Abstract
Knowledge about retinal photoreceptor signal transduction and the visual cycle required for normal eyesight has expanded exponentially over the past decade. Substantial progress in human genetics has allowed identification of candidate genes and complex networks underlying inherited retinal diseases. Natural mutations in animal models that mimic human diseases have been characterized and advanced genetic manipulation now permits generation of small mammalian models of human retinal diseases. Pharmacological repair of defective visual processes in animal models not only validates their involvement in vision but also provides great promise for developing improved therapies for the millions that are progressing towards blindness or are almost completely robbed of eyesight.
Keywords: Phototransduction, retinoid cycle, Leber congenital amaurosis, age-related macular degeneration, 9-cis-retinoid, vitamin A
Visual perception
The brain's capacity to analyze and interpret information is limited ultimately by the sensory input it receives. For the human brain, visual perception is a major input pathway and an essential sense for cognition of the environment and social communication. The performance of this sense reflects one's genetic background, withstands many environmental insults, and deteriorates with age 1-4. Molecular investigations of the fundamental processes initiating vision revealed that these can be divided into two stages: phototransduction, which propagates the light signal 5, 6, and the visual (retinoid) cycle 7, which consists of metabolic pathways that regenerate the visual chromophore and thus sustain this process 8. In reality, both pathways are fully integrated and complementary (Fig. 1) 2, 7, 9. Technical innovations and improved methodologies in proteomics and structural biology have promoted substantial advances in understanding the molecular basis of vision 10, 11. This advanced knowledge of the molecular basis of phototransduction and the visual cycle now makes it possible manipulation of these processes with highly specific retinoid-based therapies for disease states and visual deterioration due to aging and environmental insults 2, 4, 12. The remarkable anatomy and physiology of the eye enables unique and highly specific pharmacological approaches that are discussed in this review. Conceptually these approaches can be extended to other biological systems that require delivery of therapeutics in situ.
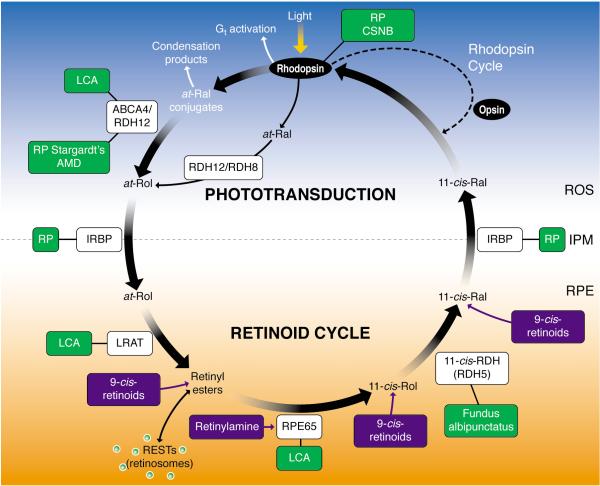
Figure 1. Phototransduction and the visual (retinoid) cycle.
In vertebrates vision is triggered by light-dependent activation of rhodopsin or other visual pigments. In rod cells, this chromophore couples to a protein opsin, forming rhodopsin. Absorption of a photon of light by rhodopsin causes photoisomerization of 11-cis-retinal to all-trans-retinal. In turn, photoactivated rhodopsin generates activation of hundreds of heterotrimeric G proteins, called transducin or Gt, in photoreceptors. This G protein-coupled receptor cascade is a classic cyclic nucleotide pathway that results in lowering cGMP levels (not depicted on the figure), and consequently hyperpolarization of the plasma membranes and ultimately reduction of glutamate secretion to the secondary neurons. The visual cycle regenerates 11-cis-retinal from released all-trans-retinal from the chromophore binding pocket of opsin. All-trans-retinal is reduced to all-trans-retinol in a reversible reaction catalyzed by RDH12 and RDH8, which are NADPH-dependent all-trans-retinol dehydrogenases. All-trans-retinol diffuses into the RPE where it is esterified in a reaction catalyzed by lecithin:retinol acyl transferase to long-chain fatty acids. As a consequence of the propensity of retinyl esters to aggregate, these esters are stored in lipid droplet-like structures called retinosomes. The all-trans-retinyl esters appear to be the substrate for RPE65 that converts it to 11-cis-retinol, which then is further oxidized back to 11-cis-retinal by retinol dehydrogenases, RDH5, RDH11, and other NAD-dependent retinol dehydrogenases. 11-cis-retinal formed in the RPE diffuses back into the ROS and COS, where it completes the cycle by recombining with opsins to form rhodopsin and cone pigments. Mutations in genes encoding proteins of phototransduction and the retinoid cycle are associated with various retinal diseases, some of which are indicated by the green boxes. Pharmacological intervention has been successful in animal models in a few instances, as indicated by the compounds in the blue boxes. IPM, interphotoreceptor matrix; IRBP, interphotoreceptor retinoid-binding protein; CSNB, congenital stationary nightblindness, ROS, rod outer segments; RPE, retinal pigment epithelium; Ral, retinal, RDH, retinol dehydrogenase; Rol, retinol; LCA, Leber congenital amaurosis; RP, retinitis pigmentosa, ABCR, ATP-binding transporter 4 .
Phototransduction and the retinoid cycle
Sensitivity and adaptation to the variable environmental light conditions are hallmarks of vision. Effective vision requires detection of light ranging from a single photon up to a trillion photons per second, and requires rapid restoration of the pre-illumination physiological state. This cyclic process is based on 11-cis-retinal, a light sensitive chromophore derived from vitamin A that enables light absorption in the visible range. When 11-cis-retinal binds to its cognate visual protein receptors (opsins), the resulting highly concentrated visual pigment is exquisitely sensitive to different wavelengths of light ranging from ~360-620 nm 13. Production of 11-cis-retinal involves several enzymatic steps, collectively called the visual (retinoid) cycle that is split between processes in photoreceptor cells and the adjacent retinal pigment epithelium (RPE).
Rhodopsin in rod photoreceptors and cone pigments in cone photoreceptors are the two classes of visual pigments that respond to light 13. Their common chromophore, 11-cis-retinal, is covalently linked via a protonated Schiff base to a lysine side chain amino group embedded within the opsin transmembrane domain, forming 11-cis-retinylidene. Upon photon absorption, the chromophore undergoes photoisomerization to all-trans-retinylidene, changing the visual pigments from an inactive to active conformation 14. In rods this active form, known as Meta II, then recruits and binds intracellular G proteins, continuing the signaling cascade that culminates in visual perception (Fig. 1)15. The various aspects of these phototransduction processes have been reviewed in-depth 5, 6,16.
The retinoid (visual) cycle is a complex enzymatic pathway essential for regeneration of 11-cis-retinal. Maintaining continuous vision and preserving the health of photoreceptors requires an adequate, continuous supply of this aldehyde, so vertebrates have evolved the retinoid cycle to achieve this objective 2. The pathway operates in sequential reactions in photoreceptor cells, in the RPE and back in photoreceptors, converting all-trans-retinal back to 11-cis-retinal by several chemical transformations. The classical vertebrate retinoid cycle contributes primarily to regeneration of rhodopsin in rod cells (Fig. 1. The key enzyme in this pathway is retinoid isomerase or RPE65 that resides in the RPE. This RPE65-dependent chromophore production may also be important for cone function 17. However, the cone retinoid cycle appears to be supplemented by another metabolic pathway that remains genetically undefined 18-20.
The series of chemical reactions comprising the classical retinoid cycle is now well established (Fig. 1). In rod cells, absorption of a photon of light by rhodopsin causes photoisomerization of 11-cis-retinylidene to all-trans-retinylidene, resulting in release of all-trans-retinal from the chromophore binding pocket of opsin. Most of the all-trans-retinal that dissociates from opsin diffuses into the cytoplasm. A fraction that dissociates into the disc lumen also reaches the cytoplasm via transfer by ATP-binding cassette, transporter 4 (ABCA4) 21. All-trans-retinal in the cytoplasm is reduced to all-trans-retinol in a reversible reaction catalyzed by an NADPH-dependent all-trans-retinol dehydrogenase (RDH). All-trans-retinol then diffuses into the RPE where it is esterified in a reaction catalyzed by lecithin:retinol acyl transferase (LRAT). The acyl group is transferred from the sn1 position of ER phospholipids 22, 23. The most common lipids in the sn1 position are saturated long-chain fatty acids, with a palmitoyl group representing the predominant species. Thus, retinyl esters are dominated by retinyl palmitate, but also contain stearate (C18) and other minor species. These esters have a propensity to aggregate and, in the RPE, they form lipophilic droplets called retinosomes, which are discussed below. These all-trans-retinyl esters are presumably converted by the retinoid isomerase RPE65 to 11-cis-retinol, which is further oxidized back to 11-cis-retinal by RDH5, RDH11 and other RDHs. After diffusion from the RPE, 11-cis-retinal combines with opsin forming light-sensitive pigment that is ready for another cycle of photoisomerization, signal transduction and regeneration.
Retinoid metabolism and retinopathies
Retinoids are required for normal growth, vision, reproduction, and maturation and maintenance of the immune system 24. More recently it was recognized that retinoids are also important regulators of metabolism in general 25. All-trans-retinol is an essential micro-nutrient because it cannot be synthesized by animals, and therefore must be absorbed as either retinol/retinyl esters from food of animal origin or generated from their precursor β,β-carotene from plants. Dietary retinyl esters are hydrolyzed in the intestinal lumen and absorbed into intestinal enterocytes, re-esterified, and incorporated into chylomicrons. Next, the retinyl esters are taken up by hepatocytes and either hydrolyzed and secreted after binding to the retinol-binding protein 4 (RBP4) complex, or stored as lipid droplet-like structures in cells called Ito or stellate cells. Thus, the liver is the largest storage place for retinyl esters in our body 26, 27. β,β-Carotene is symmetrically cleaved into two molecules of all-trans-retinal by members of the carotenoid oxygenase enzyme family 28-30. Retinal is reduced to retinol, esterified, and further processed in pathways that involve exogenous retinyl esters throughout the body 31. Retinoid metabolizing enzymes in the liver and peripheral tissues including LRAT, transferases, hydrolases, diacylglycerol acyltransferase 1 (DGAT1), acyl-coenzyme A transferase (ACAT), and other enzymes are not highly specific, so they can process retinoid analogs as well.
Considering the fundamental role of retinoids in vision, it is not surprising that many forms of retinopathies are caused by defects in genes encoding proteins of the visual cycle (Fig. 1) 2. Storage of absorbed retinoids in the liver, their transport in the plasma, and delivery to the RPE can all be impaired as a result of inactivating mutations in enzymes (such as LRAT), transport carrier proteins (like retinol-binding protein 4) or receptors (like STRA6). Progress in our understanding of these processes was possible because a large number of animals models of these defects that were either generated or occurred naturally 32. In Lrat-/- or Rpe65-/- mice, the lack of 11-cis-retinal leads to rapid degeneration of cone photoreceptors and progressive death of rods 33. This phenomenon may involve the mechanism leading to the pathology seen in Leber congenital amaurosis (LCA) patients 17. LCA has been attributed to continuous activation of visual phototransduction 34 due either to basal activity of chromophore free opsin 35-37, disordered vectorial transport of cone visual pigments lacking bound-chromophore 38, instability of unliganded cone visual pigments, or a combination of all these mechanisms.
More complete discussions of retinal diseases related to genetic alterations of phototransduction and the visual cycle can be found elsewhere 1, 2, 39, 40. Because components of the visual cycle and phototransduction are mostly non- or only partially redundant, genetic defects resulting in dysfunction of these proteins is manifested as a retinopathy (for examples, see Fig. 1). The severity of these genetic retinopathies is determined by the toxicity of the accumulated intermediates (e.g. condensation products of all-trans-retinal in Stargardt's disease) 41, the need of the product for cellular homeostasis (e.g. cGMP production in LCA caused by a mutation in guanylate cyclase) 42, the instability of the mutated protein structure (e.g. rhodopsin mutants in autosomal recessive retinitis pigmentosa (RP)) 43, and whether regulation of the protein's function is altered (e.g. Ca2+ coordination in autosomal dominant cone-rod dystrophy mutants of guanylate cyclase-activating proteins) 44.
All-trans-retinal, condensation products, and degenerative retinal diseases
In many individuals the visual system degenerates with age. Preventing vision loss requires a better understanding of the fundamental causes of age-dependant changes. Fortunately, our understanding of both retinoid metabolism outside the eye and production of 11-cis-retinal unique to the eye is accelerating 1, 2, 7. And genetic mouse models are now available to study these processes and their aberrations in vivo 45. These advances allow the central question of what compromises photoreceptor cells and the underlying RPE to be addressed. Retinoids, despite being essential for vision, can also cause certain retinal pathologies when not tightly controlled.
Typically, retinoids are complexed with soluble proteins that protect them. These reactive compounds are bound by a number of retinoid-binding proteins, and are rarely freely solubilized from membranes. Protection of retinoids also stems from their ability to cluster when esterified by long-chain fatty acids, and undergo storage in lipid-like droplets in the liver or as retinosomes in the eye 46-48.
To absorb light efficiently, visual pigments need to be very sensitive to it (11-cis-retinal requires a quantum efficiency of 0.65 14) and they need to be highly concentrated. Indeed, a large fraction of rhodopsin forms a paracrystaline structure in rod outer segments (ROS) 49-52, and cone pigments can form diffractable crystalline structures in cones 53, 54. ROS contains ~5 mM rhodopsin 55, that if completely bleached, would yield equal level of free all-trans-retinal. How can cells cope with such an aldehyde flux? Even less than a 0.5% bleach will produce toxic levels of all-trans-retinal if this retinoid is not properly cleared.
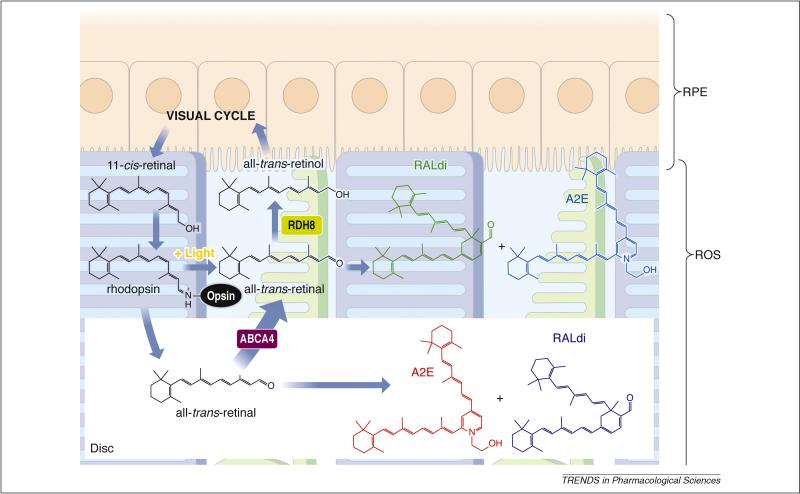
The efficiency of the mammalian visual system and the health of photoreceptors and RPE diminish significantly with age, suggesting that before cell death, there are biochemical changes that slowly promote retinal damage. For example, an abnormally high flux of retinoids through the retinoid cycle can induce retinopathies in some mouse models 56-58. This process in turn triggers host immune and other defense responses that culminate in retinal cell death. Even in the presence of an efficient and fully functional retinoid cycle, all-trans-retinal can condense producing, among a myriad of other byproducts, di-retinoid-pyridinium-ethanolamine (A2E) and all-trans-retinal dimer (RALdi) 59-61 (Fig. 2). Initial condensation products are formed in the ROS, and 10% of the ROS undergoes phagocytosis and accumulates in the RPE daily. Condensation products also accumulate with age 62, and it was proven that these compounds can cause RPE cell toxicity under experimental conditions 63-65. Patients affected by age-related macular degeneration (AMD), Stargardt's disease, or other retinal diseases associated with accumulation of surrogate markers such as A2E all develop retinal degeneration 66.
Figure 2. Retinoid flow in the visual cycle and condensation of all-trans-retinal.
After 11-cis-retinal binds to opsin, forming rhodopsin, the resulting visual chromophore, 11-cis-retinylidene, is photoisomerized to all-trans-retinylidene, the precursor of all-trans-retinal that is subsequently released. Most of the all-trans-retinal dissociates from opsin into the cytoplasm where it is reduced to all-trans-retinol by RDHs including RDH8. The fraction of all-trans-retinal that dissociates into the disc lumens is transported by ABCA4 back into the cytoplasm 21 before it is reduced. Thus, condensation products can be generated both within the disc lumens and the cytoplasm. Loss of ABCA4 and RDH8 exacerbates this condensation reminiscent of an accelerated aging process. In humans, as a result of daily phagocytosis of the part of rod outer segments, lipofuscin fluorophores accumulate with age in the RPE, especially in RPE cells underlying the cone-rich macula 109, 127. Such accumulation has been considered to constitute one of the major risk factors for AMD, the predominant cause of legal blindness in developed countries 128. Lipofuscin fluorophores also are especially abundant in Stargardt disease, the most common juvenile form of macular degeneration 72. Di-retinoid-pyridinium-ethanolamine (A2E) and all-trans-retinal dimer (RALdi), the major fluorophores of lipofuscin, is formed by condensation of phosphatidylethanolamine with two molecules of all-trans-retinal followed by oxidation and hydrolysis of the phosphate ester 129. Various mechanisms have been proposed to explain the toxicity of A2E. These include its cationic detergent properties 130, physiological interference with RPE function 131, 132, and radical reaction products induced by light-dependent oxidation 133.
Mutations in ABCA4 cause Stargardt's macular degeneration 67, cone-rod dystrophy 68, and recessive RP 69, 70. Heterozygous mutations in ABCA4 increase the risk of developing AMD as well 66. A2E 71, 72 and RALdi 61 are the major fluorophores of lipofuscins produced from all-trans-retinal 73 (Fig. 2). As a consequence of aging, both A2E and RALdi can accumulate over a lifetime of light exposure 62, and produce toxic effects on RPE cells 74, 75. Patients affected by Stargardt's disease or AMD because of a disabled ABCA4 gene, or those affected by other retinal diseases associated with lipofuscin accumulation, eventually develop retinal degeneration. ABCA4 mutations are also linked to an increased risk of AMD 66. However, no such degeneration was observed in Abca4-/- mice even though RPE atrophy was detected 21, 41, 76. Thus, mice and humans do not always exhibit identical responses to fluorophore accumulation.
Recently, we showed that mice carrying a double knockout of Abca4 21 along with retinol dehydrogenase 8 (Rdh8), one of the main enzymes that reduce all-trans-retinal in ROS and cone outer segments (COS) 77, rapidly accumulate all-trans-retinal condensation products and exhibit accentuated RPE/photoreceptor dystrophy at an early age 58. Retinas from these mice exhibited lipofuscin, drusen, basal laminar deposits, Bruch's membrane thickening, and choroidal neovascularization. Importantly, the severity of their visual dysfunction and retinopathy was exacerbated by light but attenuated by treatment with retinylamine, a visual cycle inhibitor that slows the flow of retinoids through the visual cycle, thus giving other oxidoreductase enzymes more time to detoxify retinaldehyde molecules. These findings provide direct evidence that aberrant production of toxic condensation byproducts of the visual cycle can lead to rapid and progressive retinal degeneration in mice and present a pharmacological method to ameliorate these conditions. The similarity of this retinopathy to human AMD makes these mice invaluable for research aimed at ameliorating this devastating blinding disease.
Although the above studies strongly suggest retinoid toxicity, it still is unclear if elevated levels of retinal and/or its condensation products such as A2E actually cause these retinopathies or merely constitute non-specific manifestations of impaired retinoid metabolism. Recently, we reported that all-trans-retinal is most likely responsible for photoreceptor degeneration in Rdh8-/-Abca4-/- mice 73. Toxic effects of all-trans-retinal induces apoptosis through caspase activation and mitochondrial-associated cell death 73. Therefore, excessive levels of this aldehyde must be reduced to preserve retinal health. Although A2E formation could be a surrogate marker for aberrations in all-trans-retinal clearance, it may also represent a detoxification product and not the primary toxin previously supposed. Regardless of whether all-trans-retinal or A2E are responsible for retinal degeneration, terminating all-trans-retinal accumulation will also stop production of A2E and other similar derivatives formed from this aldehyde.
Retinoids are a diverse group of compounds with different biological activity
The fact that retinoids are often grouped together in medical literature without distinction can be misleading. For example, retinol and retinoic acid display dramatic differences in biological activity, and though retinol can be converted to retinoic acid, this transformation is highly regulated. Retinol has only limited biological activity per se 78, but it can be dehydrated to anhydro-retinol, which may exert regulatory effects on the immune system 79, or it can be saturated in the 13-14 position to produce dihydroretinol precursors involved in metabolic processes 80, 81. In the retinoid cycle, retinol is an excellent substrate for LRAT and is quickly converted into fatty acid esters. Their propensity to form oil droplets excludes fatty acid esters of retinol from the circulation. Retinol can be oxidized to retina in a reaction catalyzed by a subset of short-chain alcohol dehydrogenases and medium-chain alcohol dehydrogenases. Because the redox potential of cells favors reducing conditions and the oxidation reaction is thermodynamically neutral, only a tiny fraction of retinol can be oxidized to fulfill the thermodynamic requirements of equilibrium. The all-trans-retinal formed probably does not exert direct biological activity, although there is speculation to the contrary 82. Instead, this aldehyde is subsequently oxidized by aldehyde dehydrogenase to form retinoic acid in a highly regulated process. Retinoic acid is a potent mitogen involved, via nuclear transcription factors, in controlling the expression of a large number of genes. One of the most highly up-regulated genes is CYP26, a P450 enzyme that oxidizes retinoic acid to inactive products. Thus, retinoic acid levels are kept relatively low and tightly controlled by their rates of biosynthesis and destruction.
Another issue is the differences in biological processing exhibited by geometric isomers of retinoids. For example, 9-cis-retinal and 11-cis-retinal recombine with opsin to generate visual pigments, but all-trans-retinal or 13-cis-retinal, two isomers that are in thermodynamic equilibrium, do not. Indeed, all-trans-retinal can be converted to 11-cis-retinal through the retinoid cycle, but typically all-trans-forms are preferably stored as fatty acid esters in the RPE.
Strategies for treating blinding retinal diseases caused by mutations in retinoid cycle genes
Conceptually, the simplest way to restore function is to replace defective genes by viral, nanoparticle, or other gene therapeutic methods. RPE and photoreceptor cells take up and express recombinant constructs with great efficiency in many experimental settings. This strategy was used successfully in LCA animal models such mice and dogs, and the positive effect of gene replacement therapy was sustained for several years after a single treatment of RPE65-null dogs 83, 84. Recently, several LCA patients were enrolled for gene therapy. Initial results revealed that this treatment at least partially restored vision in retinal areas of gene transfer in those individuals with blindness attributed to RPE65 mutations 85-87. However, there are currently several limitations to this method which are discussed in more detail elsewhere 2.
Retinoids undergo multiple transformations in the eye (Figs. 1, 2), and disabling mutations in genes encoding unique enzymes responsible for these transformations cause a deficiency in 11-cis-retinal production. Below we describe how such compromised transformations can be bypassed by supplying active chromophore(s).
Theoretically, reducing the production of the 11-cis-retinal chromophore by specific inhibitors of the visual cycle would be beneficial. Slowing down a fast turnover of retinoids would prevent excessive production of toxic all-trans-retinal and its condensation products. Excessive or greatly prolonged inhibition would be detrimental because conceptually, this would decrease vision at extreme conditions as observed in LCA. However, slowing the visual cycle under strong illumination conditions would be beneficial.
Retinosomes as a depot for chromophores or inhibitors of the visual cycle
Retinosomes are storage particles that were discovered by a post-doctoral fellow in my laboratory, Dr. Yoshikazu Imanishi. They bud off the ER, but the mechanism of formation has yet to be clarified. Retinosomes are composed of fatty acid retinyl esters, lipids and at least one other identified component, adipocyte differentiation-related protein 46, 47, 88, 89. As a result of the high UV sensitivity of retinoids, these structures can be imaged only under long-wavelength infrared light by using two-photon microscopy (Fig. 3A). It was then demonstrated that these particles expand under light and contract when light was removed, providing evidence that retinosomes participate in the regular visual cycle (Fig. 3B). These storage particles become light insensitive when the visual cycle is disabled by elimination of retinoid isomerase or LRAT activity. Retinosomes can also be used to store retinylamine, as this compound can be amidated by LRAT 48. Importantly, these structures also can be used to store artificial precursors of the chromophore in the form of 9-cis-retinyl esters.
Figure 3. Transformations of visual cycle retinoids in the RPE.
All-trans-retinol diffuses from photoreceptor cells into the RPE, where it is esterified by LRAT to all-trans-retinyl esters. Hydrophobic retinyl esters then form retinosomes (RESTs). All-trans-retinyl esters are isomerized to 11-cis-retinol (reaction a) in a reaction that involves an RPE-abundant protein, termed RPE65. 11-cis-Retinol is then oxidized by 11-cis-RDH to 11-cis-retinal (reaction b). 11-cis-Retinal diffuses back into the rod and cone outer segments, where it completes the retinoid cycle by recombining with opsins to reform rhodopsin and cone pigments. A. Retinosomes imaged in RPE cells by two-photon microscopy (courtesy of Grazyna Palczewska, Polgenix, Inc., Cleveland). Fluorescence emission from the isolated intact mouse eye at 560–700 nm in green pseudocolor was observed after excitation by a 730-nm mode-locked Ti:Sapphire laser. Scale bar 5 μm. B. Flash-dependent changes in fluorescence and all-trans-retinol/all-trans-retinyl esters in the RPE cell layer of isolated mouse eyes. Top: A row of images showing optical sections of the retina, perpendicular to the ocular tissue. RPE fluorescence (a.u., arbitrary unit) was quantified as a function of time. Numbers refer to minutes after the flash. Middle and bottom graphs show quantified fluorescence from retinoids and retinoid analyses by HPLC (all-trans-retinol and all-trans-retinyl esters; mean ± SD, n = 3), respectively. Dashed lines indicate half-times for formation of RESTs and the increase in all-trans-retinol and all-trans-retinyl esters. On the right, light-dependent changes in the fluorescent signal in different subcellular compartments are shown. (copied from ref.46 with permission from the Rockefeller University Press).
Pharmacological replacement of missing chromophore and its precursor
In the most severe cases, insufficient 11-cis-retinal production leads to congenital or progressive blindness in humans. LCA is an autosomal recessive, early onset, severe retinal dystrophy that accounts for 5% of all such inherited disorders 90. Pharmacological replacement of missing chromophore is applicable to diseases resulting from deficient chromophore biosynthesis. Examples include LCA arising from mutations in the LRAT and RPE65 genes (Fig. 1). Initial experiments aimed at bypassing the biochemical defect caused by absence of Rpe65 were performed by oral gavage of Rpe65−/− mice with 9-cis-retinal 91. 9-cis-Retinal, which combines with opsin to form light-sensitive iso-rhodopsin 91, was initially selected because it is easier to synthesize and more stable than 11-cis-retinal. Moreover, iso-rhodopsin has an absorbance maximum of 494 nm versus 502 nm for rhodopsin, permitting experimental identification of reconstituted iso-rhodopsin 92. Further refinement and extensive testing identified 9-cis-retinyl acetate as a useful experimental compound 93 (Fig. 4).
Figure 4. Delivery and action of 9-cis-R-Ac.
This retinyl ester is effective when taken orally. In the small intestine, 9-cis-R-Ac is either hydrolyzed and esterified with fatty acyl coenzyme A, or trans-esterified with phospholipids before being transported in chylomicrons to the liver, where these intermediate products are found in lipid droplets. This cycle of hydrolysis and esterification may occur several times before storage in hepatic stellate cells. Fatty acid (mostly palmitate) esters and free 9-cis-retinol are then secreted into the systemic circulation, either bound to RBP or albumin or incorporated into chylomicrons. In the eye, these retinoids are likely hydrolyzed again as they pass from the choroid capillaries into the RPE in both a STRA6-dependent and independent manner. Fatty acid esters of 9-cis-retinol in the RPE are stored in specific lipid droplets called retinosomes. When required, these esters are hydrolyzed and oxidized to the drug, 9-cis-retinal, which is then delivered to opsins in photoreceptors to form light-sensitive visual pigments.
Briefly, the use of cis-retinoids in the treatment of LCA 91,100 seemed mechanistically sound and encouraging results were obtained in the treatment of aging mice 101. Not only were cis-retinoids proven to chaperone the mutant opsin to allow proper in vivo folding of P23H-opsin, but in experimental cell lines, the rescued protein formed pigment, acquired mature glycosylation, and was transported to the cell surface 94. Only photoactive cis-chromophores were beneficial, whereas all-trans-retinal was ineffective.
Dietary supplementation of Rpe65−/− mice with 9-cis-retinoids restored light sensitivity to levels found in wild-type animals, as assessed by both single-cell and ERG recordings. Similar recovery of visual function was reported following intraperitoneal injection of 11-cis-retinal into Rpe65−/− mice 95. There are several advantages of 9-cis-retinoid over 11-cis-retinoid treatment. First, the 9-cis-compound is effective when taken orally. Second, because it is converted to prodrug forms that are stored in the liver, transported in the blood, selectively taken up by the eye and stored in retinosomes (Fig. 4A and 4B), storage particles that participate in the regular visual cycle 45,46,47,88,89 but can also be used to store artificial precursors of the chromophore, from which the active compound is slowly released, a single dose can produce a long-term therapeutic effect with minimal risk of toxicity. The major disadvantage is that the toxicity profile of 9-cis-retinoids has yet to be determined. If this retinoid has a narrow therapeutic window, any toxic effects are likely to be long-lasting. A particular concern is the potential for long-term toxicity during pregnancy. Fortunately, such toxicity is unlikely to emanate from conversion to retinoic acid, a potent mitogen, as the latter's biosynthesis from retinol is tightly controlled. As in aging, rhodopsin regeneration after light exposure is more delayed in humans and mice with vitamin A deficiency due either to inadequate dietary intake or intestinal absorption 9. Studies in mice have shown that age-related decreased retinal rod cell function cannot be explained by rod cell loss, abnormal retinal plasticity or any signs of retinal disease 96-98. However, a dramatic, age-associated slowing of rod-mediated dark adaptation after light exposure in humans was observed that related to delayed regeneration of rhodopsin 96. Deteriorating photoreceptor function documented in mice at 10 and 14 versus 4 months of age was improved significantly by long-term, monthly administration of the artificial chromophore, 9-cis-retinyl acetate. These findings suggest one potential therapeutic approach for prevention of age-related retinal dysfunction 3.
In a highly discussed study several years ago, all-trans-retinol together with vitamin E was tested as a remedy for patients with retinitis pigmentosa but lacking genotypic characterization. Results of this study support a beneficial effect of 15,000 IU/d of vitamin A on the course of retinitis pigmentosa.99 As there was no indication that these patients were vitamin deficient, it appears inconceivable that the role of this treatment differed mechanistically from the known anti-oxidant role of vitamin A on the retinas of this genetically heterogeneous diseased population. Surprisingly, vitamin A supplementation slows the rate of photoreceptor degeneration caused by a threonine-17 to methionine (T17M) mutation in the opsin gene. The authors speculated that vitamin A supplementation could confer therapeutic benefit by stabilizing mutant opsins through increased availability of the chromophore 100, but chromophore production does not depend on the further elevation of this precursor because it exists in significant excess in the mature retina.
An observation made more recently using Abca4-/- mice is also puzzling. These mice, when supplemented with vitamin A, exhibited dramatically higher levels of retinyl esters in their liver and RPE, and more importantly, lipofuscin pigments such as A2E were significantly increased as well. Photoreceptor degeneration also was observed in 11-month-old albino mice. The author recommended that “vitamin A supplementation should be avoided in patients with ABCA4 mutations or other retinal or macular dystrophies associated with lipofuscin accumulation in the retinal pigment epithelium” 101. However, A2E formation is related only to high levels of flux through the visual cycle, and high levels of the chromophore ester precursor have nothing in common with A2E formation as evidenced by Rpe65-/- mice that completely lack A2E, but still accumulate huge amounts of all-trans-retinyl esters 102.
The situation differs for Sorsby's fundus dystrophy (SFD), which is an autosomal dominant retinal degeneration caused by mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) gene. During the course of this disease, a thickened membrane barrier between photoreceptor layers causes local vitamin A-deprivation. Administration of vitamin A led to dramatic restoration of photoreceptor function 103. Insufficient dietary vitamin A also can cause progressive deterioration of vision and ultimately blindness without genetic abnormalities 104, a major problem in underdeveloped countries. Dietary supplementation with vitamin A typically reverses this problem.
Pharmacological inhibition of the retinoid cycle
Another treatment strategy is to slow the biosynthesis of chromophore by either inhibiting steps in the visual cycle or limiting availability of the all-trans-retinol precursor. This approach is applicable to diseases associated with accumulation of retinoid cycle intermediates. As noted above, impaired clearance of all-trans-retinal has been shown to cause both acute light-induced retinal toxicity 73 and induce formation of fluorescent lipofuscin pigments such as A2E in RPE cells. Acute all-trans-retinal toxicity seen in light-induced photoreceptor damage may involve increased plasma membrane permeability and mitochondrial poisoning that leads to caspase activation and mitochondrial-associated cell death 73. While accumulation of lipofuscin pigments is an important pathological feature of Stargardt's disease 105, 106, it is not limited to this inherited condition. Fundus autofluorescence is non-invasive technique developed in that past decade that utilizes fluorescent properties of lipofuscin to study the health and viability of the retina (RPE and photoreceptors). Increased fundus autofluorescence by scanning laser ophthalmoscopy is commonly seen in patients with AMD 107, 108.
Fluorescent material acquired in aged RPE has spectral properties similar to A2P identified in Abca4−/− mice 59, 109. Strong fundus autofluorescence is also seen in patients with Best vitelliform macular dystrophy and in a subset of patients with cone-rod dystrophy 110. Patients with dominant Stargardt's disease, caused by mutations in the ELOVL4 gene, show a dark choroid by fluorescein angiography, also owing to lipofuscin in RPE cells 111 112. The major disadvantage of inhibiting the retinoid cycle is night-blindness, with resulting inability to drive. Also of concern is that prolonged inadequate production of chromophore will adversely affect the health of rod and, more severely, cone cells. The most potent and efficacious retinoid cycle inhibitor identified to date is retinylamine, a transition state inhibitor of RPE65 19, 48, 58, 113-118. The unusual fate of this retinoid, including its route of administration, hepatic storage, release into the circulation, uptake by the eye, storage, and eventual release in retinosomes of the retina is illustrated in Fig. 5.
Figure 5. Delivery and action of retinylamide.
This amide ester can be taken orally. Once in the intestine it is hydrolyzed and re-amidated with fatty acid coenzyme A, and transported in chylomicrons to the liver, where it is stored as lipid droplets in hepatic stellate cells. This cycle of hydrolysis and re-amidation can occur several times. Fatty acid (mostly palmitate) amide and free retinylamine are then secreted into the systemic circulation, either bound to RBP or albumin, or incorporated into chylomicrons. The amides likely are hydrolyzed again as they pass from the choroid capillaries into the RPE in a STRA6-independent manner. There, retinylamine is stored in specific lipid droplets called retinosomes. When required, retinylamine is released and acts as a very potent transition state inhibitor of RPE65, which catalyses the hydrolytic isomerization of retinyl esters. Suppression of this isomerization can last for weeks because of long-term storage. Retinylamine also conjugates with free retinal, preventing accumulation of other toxic retinal condensation products. Eventually retinylamine is metabolized to retinol.
We systematically studied the effects of retinylamine and other potential inhibitors on visual function in mice. Prolonged, sustainable, but reversible suppression of visual function was observed with retinylamine (Fig. 5) as a result of its storage in a prodrug form, N-retinylamide 117. Direct comparison of other inhibitors was tested to assess their prevention of light-induced retinal damage. Retinylamine displayed a higher efficacy, specificity, potency and lower transcriptional activation as compared to N-(4-hydroxyphenyl)retinamide 119 and 13-cis-retinoic acid 120, 121, whereas other tested compounds were ineffective 114. As a note, other investigators have proposed that N-(4-hydroxyphenyl)retinamide would cause immediate, dose-dependent reductions in serum retinol and RBP in Abca4-/- mice 119. This prediction is puzzling because excess retinoids are stored in the eye, which is highly resistant to vitamin A deprivation. In mice, it takes two generations, even after liver stores are depleted, for retinoids to be depleted from the eye. Methods to reduce circulating retinol would be inadequate to deplete retinoids in the eye because of the powerful protective mechanisms involved in retinoid transport (Fig. 4, 5) and retinoid retention within the RPE (Fig. 5). Thus, it is possible that a small effect on retinoid metabolism in the eye could be accomplished by weak inhibition of RPE65 by N-(4-hydroxyphenyl)retinamide and 11-cis-retinol dehydogenase by 13-cis-retinoic acid 122. On the other hand, dramatic reduction of retinol delivery to peripheral tissues for a prolonged period could be detrimental to the health of the individual.
Is there a future for use of inhibitors of the visual cycle for treatment of retinal degenerative diseases? We believe these inhibitors could be effective therapies based on what we have learned about their effects in animal models of these diseases. For example, it was recognized that cone photoreceptor cells in Rpe65-/- mice degenerate more rapidly than rod photoreceptors 123, and similar observations were published for Lrat-/- mice 38, 124. In Rpe65-/-rhodopsin-/- mice, addition of chromophore enhanced proper transport of cone opsins to outer segments while partially preserving cone structure and function in a compromised retina lacking rods because of rhodopsin elimination 38. These findings are critical both because of the importance of cones for human high resolution spatial vision and color perception 125 and the need to evaluate cone status for any potential therapy of LCA 126. Children with RPE65-LCA manifest cone photoreceptor loss in the first decade of life 118. The central retinal RPE layer of the normal primate retina also shows higher retinoid isomerase activity than the more peripheral RPE, so we speculated that early cone photoreceptor loss in RPE65-LCA indicates that robust RPE65-based visual chromophore production is vital for cones 17. Residual cone structure and function could be supported by a retinal-based alternative pathway for chromophore production 18. Mice chronically treated with retinylamine showed a decline in the number of cones that was ameliorated by administration of 9-cis-retinoids. Together these results suggest that chronic lack of chromophore leads to progressive loss of cones in mice and humans 118. Thus, prolonged inhibition of the visual cycle, a currently tested approach for treatment of Stargardt's and AMD patients, poses a major unresolved problem. Moreover, night blindness that accompanies this treatment might severely limit patients who can no longer drive at night, along with other serious inconveniences.
Conclusions
Classical approaches can be combined with emerging technologies to address previously challenging therapeutic questions. By obtaining a proper molecular framework with which to view biological systems, new strategies can be evolved to develop better pharmacological agents to combat blinding diseases. Further progress in vision research and medicine will require a combination of multiple approaches and techniques to solve the complexities of retinal diseases. Only selected retinoids, when properly used, have the potential to combat retinal diseases. Compounds that can either inhibit the trans-to-cis isomerization step of the retinoid cycle or that recombine with opsin to form light-sensitive pigments could be stored in the liver and eye as prodrugs. This is highly unusual, but it would allow novel and very powerful pharmacology to be employed. These properties rely on the ability of the vertebrates to store inactive fatty acid acylated chromophore or retinoid inhibitors in cellular structures such as stellate cells in the liver and retinosomes in the eye.
Acknowledgements
We thank Drs. Leslie T. Webster and John C. Saari, and members of Palczewski's laboratory for critical comments on the manuscript. This research was supported in part by grant EY008061 (KP), and a core grant P30 EY11373 from the National Institutes of Health, and Foundation Fighting Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
University of Washington, Acucela Inc. Retinagenix Inc. and QLT Inc. may commercialize some of the technology described in this work. KP is a consultant for QLT Inc and Acucela Inc. and a co-founder of Retinagenix Inc.
Reference
- 1.Thompson DA, Gal A. Vitamin A metabolism in the retinal pigment epithelium: genes, mutations, and diseases. Progress in retinal and eye research. 2003;22:683–703. doi: 10.1016/s1350-9462(03)00051-x. [DOI] [PubMed] [Google Scholar]
- 2.Travis GH, et al. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda T, et al. Effects of long-term administration of 9-cis-retinyl acetate on visual function in mice. Investigative ophthalmology & visual science. 2009;50:322–333. doi: 10.1167/iovs.08-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson GR, et al. Impact of aging and age-related maculopathy on inactivation of the a-wave of the rod-mediated electroretinogram. Vision research. 2006;46:1422–1431. doi: 10.1016/j.visres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139:246–264. doi: 10.1016/j.cell.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arshavsky VY, et al. G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 7.McBee JK, et al. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Progress in retinal and eye research. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 8.Rando RR. Polyenes and vision. Chem Biol. 1996;3:255–262. doi: 10.1016/s1074-5521(96)90105-2. [DOI] [PubMed] [Google Scholar]
- 9.Lamb TD, Pugh EN., Jr. Dark adaptation and the retinoid cycle of vision. Progress in retinal and eye research. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Ridge KD, et al. Phototransduction: crystal clear. Trends in biochemical sciences. 2003;28:479–487. doi: 10.1016/S0968-0004(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 11.Ridge KD, Palczewski K. Visual rhodopsin sees the light: structure and mechanism of G protein signaling. J Biol Chem. 2007;282:9297–9301. doi: 10.1074/jbc.R600032200. [DOI] [PubMed] [Google Scholar]
- 12.Moise AR, et al. Delivery of retinoid-based therapies to target tissues. Biochemistry. 2007;46:4449–4458. doi: 10.1021/bi7003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filipek S, et al. G protein-coupled receptor rhodopsin: A Prospectus. Annu Rev Physiol. 2003;65:851–879. doi: 10.1146/annurev.physiol.65.092101.142611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palczewski K. G protein-coupled receptor rhodopsin. Annual review of biochemistry. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Lintig J, et al. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends in biochemical sciences. 2010 doi: 10.1016/j.tibs.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polans A, et al. Turned on by Ca2+! The physiology and pathology of Ca2+-binding proteins in the retina. Trends in neurosciences. 1996;19:547–554. doi: 10.1016/s0166-2236(96)10059-x. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson SG, et al. Human cone photoreceptor dependence on RPE65 isomerase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mata NL, et al. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schonthaler HB, et al. Evidence for RPE65-independent vision in the cone-dominated zebrafish retina. The European journal of neuroscience. 2007;26:1940–1949. doi: 10.1111/j.1460-9568.2007.05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JS, et al. Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nature neuroscience. 2009;12:295–302. doi: 10.1038/nn.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molday RS. ATP-binding cassette transporter ABCA4: Molecular properties and role in vision and macular degeneration. Journal of bioenergetics and biomembranes. 2007;39:507–517. doi: 10.1007/s10863-007-9118-6. [DOI] [PubMed] [Google Scholar]
- 22.Saari JC, Bredberg DL. Lecithin:retinol acyltransferase in retinal pigment epithelial microsomes. J Biol Chem. 1989;264:8636–8640. [PubMed] [Google Scholar]
- 23.Saari JC, et al. Retinol esterification in bovine retinal pigment epithelium: reversibility of lecithin:retinol acyltransferase. The Biochemical journal. 1993;291:697–700. doi: 10.1042/bj2910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940–954. [PubMed] [Google Scholar]
- 25.Ross AC. Retinoid production and catabolism: role of diet in regulating retinol esterification and retinoic Acid oxidation. The Journal of nutrition. 2003;133:291S–296S. doi: 10.1093/jn/133.1.291S. [DOI] [PubMed] [Google Scholar]
- 26.Paik J, et al. Vitamin A: overlapping delivery pathways to tissues from the circulation. The Journal of nutrition. 2004;134:276S–280S. doi: 10.1093/jn/134.1.276S. [DOI] [PubMed] [Google Scholar]
- 27.Blaner WS, et al. Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochimica et biophysica acta. 2009;1791:467–473. doi: 10.1016/j.bbalip.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voolstra O, et al. NinaB is essential for Drosophila vision but induces retinal degeneration in opsin-deficient photoreceptors. J Biol Chem. 2009;285:2130–2139. doi: 10.1074/jbc.M109.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fierce Y, et al. In vitro and in vivo characterization of retinoid synthesis from beta-carotene. Archives of biochemistry and biophysics. 2008;472:126–138. doi: 10.1016/j.abb.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moise AR, et al. Related enzymes solve evolutionarily recurrent problems in the metabolism of carotenoids. Trends Plant Sci. 2005;10:178–186. doi: 10.1016/j.tplants.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Yeum KJ, Russell RM. Carotenoid bioavailability and bioconversion. Annu Rev Nutr. 2002;22:483–504. doi: 10.1146/annurev.nutr.22.010402.102834. [DOI] [PubMed] [Google Scholar]
- 32.Baehr W, Frederick JM. Naturally occurring animal models with outer retina phenotypes. Vision research. 2009;49:2636–2652. doi: 10.1016/j.visres.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohrer B, et al. Cone Opsin Mislocalization in Rpe65-/- Mice: A Defect That Can Be Corrected by 11-cis Retinal. Investigative ophthalmology & visual science. 2005;46:3876–3882. doi: 10.1167/iovs.05-0533. [DOI] [PubMed] [Google Scholar]
- 34.Fan J, et al. Opsin activation of transduction in the rods of dark-reared Rpe65 knockout mice. J Physiol. 2005;568:83–95. doi: 10.1113/jphysiol.2005.091942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofmann KP, et al. The role of arrestin and retinoids in the regeneration pathway of rhodopsin. J Biol Chem. 1992;267:15701–15706. [PubMed] [Google Scholar]
- 36.Palczewski K, et al. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 1994;33:13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- 37.Jager S, et al. Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry. 1996;35:2901–2908. doi: 10.1021/bi9524068. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, et al. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci. 2008;28:4008–4014. doi: 10.1523/JNEUROSCI.0317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dryja TP. Doyne Lecture. Rhodopsin and autosomal dominant retinitis pigmentosa. Eye. 1992;6(Pt 1):1–10. doi: 10.1038/eye.1992.2. [DOI] [PubMed] [Google Scholar]
- 40.Rattner A, et al. Molecular genetics of human retinal disease. Annu Rev Genet. 1999;33:89–131. doi: 10.1146/annurev.genet.33.1.89. [DOI] [PubMed] [Google Scholar]
- 41.Weng J, et al. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 42.Perrault I, et al. Retinal-specific guanylate cyclase gene mutations in Leber's congenital amaurosis. Nature genetics. 1996;14:461–464. doi: 10.1038/ng1296-461. [DOI] [PubMed] [Google Scholar]
- 43.Dryja TP, et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- 44.Sokal I, et al. GCAP1 (Y99C) mutant is constitutively active in autosomal dominant cone dystrophy. Mol Cell. 1998;2:129–133. doi: 10.1016/s1097-2765(00)80121-5. [DOI] [PubMed] [Google Scholar]
- 45.Baehr W, et al. The retinoid cycle and retina disease. Vision research. 2003;43:2957–2958. doi: 10.1016/j.visres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Imanishi Y, et al. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. The Journal of cell biology. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imanishi Y, et al. Retinosomes: new insights into intracellular managing of hydrophobic substances in lipid bodies. The Journal of cell biology. 2004;166:447–453. doi: 10.1083/jcb.200405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Golczak M, et al. Lecithin:retinol acyltransferase is responsible for amidation of retinylamine, a potent inhibitor of the retinoid cycle. J Biol Chem. 2005;280:42263–42273. doi: 10.1074/jbc.M509351200. [DOI] [PubMed] [Google Scholar]
- 49.Fotiadis D, et al. The G protein-coupled receptor rhodopsin in the native membrane. FEBS letters. 2004;564:281–288. doi: 10.1016/S0014-5793(04)00194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fotiadis D, et al. Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature. 2003;421:127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- 51.Liang Y, et al. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J Biol Chem. 2003;278:21655–21662. doi: 10.1074/jbc.M302536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Govardovskii VI, et al. Lateral diffusion of rhodopsin in photoreceptor membrane: a reappraisal. Molecular vision. 2009;15:1717–1729. [PMC free article] [PubMed] [Google Scholar]
- 53.Corless JM, et al. Two-dimensional rhodopsin crystals from disk membranes of frog retinal rod outer segments. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:1116–1120. doi: 10.1073/pnas.79.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corless JM, et al. Three-dimensional membrane crystals in amphibian cone outer segments. 1. Light-dependent crystal formation in frog retinas. Journal of structural biology. 1994;113:64–86. doi: 10.1006/jsbi.1994.1033. [DOI] [PubMed] [Google Scholar]
- 55.Nickell S, et al. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. The Journal of cell biology. 2007;177:917–925. doi: 10.1083/jcb.200612010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maeda A, et al. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J Biol Chem. 2006;281:37697–37704. doi: 10.1074/jbc.M608375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wenzel A, et al. The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J Neurosci. 2001;21:53–58. doi: 10.1523/JNEUROSCI.21-01-00053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maeda A, et al. Retinopathy in Mice Induced by Disrupted All-trans-retinal Clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mata NL, et al. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim SR, et al. Characterization of dihydro-A2PE: an intermediate in the A2E biosynthetic pathway. Biochemistry. 2007;46:10122–10129. doi: 10.1021/bi7009635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fishkin NE, et al. Isolation and characterization of a retinal pigment epithelial cell fluorophore: an all-trans-retinal dimer conjugate. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7091–7096. doi: 10.1073/pnas.0501266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yannuzzi LA, et al. Ophthalmic fundus imaging: today and beyond. American journal of ophthalmology. 2004;137:511–524. doi: 10.1016/j.ajo.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 63.Finnemann SC, et al. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3842–3847. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim SR, et al. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Experimental eye research. 2006;82:828–839. doi: 10.1016/j.exer.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Kim SR, et al. Mechanisms involved in A2E oxidation. Experimental eye research. 2008;86:975–982. doi: 10.1016/j.exer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allikmets R. Further evidence for an association of ABCR alleles with age-related macular degeneration. The International ABCR Screening Consortium. American journal of human genetics. 2000;67:487–491. doi: 10.1086/303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allikmets R, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 68.Cremers FP, et al. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Human molecular genetics. 1998;7:355–362. doi: 10.1093/hmg/7.3.355. [DOI] [PubMed] [Google Scholar]
- 69.Martinez-Mir A, et al. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nature genetics. 1998;18:11–12. doi: 10.1038/ng0198-11. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, et al. Expression of Functional G Protein-Coupled Receptors in Photoreceptors of Transgenic Xenopus laevis. Biochemistry. 2005;44:14509–14518. doi: 10.1021/bi051386z. [DOI] [PubMed] [Google Scholar]
- 71.Parish CA, et al. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delori FC, et al. In vivo measurement of lipofuscin in Stargardt's disease--Fundus flavimaculatus. Investigative ophthalmology & visual science. 1995;36:2327–2331. [PubMed] [Google Scholar]
- 73.Maeda A, et al. Involvement of All-trans-retinal in Acute Light-induced Retinopathy of Mice. J Biol Chem. 2009;284:15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Radu RA, et al. Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt's macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5928–5933. doi: 10.1073/pnas.0308302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De S, Sakmar TP. Interaction of A2E with model membranes. Implications to the pathogenesis of age-related macular degeneration. The Journal of general physiology. 2002;120:147–157. doi: 10.1085/jgp.20028566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Allikmets R, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nature genetics. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 77.Rattner A, et al. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000;275:11034–11043. doi: 10.1074/jbc.275.15.11034. [DOI] [PubMed] [Google Scholar]
- 78.Chiu HJ, et al. Vitamin A depletion causes oxidative stress, mitochondrial dysfunction, and PARP-1-dependent energy deprivation. Faseb J. 2008;22:3878–3887. doi: 10.1096/fj.08-112375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O'Connell MJ, et al. Retro-retinoids in regulated cell growth and death. J Exp Med. 1996;184:549–555. doi: 10.1084/jem.184.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moise AR, et al. Metabolism and transactivation activity of 13,14-dihydroretinoic Acid. J Biol Chem. 2005;280:27815–27825. doi: 10.1074/jbc.M503520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moise AR, et al. Stereospecificity of retinol saturase: absolute configuration, synthesis, and biological evaluation of dihydroretinoids. Journal of the American Chemical Society. 2008;130:1154–1155. doi: 10.1021/ja710487q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ziouzenkova O, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nature medicine. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Acland GM, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Acland GM, et al. Gene therapy restores vision in a canine model of childhood blindness. Nature genetics. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 85.Bainbridge JW, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. The New England journal of medicine. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 86.Maguire AM, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. The New England journal of medicine. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cideciyan AV, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Imanishi Y, Palczewski K. Visualization of Retinoid Storage and Trafficking by Two-Photon Microscopy. Methods Cell Biol. 2010 doi: 10.1007/978-1-60327-325-1_14. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Imanishi Y, et al. Retinyl Ester Homeostasis in the Adipose Differentiation-related Protein-deficient Retina. J Biol Chem. 2008;283:25091–25102. doi: 10.1074/jbc.M802981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hanein S, et al. Leber congenital amaurosis: comprehensive survey of the genetic heterogeneity, refinement of the clinical definition, and genotype-phenotype correlations as a strategy for molecular diagnosis. Human mutation. 2004;23:306–317. doi: 10.1002/humu.20010. [DOI] [PubMed] [Google Scholar]
- 91.Van Hooser JP, et al. Recovery of visual functions in a mouse model of Leber congenital amaurosis. J Biol Chem. 2002;277:19173–19182. doi: 10.1074/jbc.M112384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fan J, et al. Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13662–13667. doi: 10.1073/pnas.2234461100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Batten ML, et al. Pharmacological and rAAV Gene Therapy Rescue of Visual Functions in a Blind Mouse Model of Leber Congenital Amaurosis. PLoS Med. 2005;2:e333. doi: 10.1371/journal.pmed.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Noorwez SM, et al. Pharmacological Chaperone-mediated in Vivo Folding and Stabilization of the P23H-opsin Mutant Associated with Autosomal Dominant Retinitis Pigmentosa. J Biol Chem. 2003;278:14442–14450. doi: 10.1074/jbc.M300087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ablonczy Z, et al. 11-cis-retinal reduces constitutive opsin phosphorylation and improves quantum catch in retinoid-deficient mouse rod photoreceptors. J Biol Chem. 2002;277:40491–40498. doi: 10.1074/jbc.M205507200. [DOI] [PubMed] [Google Scholar]
- 96.Jackson GR, et al. Aging and dark adaptation. Vision research. 1999;39:3975–3982. doi: 10.1016/s0042-6989(99)00092-9. [DOI] [PubMed] [Google Scholar]
- 97.Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Investigative ophthalmology & visual science. 1992;33:1–17. [PubMed] [Google Scholar]
- 98.Jackson GR, et al. Aging and scotopic sensitivity. Vision research. 1998;38:3655–3662. doi: 10.1016/s0042-6989(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 99.Berson EL, et al. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Archives of ophthalmology. 1993;111:761–772. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- 100.Li T, et al. Effect of vitamin A supplementation on rhodopsin mutants threonine-17 --> methionine and proline-347 --> serine in transgenic mice and in cell cultures. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:11933–11938. doi: 10.1073/pnas.95.20.11933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Radu RA, et al. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Investigative ophthalmology & visual science. 2008;49:3821–3829. doi: 10.1167/iovs.07-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Katz ML, Redmond TM. Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Investigative ophthalmology & visual science. 2001;42:3023–3030. [PubMed] [Google Scholar]
- 103.Jacobson SG, et al. Night blindness in Sorsby's fundus dystrophy reversed by vitamin A. Nature genetics. 1995;11:27–32. doi: 10.1038/ng0995-27. [DOI] [PubMed] [Google Scholar]
- 104.Schoeff L. Vitamin A. Am J Med Technol. 1983;49:447–452. [PubMed] [Google Scholar]
- 105.Birnbach CD, et al. Histopathology and immunocytochemistry of the neurosensory retina in fundus flavimaculatus. Ophthalmology. 1994;101:1211–1219. doi: 10.1016/s0161-6420(13)31725-4. [DOI] [PubMed] [Google Scholar]
- 106.De Laey JJ, Verougstraete C. Hyperlipofuscinosis and subretinal fibrosis in Stargardt's disease. Retina (Philadelphia, Pa. 1995;15:399–406. doi: 10.1097/00006982-199515050-00005. [DOI] [PubMed] [Google Scholar]
- 107.Lois N, et al. Fundus autofluorescence in patients with age-related macular degeneration and high risk of visual loss. American journal of ophthalmology. 2002;133:341–349. doi: 10.1016/s0002-9394(01)01404-0. [DOI] [PubMed] [Google Scholar]
- 108.Bindewald A, et al. Classification of fundus autofluorescence patterns in early age-related macular disease. Investigative ophthalmology & visual science. 2005;46:3309–3314. doi: 10.1167/iovs.04-0430. [DOI] [PubMed] [Google Scholar]
- 109.Delori FC, et al. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Investigative ophthalmology & visual science. 2001;42:1855–1866. [PubMed] [Google Scholar]
- 110.Wabbels B, et al. Fundus autofluorescence in children and teenagers with hereditary retinal diseases. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2006;244:36–45. doi: 10.1007/s00417-005-0043-2. [DOI] [PubMed] [Google Scholar]
- 111.Zhang K, et al. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nature genetics. 2001;27:89–93. doi: 10.1038/83817. [DOI] [PubMed] [Google Scholar]
- 112.Karan G, et al. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4164–4169. doi: 10.1073/pnas.0407698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Golczak M, et al. Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8162–8167. doi: 10.1073/pnas.0503318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maeda A, et al. Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Molecular pharmacology. 2006;70:1220–1229. doi: 10.1124/mol.106.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maeda A, et al. Aberrant metabolites in mouse models of congenital blinding diseases: formation and storage of retinyl esters. Biochemistry. 2006;45:4210–4219. doi: 10.1021/bi052382x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tu DC, et al. Inner retinal photoreception independent of the visual retinoid cycle. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10426–10431. doi: 10.1073/pnas.0600917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Golczak M, et al. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J Biol Chem. 2008;283:9543–9554. doi: 10.1074/jbc.M708982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maeda T, et al. Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug, 9-cis-retinyl acetate. Human molecular genetics. 2009;18:2277–2287. doi: 10.1093/hmg/ddp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Radu RA, et al. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Investigative ophthalmology & visual science. 2005;46:4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- 120.Radu RA, et al. Isotretinoin treatment inhibits lipofuscin accumulation in a mouse model of recessive Stargardt's macular degeneration. Novartis Found Symp. 2004;255:51–63. discussion 63-57, 177-178. [PubMed] [Google Scholar]
- 121.Radu RA, et al. Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt's macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4742–4747. doi: 10.1073/pnas.0737855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Law WC, Rando RR. The molecular basis of retinoic acid induced night blindness. Biochem Biophys Res Commun. 1989;161:825–829. doi: 10.1016/0006-291x(89)92674-0. [DOI] [PubMed] [Google Scholar]
- 123.Znoiko SL, et al. Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the rpe65-/- mouse at early ages. Investigative ophthalmology & visual science. 2005;46:1473–1479. doi: 10.1167/iovs.04-0653. [DOI] [PubMed] [Google Scholar]
- 124.Fan J, et al. Rpe65-/- and Lrat-/- mice: comparable models of leber congenital amaurosis. Investigative ophthalmology & visual science. 2008;49:2384–2389. doi: 10.1167/iovs.08-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rodieck RW. The first steps in seeing. Sinauer Associates; 1998. [Google Scholar]
- 126.Jacobson SG, et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: Prerequisite for human gene therapy success. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wing GL, et al. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Investigative ophthalmology & visual science. 1978;17:601–607. [PubMed] [Google Scholar]
- 128.Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nature reviews. 2006;7:860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 129.Sparrow JR, et al. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Investigative ophthalmology & visual science. 1999;40:2988–2995. [PubMed] [Google Scholar]
- 130.Eldred GE. Age pigment structure. Nature. 1993;364:396. doi: 10.1038/364396a0. [DOI] [PubMed] [Google Scholar]
- 131.Yasukawa T, et al. Glycoxidized particles mimic lipofuscin accumulation in aging eyes: a new age-related macular degeneration model in rabbits. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2007;245:1475–1485. doi: 10.1007/s00417-007-0571-z. [DOI] [PubMed] [Google Scholar]
- 132.Vives-Bauza C, et al. The age lipid A2E and mitochondrial dysfunction synergistically impair phagocytosis by retinal pigment epithelial cells. J Biol Chem. 2008;283:24770–24780. doi: 10.1074/jbc.M800706200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sparrow JR, et al. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Investigative ophthalmology & visual science. 2000;41:1981–1989. [PubMed] [Google Scholar]