [DOI] [PubMed] [Google Scholar]
- Arabia G, Zappia M, Bosco D, Crescibene L, Bagala A, Bastone L, et al. Body weight, levodopa pharmacokinetics and dyskinesia in Parkinson’s disease. Neurological Sciences. 2002;23(Suppl 2):S53–S54. doi: 10.1007/s100720200066. [DOI] [PubMed] [Google Scholar]
- Asenbaum S, Brucke T, Pirker W, Podreka I, Angelberger P, Wenger S, et al. Imaging of dopamine transporters with iodine-123-beta-CIT and SPECT in Parkinson’s disease. Journal of Nuclear Medicine. 1997;38(1):1–6. [PubMed] [Google Scholar]
- Askenasy JJ. Sleep in Parkinson’s disease. Acta Neurologica Scandinavica. 1993;87(3):167–170. doi: 10.1111/j.1600-0404.1993.tb04095.x. [DOI] [PubMed] [Google Scholar]
- Askenasy JJ. Approaching disturbed sleep in late Parkinson’s Disease: first step toward a proposal for a revised UPDRS. Parkinsonism & Related Disorders. 2001;8(2):123–131. doi: 10.1016/s1353-8020(01)00026-8. [DOI] [PubMed] [Google Scholar]
- Azulay JP, Mesure S, Amblard B, Blin O, Sangla I, Pouget J. Visual control of locomotion in Parkinson’s disease. Brain. 1999;122(Pt 1):111–120. doi: 10.1093/brain/122.1.111. [DOI] [PubMed] [Google Scholar]
- Azulay JP, Mesure S, Amblard B, Pouget J. Increased visual dependence in Parkinson’s disease. Perceptual and Motor Skills. 2002;95(3 Pt 2):1106–1114. doi: 10.2466/pms.2002.95.3f.1106. [DOI] [PubMed] [Google Scholar]
- Baba Y, Putzke JD, Whaley NR, Wszolek ZK, Uitti RJ. Gender and the Parkinson’s disease phenotype. Journal de Neurologie. 2005;252(10):1201–1205. doi: 10.1007/s00415-005-0835-7. [DOI] [PubMed] [Google Scholar]
- Bartels AL, de Jong BM, Giladi N, Schaafsma JD, Maguire RP, Veenma L, et al. Striatal dopa and glucose metabolism in PD patients with freezing of gait. Movement Disorders. 2006;21(9):1326–1332. doi: 10.1002/mds.20952. [DOI] [PubMed] [Google Scholar]
- Bearden TS, Cassisi JE, White JN. Electrophysiological correlates of vigilance during a continuous performance test in healthy adults. Applied Psychophysiology and Biofeedback. 2004;29(3):175–188. doi: 10.1023/b:apbi.0000039056.58787.76. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(Pt 5):883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blonder LX, Gur RE, Gur RC, Saykin AJ, Hurtig HI. Neuropsychological functioning in hemiparkinsonism. Brain and Cognition. 1989;9(2):244–257. doi: 10.1016/0278-2626(89)90034-1. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Kaszniak AW, Bayles K, Vance K. Contributions of frontal system dysfunction to memory and perceptual abilities in Parkinson’s disease. Neuropsychology. 1991;7:89–102. [Google Scholar]
- Booij J, Tissingh G, Boer GJ, Speelman JD, Stoof JC, Janssen AG, et al. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 1997;62(2):133–140. doi: 10.1136/jnnp.62.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen FP, Burns MM, Brady EM, Yahr MD. A note of alterations of personal orientation in Parkinsonism. Neuropsychologia. 1976;14(4):425–429. doi: 10.1016/0028-3932(76)90071-3. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Alzheimer’s disease: intraneuronal alterations precede insoluble amyloid-beta formation. Neurobiology of Aging. 2004;25(6):713–718. doi: 10.1016/j.neurobiolaging.2003.12.015. discussion 743–716. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell and Tissue Research. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Hood JD, Gresty MA, Panagi C. Visual control of balance in cerebellar and parkinsonian syndromes. Brain. 1990;113(Pt 3):767–779. doi: 10.1093/brain/113.3.767. [DOI] [PubMed] [Google Scholar]
- Brown HD, Kosslyn SM, Dror IE. Aging and scanning of imagined and perceived visual images. Experimental Aging Research. 1998;24(2):181–194. doi: 10.1080/036107398244319. [DOI] [PubMed] [Google Scholar]
- Chokron S, Bartolomeo P. Patterns of dissociation between left hemineglect and deviation of the egocentric reference. Neuropsychologia. 1997;35(11):1503–1508. doi: 10.1016/s0028-3932(97)00079-1. [DOI] [PubMed] [Google Scholar]
- Clark U, Neargarder S, Cronin-Golomb A. Specific impairments in the recognition of emotional facial expressions in Parkinson’s disease. Neuropsychologia. 2008;46:2300–2309. doi: 10.1016/j.neuropsychologia.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Neargarder S, Cronin-Golomb A. Visual exploration of emotional facial expressions in Parkinson’s disease. Neuropsychologia. 2010 doi: 10.1016/j.neuropsychologia.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to ‘AIP’. Cerebral Cortex. 2005;15(7):913–920. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- Cooke JD, Brown JD, Brooks VB. Increased dependence on visual information for movement control in patients with Parkinson’s disease. Canadian Journal of Neurological Sciences. 1978;5(4):413–415. doi: 10.1017/s0317167100024197. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain. 1991;114(Pt 5):2095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A. Subcortical transfer of cognitive information in subjects with complete forebrain commissurotomy. Cortex. 1986;22(4):499–519. doi: 10.1016/s0010-9452(86)80012-0. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A, Amick MM. Spatial abilities in aging, Alzheimer’s disease, and Parkinson’s disease. In: Boller F, Cappa S, editors. Handbook of Neuropsychology. 2. Vol. 6. Amsterdam: Elsevier; 2001. pp. 119–143. [Google Scholar]
- Cronin-Golomb A, Braun AE. Visuospatial dysfunction and problem solving in Parkinson’s disease. Neuropsychology. 1997;11(1):44–52. doi: 10.1037//0894-4105.11.1.44. [DOI] [PubMed] [Google Scholar]
- Danta G, Hilton RC. Judgment of the visual vertical and horizontal in patients with Parkinsonism. Neurology. 1975;25(1):43–47. doi: 10.1212/wnl.25.1.43. [DOI] [PubMed] [Google Scholar]
- Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson’s disease. Vis Res. 2005;45(10):1285–1296. doi: 10.1016/j.visres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Davidsdottir S, Wagenaar R, Young D, Cronin-Golomb A. Impact of optic flow perception and egocentric coordinates on veering in Parkinson’s disease. Brain. 2008;131(Pt 11):2882–2893. doi: 10.1093/brain/awn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science. 1982;218(4578):1235–1237. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Goetz CG, Stebbins GT. Repeated visual hallucinations in Parkinson’s disease as disturbed external/internal perceptions: focused review and a new integrative model. Movement Disorders. 2005;20(2):130–140. doi: 10.1002/mds.20308. [DOI] [PubMed] [Google Scholar]
- Disshon KA, Dluzen DE. Use of in vitro superfusion to assess the dynamics of striatal dopamine clearance: influence of estrogen. Brain Research. 1999;842(2):399–407. doi: 10.1016/s0006-8993(99)01863-6. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL. Gender differences in neurotoxicity of the nigrostriatal dopaminergic system: implications for Parkinson’s disease. Journal of Gender-Specific Medicine. 2000;3(6):36–42. [PubMed] [Google Scholar]
- Dominey P, Decety J, Broussolle E, Chazot G, Jeannerod M. Motor imagery of a lateralized sequential task is asymmetrically slowed in hemi-Parkinson’s patients. Neuropsychologia. 1995;33(6):727–741. doi: 10.1016/0028-3932(95)00008-q. [DOI] [PubMed] [Google Scholar]
- Duchon AP, Warren WH., Jr A visual equalization strategy for locomotor control: of honeybees, robots, and humans. Psychological Science. 2002;13(3):272–278. doi: 10.1111/1467-9280.00450. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Blairy S, Defebvre L, Duhem S, Noel Y, Hess U, et al. Deficits in decoding emotional facial expressions in Parkinson’s disease. Neuropsychologia. 2004;42(2):239–250. doi: 10.1016/s0028-3932(03)00154-4. [DOI] [PubMed] [Google Scholar]
- Dyre BP, Andersen GJ. Image velocity magnitudes and perception of heading. Journal of Experimental Psychology. Human Perception and Performance. 1997;23(2):546–565. doi: 10.1037//0096-1523.23.2.546. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Antonova E, Crawford TJ, Mitterschiffthaler MT, Goswani S, Sharma T, et al. Structural neural correlates of prosaccade and antisaccade eye movements in healthy humans. Neuroimage. 2005;24(2):487–494. doi: 10.1016/j.neuroimage.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Factor SA, McAlarney T, Sanchez-Ramos JR, Weiner WJ. Sleep disorders and sleep effect in Parkinson’s disease. Movement Disorders. 1990;5(4):280–285. doi: 10.1002/mds.870050404. [DOI] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson’s disease as a clinical syndrome. Annals of the New York Academy of Sciences. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Fajen BR, Warren WH. Visual guidance of intercepting a moving target on foot. Perception. 2004;33(6):689–715. doi: 10.1068/p5236. [DOI] [PubMed] [Google Scholar]
- Farabaugh AH, Locascio JJ, Yap L, Weintraub D, McDonald WM, Agoston M, et al. Pattern of depressive symptoms in Parkinson’s disease. Psychosomatics. 2009;50(5):448–454. doi: 10.1176/appi.psy.50.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382(6592):626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli. Brain. 1997a;120(Pt 10):1779–1791. doi: 10.1093/brain/120.10.1779. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Halligan PW, Frith CD, Frackowiak RS, Dolan RJ. Hemispheric specialization for global and local processing: the effect of stimulus category. Proceedings of the Royal Society B: Biological Sciences. 1997b;264(1381):487–494. doi: 10.1098/rspb.1997.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CM. Alien hand phenomena: a review with the addition of six personal cases. Canadian Journal of Neurological Sciences. 2000;27(3):192–203. doi: 10.1017/s0317167100000834. [DOI] [PubMed] [Google Scholar]
- Friedman JH, Chou KL. Sleep and fatigue in Parkinson’s disease. Parkinsonism & Related Disorders. 2004;10(Suppl 1):S27–S35. doi: 10.1016/j.parkreldis.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Ganis G, Keenan JP, Kosslyn SM, Pascual-Leone A. Transcranial magnetic stimulation of primary motor cortex affects mental rotation. Cerebral Cortex. 2000;10(2):175–180. doi: 10.1093/cercor/10.2.175. [DOI] [PubMed] [Google Scholar]
- Gattellaro G, Minati L, Grisoli M, Mariani C, Carella F, Osio M, et al. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. AJNR American Journal of Neuroradiology. 2009;30(6):1222–1226. doi: 10.3174/ajnr.A1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965a;88(2):237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965b;88(3):585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- Girotti F, Soliveri P, Carella F, Geminiani G, Aiello G, Caraceni T. Role of motor performance in cognitive processes of parkinsonian patients. Neurology. 1988;38(4):537–540. doi: 10.1212/wnl.38.4.537. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Wuu J, Curgian LM, Leurgans S. Hallucinations and sleep disorders in PD: six-year prospective longitudinal study. Neurology. 2005;64(1):81–86. doi: 10.1212/01.WNL.0000148479.10865.FE. [DOI] [PubMed] [Google Scholar]
- Gunn DG, Naismith SL, Lewis SJ. Sleep disturbances in Parkinson disease and their potential role in heterogeneity. Journal of Geriatric Psychiatry and Neurology. 2010 doi: 10.1177/0891988709358591. [DOI] [PubMed] [Google Scholar]
- Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, et al. Gender differences in Parkinson’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 2007;78(8):819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Harris JM, Bonas W. Optic flow and scene structure do not always contribute to the control of human walking. Vision Research. 2002;42(13):1619–1626. doi: 10.1016/s0042-6989(02)00066-4. [DOI] [PubMed] [Google Scholar]
- Harris MG, Carre G. Is optic flow used to guide walking while wearing a displacing prism? Perception. 2001;30(7):811–818. doi: 10.1068/p3160. [DOI] [PubMed] [Google Scholar]
- Harris IM, Egan GF, Sonkkila C, Tochon-Danguy HJ, Paxinos G, Watson JD. Selective right parietal lobe activation during mental rotation: a parametric PET study. Brain. 2000;123(Pt 1):65–73. doi: 10.1093/brain/123.1.65. [DOI] [PubMed] [Google Scholar]
- Harris JP, Atkinson EA, Lee AC, Nithi K, Fowler MS. Hemispace differences in the visual perception of size in left hemiParkinson’s disease. Neuropsychologia. 2003;41(7):795–807. doi: 10.1016/s0028-3932(02)00285-3. [DOI] [PubMed] [Google Scholar]
- Hasselbach-Heitzeg MM, Reuter-Lorenz PA. Egocentric body-centered coordinates modulate visuomotor performance. Neuropsychologia. 2002;40(11):1822–1833. doi: 10.1016/s0028-3932(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Hasselbach M, Butter CM. Ipsilesional displacement of egocentric midline in neglect patients with, but not in those without, extensive right parietal damage. In: Thier P, Karnath H-O, editors. Parietal lobe contributions to orientation in 3D space. Heidelberg: Springer-Verlag; 1997. pp. 217–228. [Google Scholar]
- Hobson JA, Stickgold R, Pace-Schott EF. The neuropsychology of REM sleep dreaming. NeuroReport. 1998;9(3):R1–R14. doi: 10.1097/00001756-199802160-00033. [DOI] [PubMed] [Google Scholar]
- Hof PR, Bouras C. Object recognition deficit in Alzheimer’s disease: possible disconnection of the occipito-temporal component of the visual system. Neuroscience Letters. 1991;122(1):53–56. doi: 10.1016/0304-3940(91)90191-u. [DOI] [PubMed] [Google Scholar]
- Imamura T, Ishii K, Hirono N, Hashimoto M, Tanimukai S, Kazuai H, et al. Visual hallucinations and regional cerebral metabolism in dementia with Lewy bodies (DLB) NeuroReport. 1999;10(9):1903–1907. doi: 10.1097/00001756-199906230-00020. [DOI] [PubMed] [Google Scholar]
- Innis RB, Seibyl JP, Scanley BE, Laruelle M, Abi-Dargham A, Wallace E, et al. Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(24):11965–11969. doi: 10.1073/pnas.90.24.11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DH, Shuren J, Bowers D, Heilman KM. Emotional facial imagery, perception, and expression in Parkinson’s disease. Neurology. 1995;45(9):1696–1702. doi: 10.1212/wnl.45.9.1696. [DOI] [PubMed] [Google Scholar]
- Johnsen BH, Laberg JC, Eid J, Hugdahl K. Dichotic listening and sleep deprivation: vigilance effects. Scandinavian Journal of Psychology. 2002;43(5):413–417. doi: 10.1111/1467-9450.00309. [DOI] [PubMed] [Google Scholar]
- Kan Y, Kawamura M, Hasegawa Y, Mochizuki S, Nakamura K. Recognition of emotion from facial, prosodic and written verbal stimuli in Parkinson’s disease. Cortex. 2002;38(4):623–630. doi: 10.1016/s0010-9452(08)70026-1. [DOI] [PubMed] [Google Scholar]
- Karagulle Kendi AT, Lehericy S, Luciana M, Ugurbil K, Tuite P. Altered diffusion in the frontal lobe in Parkinson disease. AJNR American Journal of Neuroradiology. 2008;29(3):501–505. doi: 10.3174/ajnr.A0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO. Subjective body orientation in neglect and the interactive contribution of neck muscle proprioception and vestibular stimulation. Brain. 1994;117(Pt 5):1001–1012. doi: 10.1093/brain/117.5.1001. [DOI] [PubMed] [Google Scholar]
- Karnath HO. Spatial orientation and the representation of space with parietal lobe lesions. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1997;352(1360):1411–1419. doi: 10.1098/rstb.1997.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Schenkel P, Fischer B. Trunk orientation as the determining factor of the ‘contralateral’ deficit in the neglect syndrome and as the physical anchor of the internal representation of body orientation in space. Brain. 1991;114(Pt 4):1997–2014. doi: 10.1093/brain/114.4.1997. [DOI] [PubMed] [Google Scholar]
- Kearns MJ, Warren WH, Duchon AP, Tarr MJ. Path integration from optic flow and body senses in a homing task. Perception. 2002;31(3):349–374. doi: 10.1068/p3311. [DOI] [PubMed] [Google Scholar]
- Kempster PA, Gibb WR, Stern GM, Lees AJ. Asymmetry of substantia nigra neuronal loss in Parkinson’s disease and its relevance to the mechanism of levodopa related motor fluctuations. Journal of Neurology, Neurosurgery and Psychiatry. 1989;52(1):72–76. doi: 10.1136/jnnp.52.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Lee WY, Choe YS, Kim JH. SPECT measurement of iodine-123-beta-CIT binding to dopamine and serotonin transporters in Parkinson’s disease: correlation with symptom severity. Neurological Research. 1999;21(3):255–261. doi: 10.1080/01616412.1999.11740928. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, DiGirolamo GJ, Thompson WL, Alpert NM. Mental rotation of objects versus hands: neural mechanisms revealed by positron emission tomography. Psychophysiology. 1998;35(2):151–161. [PubMed] [Google Scholar]
- Laulumaa V, Kuikka JT, Soininen H, Bergstrom K, Lansimies E, Riekkinen P. Imaging of D2 dopamine receptors of patients with Parkinson’s disease using single photon emission computed tomography and iodobenzamide I 123. Archives of Neurology. 1993;50(5):509–512. doi: 10.1001/archneur.1993.00540050059016. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Goerendt IK, Brooks DJ. Impaired recognition of facial expressions of anger in Parkinson’s disease patients acutely withdrawn from dopamine replacement therapy. Neuropsychologia. 2007;45(1):65–74. doi: 10.1016/j.neuropsychologia.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Lee AC, Harris JP, Calvert JE. Impairments of mental rotation in Parkinson’s disease. Neuropsychologia. 1998;36(1):109–114. doi: 10.1016/s0028-3932(97)00017-1. [DOI] [PubMed] [Google Scholar]
- Lee AC, Harris JP, Atkinson EA, Fowler MS. Disruption of estimation of body-scaled aperture width in Hemiparkinson’s disease. Neuropsychologia. 2001a;39(10):1097–1104. doi: 10.1016/s0028-3932(01)00032-x. [DOI] [PubMed] [Google Scholar]
- Lee AC, Harris JP, Atkinson EA, Fowler MS. Evidence from a line bisection task for visuospatial neglect in left hemiparkinson’s disease. Vision Research. 2001b;41(20):2677–2686. doi: 10.1016/s0042-6989(01)00129-8. [DOI] [PubMed] [Google Scholar]
- Leenders KL, Salmon EP, Tyrrell P, Perani D, Brooks DJ, Sager H, et al. The nigrostriatal dopaminergic system assessed in vivo by positron emission tomography in healthy volunteer subjects and patients with Parkinson’s disease. Archives of Neurology. 1990;47(12):1290–1298. doi: 10.1001/archneur.1990.00530120034007. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson’s disease. Clinical Neuropharmacology. 1988;11(6):512–519. doi: 10.1097/00002826-198812000-00004. [DOI] [PubMed] [Google Scholar]
- Liotti M, Tucker DM. Right hemisphere sensitivity to arousal and depression. Brain and Cognition. 1992;18(2):138–151. doi: 10.1016/0278-2626(92)90075-w. [DOI] [PubMed] [Google Scholar]
- Liu B, Dluzen DE. Oestrogen and nigrostriatal dopaminergic neurodegeneration: animal models and clinical reports of Parkinson’s disease. Clinical and Experimental Pharmacology and Physiology. 2007;34(7):555–565. doi: 10.1111/j.1440-1681.2007.04616.x. [DOI] [PubMed] [Google Scholar]
- Maquet P, Peters J, Aerts J, Delfiore G, Degueldre C, Luxen A, et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383(6596):163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- Marek KL, Seibyl JP, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Fussell B, et al. [123I] beta-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson’s disease. Neurology. 1996;46(1):231–237. doi: 10.1212/wnl.46.1.231. [DOI] [PubMed] [Google Scholar]
- Martin WR, Wieler M, Gee M, Camicioli R. Temporal lobe changes in early, untreated Parkinson’s disease. Movement Disorders. 2009;24(13):1949–1954. doi: 10.1002/mds.22680. [DOI] [PubMed] [Google Scholar]
- McNamara P, Stavitsky K, Harris E, Szent-Imrey O, Durso R. Mood, side of motor symptom onset and pain complaints in Parkinson’s disease. International Journal of Geriatric Psychiatry. 2009 doi: 10.1002/gps.2374. [DOI] [PubMed] [Google Scholar]
- Meppelink AM, de Jong BM, Renken R, Leenders KL, Cornelissen FW, van Laar T. Impaired visual processing preceding image recognition in Parkinson’s disease patients with visual hallucinations. Brain. 2009;132:2980–2993. doi: 10.1093/brain/awp223. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. The temporal lobe is a target of output from the basal ganglia. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(16):8683–8687. doi: 10.1073/pnas.93.16.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research. Brain Research Reviews. 2000a;31(2–3):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain and Cognition. 2000b;42(2):183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Morris M, Iansek R, McGinley J, Matyas T, Huxham F. Three-dimensional gait biomechanics in Parkinson’s disease: evidence for a centrally mediated amplitude regulation disorder. Movement Disorders. 2005;20(1):40–50. doi: 10.1002/mds.20278. [DOI] [PubMed] [Google Scholar]
- Natale M, Gur RE, Gur RC. Hemispheric asymmetries in processing emotional expressions. Neuropsychologia. 1983;21(5):555–565. doi: 10.1016/0028-3932(83)90011-8. [DOI] [PubMed] [Google Scholar]
- Navon G, Ogawa S, Shulman RG, Yamane T. High-resolution 31P nuclear magnetic resonance studies of metabolism in aerobic Escherichia coli cells. Proceedings of the National Academy of Sciences of the United States of America. 1977;74(3):888–891. doi: 10.1073/pnas.74.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden JA, Growdon JH, Corkin S. Deficits on visuospatial tests involving forward planning in high-functioning Parkinson’s. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1990;3:125–139. [Google Scholar]
- Ohayon MM. Prevalence and correlates of nonrestorative sleep complaints. Archives of Internal Medicine. 2005;165(1):35–41. doi: 10.1001/archinte.165.1.35. [DOI] [PubMed] [Google Scholar]
- Oishi N, Udaka F, Kameyama M, Sawamoto N, Hashikawa K, Fukuyama H. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology. 2005;65(11):1708–1715. doi: 10.1212/01.wnl.0000187116.13370.e0. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Gabrieli JD, Phelps EA, Gazzaniga MS. Cerebrally lateralized mental representations of hand shape and movement. Journal of Neuroscience. 1998;18(16):6539–6548. doi: 10.1523/JNEUROSCI.18-16-06539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bargallo N, Tolosa E. Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson’s disease. Movement Disorders. 2009;24(8):1193–1199. doi: 10.1002/mds.22560. [DOI] [PubMed] [Google Scholar]
- Peuskens H, Sunaert S, Dupont P, Van Hecke P, Orban GA. Human brain regions involved in heading estimation. Journal of Neuroscience. 2001;21(7):2451–2461. doi: 10.1523/JNEUROSCI.21-07-02451.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontone GM, Williams JR, Anderson KE, Chase G, Goldstein SA, Grill S, et al. Prevalence of anxiety disorders and anxiety subtypes in patients with Parkinson’s disease. Movement Disorders. 2009;24(9):1333–1338. doi: 10.1002/mds.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends in Neurosciences. 1994;17(2):75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Rakshi JS, Uema T, Ito K, Bailey DL, Morrish PK, Ashburner J, et al. Frontal, midbrain and striatal dopaminergic function in early and advanced Parkinson’s disease A 3D [(18)F]dopa-PET study. Brain. 1999;122(Pt 9):1637–1650. doi: 10.1093/brain/122.9.1637. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P, Davidson RJ. Differential contributions of the two cerebral hemispheres to the perception of happy and sad faces. Neuropsychologia. 1981;19(4):609–613. doi: 10.1016/0028-3932(81)90030-0. [DOI] [PubMed] [Google Scholar]
- Richard IH, Schiffer RB, Kurlan R. Anxiety and Parkinson’s disease. Journal of Neuropsychiatry and Clinical Neurosciences. 1996;8(4):383–392. doi: 10.1176/jnp.8.4.383. [DOI] [PubMed] [Google Scholar]
- Richard C, Rousseaux M, Saj A, Honore J. Straight ahead in spatial neglect: evidence that space is shifted, not rotated. Neurology. 2004;63(11):2136–2138. doi: 10.1212/01.wnl.0000145664.09078.83. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR. Neuropsychological contributions to theories of part/whole organization. Cognitive Psychology. 1991;23(2):299–330. doi: 10.1016/0010-0285(91)90012-d. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR, Knight RT. Effects of lesions of temporal-parietal junction on perceptual and attentional processing in humans. Journal of Neuroscience. 1988;8(10):3757–3769. doi: 10.1523/JNEUROSCI.08-10-03757.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR, Knight RT. Normal global-local analysis in patients with dorsolateral frontal lobe lesions. Neuropsychologia. 1991;29(10):959–967. doi: 10.1016/0028-3932(91)90060-l. [DOI] [PubMed] [Google Scholar]
- Rorden C, Fruhmann Berger M, Karnath HO. Disturbed line bisection is associated with posterior brain lesions. Brain Research. 2006;1080(1):17–25. doi: 10.1016/j.brainres.2004.10.071. [DOI] [PubMed] [Google Scholar]
- Rushton SK, Harris JM, Lloyd MR, Wann JP. Guidance of locomotion on foot uses perceived target location rather than optic flow. Current Biology. 1998;8(21):1191–1194. doi: 10.1016/s0960-9822(07)00492-7. [DOI] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, et al. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. Neuroimage. 2009;44(4):1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletu M, Anderer P, Saletu-Zyhlarz GM, Mandl M, Arnold O, Zeitlhofer J, et al. EEG-tomographic studies with LORETA on vigilance differences between narcolepsy patients and controls and subsequent double-blind, placebo-controlled studies with modafinil. Journal de Neurologie. 2004;251(11):1354–1363. doi: 10.1007/s00415-004-0543-8. [DOI] [PubMed] [Google Scholar]
- Scepkowski LA, Cronin-Golomb A. The alien hand: cases, categorizations, and anatomical correlates. Behavioral & Cognitive Neuroscience Reviews. 2003;2(4):261–277. doi: 10.1177/1534582303260119. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Agid Y, Barone P, Jenner P, Lemke MR, Poewe W, et al. Perspectives on recent advances in the understanding and treatment of Parkinson’s disease. European Journal of Neurology. 2009;16(10):1090–1099. doi: 10.1111/j.1468-1331.2009.02793.x. [DOI] [PubMed] [Google Scholar]
- Schendan HE, Amick MM, Cronin-Golomb A. Role of a lateralized parietal-basal ganglia circuit in hierarchical pattern perception: evidence from Parkinson’s disease. Behavioral Neuroscience. 2009;123(1):125–136. doi: 10.1037/a0013734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B, Borgman A, Engler H, Johnels B, Aquilonius SM. Gender differences in Parkinson’s disease symptom profile. Acta Neurologica Scandinavica. 2000;102(1):37–43. doi: 10.1034/j.1600-0404.2000.102001037.x. [DOI] [PubMed] [Google Scholar]
- Sergent J. Basic determinants in visual-field effects with special reference to the Hannay et al. (1981) study. Brain and Language. 1982;16(1):158–164. doi: 10.1016/0093-934x(82)90079-7. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Cooper LA. Mental images and their transformations. Cambridge: MIT Press; 1982. [Google Scholar]
- Silberman EK, Weingartner H. Hemispheric lateralization of functions related to emotion. Brain and Cognition. 1986;5(3):322–353. doi: 10.1016/0278-2626(86)90035-7. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proceedings of the Royal Society B: Biological Sciences. 1998;265(1409):1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Mahn K, Schroeder U, Woitalla D, Buttner T, et al. Facial expression recognition in people with medicated and unmedicated Parkinson’s disease. Neuropsychologia. 2003;41(8):1047–1057. doi: 10.1016/s0028-3932(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Srinivasan MV, Lehrer M, Kirchner WH, Zhang SW. Range perception through apparent image speed in freely flying honeybees. Visual Neuroscience. 1991;6(5):519–535. doi: 10.1017/s095252380000136x. [DOI] [PubMed] [Google Scholar]
- Stavitsky K, McNamara P, Durso R, Harris E, Auerbach S, Cronin-Golomb A. Hallucinations, dreaming, and frequent dozing in Parkinson disease: impact of right-hemisphere neural networks. Cognitive and Behavioral Neurology. 2008;21(3):143–149. doi: 10.1097/WNN.0b013e318185e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GT, Murphy CM. Diffusion tensor imaging in Alzheimer’s disease and mild cognitive impairment. Behavioural Neurology. 2009;21(1):39–49. doi: 10.3233/BEN-2009-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, et al. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75(1):454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Thobois S, Dominey PF, Decety J, Pollak PP, Gregoire MC, Le Bars PD, et al. Motor imagery in normal subjects and in asymmetrical Parkinson’s disease: a PET study. Neurology. 2000;55(7):996–1002. doi: 10.1212/wnl.55.7.996. [DOI] [PubMed] [Google Scholar]
- Tissingh G, Booij J, Bergmans P, Winogrodzka A, Janssen AG, van Royen EA, et al. Iodine-123-N-omega-fluoropropyl-2beta-carbomethoxy-3beta-(4-iod ophenyl)tropane SPECT in healthy controls and early-stage, drug-naive Parkinson’s disease. Journal of nuclear medicine. 1998;39(7):1143–1148. [PubMed] [Google Scholar]
- Torstenson R, Hartvig P, Langstrom B, Westerberg G, Tedroff J. Differential effects of levodopa on dopaminergic function in early and advanced Parkinson’s disease. Annals of Neurology. 1997;41(3):334–340. doi: 10.1002/ana.410410308. [DOI] [PubMed] [Google Scholar]
- Turano KA, Yu D, Hao L, Hicks JC. Optic-flow and egocentric-direction strategies in walking: central vs peripheral visual field. Vision Research. 2005;45(25–26):3117–3132. doi: 10.1016/j.visres.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Ukmar M, Makuc E, Onor ML, Garbin G, Trevisiol M, Cova MA. Evaluation of white matter damage in patients with Alzheimer’s disease and in patients with mild cognitive impairment by using diffusion tensor imaging. Radiologia Medica. 2008;113(6):915–922. doi: 10.1007/s11547-008-0286-1. [DOI] [PubMed] [Google Scholar]
- Vallar G, Lobel E, Galati G, Berthoz A, Pizzamiglio L, Le Bihan D. A fronto-parietal system for computing the egocentric spatial frame of reference in humans. Experimental Brain Research. 1999;124(3):281–286. doi: 10.1007/s002210050624. [DOI] [PubMed] [Google Scholar]
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. American Journal of Epidemiology. 2003;157(11):1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- Warren WH, Jr, Kay BA, Zosh WD, Duchon AP, Sahuc S. Optic flow is used to control human walking. Nature Neuroscience. 2001;4(2):213–216. doi: 10.1038/84054. [DOI] [PubMed] [Google Scholar]
- Wilkinson DT, Halligan PW, Marshall JC, Buchel C, Dolan RJ. Switching between the forest and the trees: brain systems involved in local/global changed-level judgments. Neuroimage. 2001;13(1):56–67. doi: 10.1006/nimg.2000.0678. [DOI] [PubMed] [Google Scholar]
- Yang TT, Menon V, Eliez S, Blasey C, White CD, Reid AJ, et al. Amygdalar activation associated with positive and negative facial expressions. NeuroReport. 2002;13(14):1737–1741. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Striatal connections of the parietal association cortices in rhesus monkeys. Journal of Comparative Neurology. 1993;332(2):175–197. doi: 10.1002/cne.903320204. [DOI] [PubMed] [Google Scholar]