Abstract
Bronchopulmonary C-fibers and acid-sensitive, capsaicin-insensitive mechanoreceptors innervating the larynx, trachea and large bronchi regulate the cough reflex. These vagal afferent nerves may interact centrally with sensory input arising from afferent nerves innervating the intrapulmonary airways or even extrapulmonary afferents such as those innervating the nasal mucosa and esophagus to produce chronic cough or enhanced cough responsiveness. The mechanisms of cough initiation in health and in disease are briefly described.
Keywords: capsaicin, vagal, bradykinin, NMDA
The cough reflex protects the airways and lungs from aspiration, inhaled irritants, particulates and pathogens and clears the air spaces of accumulated secretions. Studies in animals provide conclusive evidence that cough is initiated by activation of vagal afferent nerves. Precisely which afferent nerve subtypes regulate cough has been debated and reviewed elsewhere [1]. In this review, we describe the stimuli that initiate cough and the mechanisms by which these stimuli activate airway sensory nerves. These data are related to the known physiological properties of bronchopulmonary afferent nerve subtypes. The review concludes with a discussion of the potential interactions between afferent nerve subtypes and the possible mechanisms of altered cough reflexes in disease.
Chemical and Mechanical Stimuli That Initiate Coughing
Multiple chemical and mechanical stimuli have been shown to initiate coughing in human subjects and in animals [1-4]. Several of these stimuli, including capsaicin, citric acid, hypertonic saline and low chloride buffers/ solutions, are often used to evoke cough experimentally. Other stimuli known to initiate coughing in animals and in human subjects include particulate/ dust, mechanical/ vibratory stimulation of the airway mucosa larynx or chest wall, chemical irritants such as resiniferetoxin, cinnamaldehyde and allylisothiocyanate (AITC), and the autacoids bradykinin, anandamide and prostaglandin E2 (PGE2). Although seemingly varied in origin and chemical composition, many of these stimuli share common modes of action. For example, acids/ protons, capsaicin, resiniferetoxin and anandamide all act in part or entirely by activation of the ion channel/ receptor TRPV1 [3, 5-12]. Bradykinin and PGE2 may also act in part through TRPV1 activation [13-16]. Cinnamaldehyde, AITC, and several other known respiratory irritants (e.g. cigarette smoke, toluene diisocyanate) activate the ion channel TRPA1 [17-19]. Knowing the specific ion channels and receptors for the stimuli that initiate cough is important, as this information can be used to identify which afferent nerves express these ion channels and receptors. This information can then be used to identify possible mechanisms for coughing in disease but may also suggest therapeutic approaches to restore normal cough function and sensitivity (Table 1).
Table 1.
Stimuli Initiating Cough: Mode of Action and Afferent Nerve Targets.
| Stimulus | Mode of Action | Afferent nerves targeted |
|---|---|---|
| Capsaicin | TRPV1 | C-fibers |
| Bradykinin | Bradykinin B2 receptors | C-fibers |
| Acid | TRPV1, Acid-Sensing Ion Channels | C-fibers, cough receptors |
| Particulates | Unknown | Cough receptors, C-fibers |
| TRPA1 agonists | TRPA1 | C-fibers |
| Prostaglandin E2 | Prostaglandin EP3 receptors | C-fibers |
| Nicotine | Nicotinic Receptors | C-fibers |
Abbreviations: TRPV1: transient receptor potential vanilloid 1; TRPA1: transient receptor potential ankyrin 1
The stimuli that do not initiate coughing are equally helpful when attempting to identify the specific vagal afferent nerve subtypes that regulate this reflex (Table 2). These stimuli may also suggest a great complexity underlying the cough associated with disease. Consider, for example, three of the most common causes of chronic cough:
Asthma
Gastroesophageal reflux disease (GERD)
Upper airway inflammatory diseases (e.g. allergic rhinitis, sinusitis)
Table 2.
Stimuli that do not initiate cough: Reflexes evoked, afferent nerves activated.
| Stimulus | Reflexes evoked | Afferent nerves targeted |
|---|---|---|
| Bronchoconstriction (1) | mucus secretion, tachypnea | RARs |
| Esophageal acid | bronchospasm, mucus secretion | Esophageal nociceptors |
| Upper airway stimulation | Sneeze, mucus secretion | Trigeminal afferent nerves |
| Inc. airway luminal pressure | Respiratory slowing | SARs |
| Dec. airway luminal pressure | Tachypnea | RARs |
| Adenosine | Tachypnea, dyspnea | RARs, C-fibers |
| Pulmonary embolism | Tachypnea, dyspnea | RARs, C-fibers |
Bronchoconstrictors including histamine, methacholine, leukotriene D4, thromboxane A2 and neurokinin A fail to reliably evoke coughing in humans or in animals despite initiating reflex bronchospasm and mucus secretion, an increase in respiratory rate and dyspnea. Abbreviations: Inc.: Increase; Dec.: Decrease; RARs: Rapidly adapting receptors; SARs: slowly adapting receptors
These conditions are often found simultaneously in patients with chronic cough [20, 21]. While none of these disorders are adequately described by a single presenting symptom, it is generally agreed that reversible airways obstruction, acidic refluxate in the esophagus and inflammation of the upper airways are characteristics of asthma, GERD and upper airway diseases, respectively. Remarkably, however, bronchospasm, acid in the esophagus and upper airway challenges with a variety of inflammatory mediators are consistently ineffective at initiating cough in animals or human subjects [1, 22-24]. The inability of these stimuli to initiate cough is not because they fail to activate sensory nerves. Histamine, capsaicin, allergen and bradykinin all initiate sneezing and reflex-dependent mucus secretion when delivered selectively to the upper airways, while the bronchoconstrictors histamine, substance P and even methacholine evoke reflex dependent changes in autonomic tone in the airways, changes in breathing pattern and respiratory sensations such as chest tightness and dyspnea [1, 22, 25, 26]. Esophageal acidification is known to evoke reflex bronchospsam in animals and in human subjects but has rarely if ever been reported to initiate coughing [26-31]. Observations such as these argue against the afferent nerves responding to these stimuli as the primary initiators of cough in disease. This implies the involvement of other afferent nerves and afferent nerve subtype interactions in disease. Defining precisely how these afferent nerves interact to produce the coughing observed in diseases such as asthma and GERD are likely be critical to the development of better therapeutic strategies for the treatment of chronic cough.
Afferent nerves regulating the cough reflex
Coughing can be partitioned into at least four phases.
The initial phase comprises the encoding of action potentials by the afferent nerves directly responding to the tussive stimulus, and the subsequent reconfiguration of the respiratory motor drive within the brainstem. This initial phase immediately precedes any change in respiratory muscle activity.
The second phase is the enhanced inspiratory effort that accompanies cough.
The expiratory phase of cough has 2 components:
an initial compressive phase when expiration is initiated against a restricted or occluded upper airway
the expulsive phase, when the upper airways are dilated, allowing forceful expiration and the high airflow velocities that facilitate airway clearance
Depending on the stimulus and social situation, the number and forcefulness of the resulting coughs can vary substantially. Given this complexity and the multiple elements involved (lower and upper airways, respiratory muscles, brain stem), it is apparent that multiple afferent nerve subtypes act in concert to regulate the sensitivity, forcefulness and repetitions of coughs in response to all tussive stimuli. The discussion below focuses only on those afferents involved in the initial encoding phase of cough (Figure 1).
Figure 1.
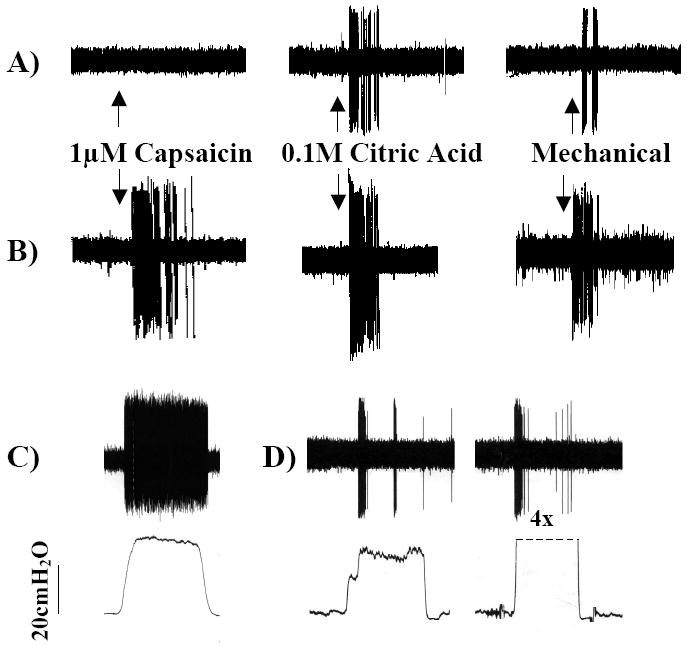
Representative extracellular recordings from the vagal afferent nerve subtypes innervating the airways and lungs. A) The cough receptors innervate the larynx, trachea and mainstem bronchi. They are insensitive to capsaicin, airway smooth muscle contraction (not shown) and distending or collapsing airway luminal pressures (not shown) but are activated by punctate mechanical stimulation and acid. When activated, these afferent nerves initiate coughing. B) Bronchopulmonary C-fibers terminate throughout the airways and lungs. C-fibers are less sensitive to mechanical stimulation than other airway afferent nerves but are activated by chemical stimuli such as capsaicin, bradykinin, acid and adenosine. When activated, C-fibers initiate coughing, changes in respiratory pattern and autonomic reflexes (e.g. airway smooth muscle contraction, mucus secretion. C-fiber subtypes have been described. C) Slowly adapting receptors are active during the dynamic and static phases of lung inflation. Slowly adapting receptor activation initiates respiratory slowing but does not initiate coughing. D) Rapidly adapting receptors are active during the dynamic phases of lung inflation and deflation and are activated by airway smooth muscle contraction/ bronchospasm and lung collapse/ negative airway luminal pressures. Rapidly adapting receptor activation initiates parasympathetic reflexes such as mucus secretion and airway smooth muscle contraction as well as tachypnea.
Figures are reproduced with permission from Canning et al., 2004 (49). Canning BJ, Mazzone SB, Meeker SN et al. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 2004;557:543-58.
C-fibers
Bronchopulmonary C-fibers are identified physiologically by their action potential conduction velocity, which falls in the “C” range (≤1 m/ sec) of the compound action potential. C-fibers comprise the majority of afferent nerves innervating the airways and lungs, terminating in the mucosa and submucosa of the nose, pharynx, larynx, trachea, bronchi and throughout the lungs. C-fibers project to the airways from the jugular and nodose ganglia of the vagus nerves, and from thoracic dorsal root ganglia [1, 32-34].
Bronchopulmonary C-fibers are activated by capsaicin, bradykinin (Figure 2), protons, nicotine and the TRPA1 agonists cinnamaldehyde and AITC. C-fibers are selectively desensitized by high dose capsaicin treatment, which also prevents coughing evoked by citric acid [35]. In guinea pigs, C-fibers utilize the peptide neurotransmitters substance P and neurokinin A, which act primarily via neurokinin1 (NK1), NK2 and NK3 receptors. Neurokinin receptor antagonists prevent coughing evoked in guinea pigs by capsaicin and citric acid [36-39]. These results and observations provide conclusive evidence that C-fiber activation initiates coughing.
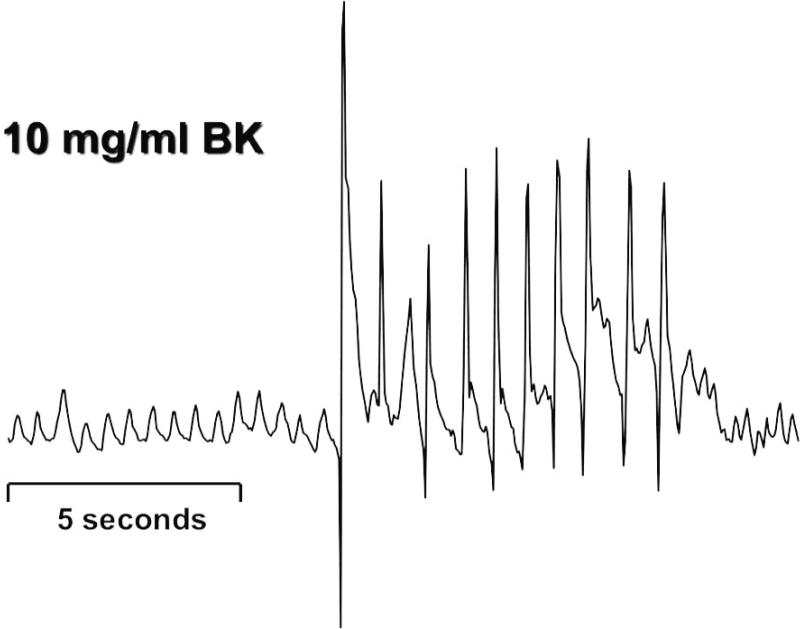
Figure 2.
A representative trace of coughing recorded from a guinea pig following exposure to an aerosol of bradykinin aerosol is shown. Many animal species cough in response to the same stimuli that initiate coughing in human subjects. This permits more mechanistic studies of the cough reflex and has led to the development of novel therapeutic strategies for the treatment of cough.
The figure is reproduced with permission from Canning et al. (49). Canning BJ, Mazzone SB, Meeker SN et al. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 2004;557:543-58.
Despite the overwhelming evidence that C-fiber activation can initiate coughing, the role of C-fibers in cough remains controversial. Much of the controversy arises from the inability of C-fiber selective stimuli to initiate coughing in anesthetized animals [1, 22, 26, 40, 41]. It seems likely that anesthesia selectively inhibits C-fiber dependent coughing. But anesthesia does not readily explain the observation that C-fiber activation not only fails to initiate coughing in anesthetized animals, but also actively inhibits coughing evoked by activation of other afferent nerves subtypes [40, 41]. We speculated that these opposing effects of C-fibers on cough might be attributable to the opposing actions of C-fiber subtypes. Studies carried out in guinea pigs using stimuli that are selective for vagal C-fiber subtypes suggest that C-fibers arising from the nodose ganglia can acutely inhibit coughing while activation of C-fibers arising from the jugular ganglia sensitize or initiate coughing [42, 43]. Circumstantial evidence suggests that C-fiber subtypes in humans play similar opposing roles [2, 44-46].
Rapidly Adapting Receptors
Rapidly adapting receptors (RARs) are mechanoreceptors that respond to the dynamic physical forces associated with lung inflation and deflation. RARs are also activated by punctate mechanical stimuli and airway smooth muscle contraction [32, 47-49]. The seminal work of Widdicombe has been interpreted as evidence that RARs play an essential role in the initiation of cough [47, 50]. A critical reassessment of Widdicombe’s studies suggests otherwise [51].
Central to the thesis that RARs play a role in the initiation of cough in anesthetized animals has been the inability of C-fiber selective stimuli to initiate cough, and the sensitivity of the cough reflex to vagal cooling temperatures that target myelinated afferent nerves such as RARs [41, 47, 50]. Slowly adapting receptors (SARs), the stretch receptors that regulate the Hering Breuer reflex, are not implicated in the initiation of cough [1, 47, 50, 51], and with C-fiber-selective stimuli failing to evoke cough and nearly all airway sensory nerve classification schemes limited to 3 types (C-fibers, RARs and SARs), RARs have been implicated largely by default [1, 32, 48-51]. But there is overwhelming evidence against a role for RARs in cough. Hyperventilation and maximal inspiratory efforts against a closed glottis, for example, are very effective at activating RARs and consistently ineffective at initiating cough [1, 51]. Bronchoconstrictors such as histamine, neurokinin A, the cysteinyl-leukotrienes and even methacholine are also effective stimulants of RARs but rarely if ever initiate coughing [1, 22, 49, 51, 52].
The imprecise semantics often used in describing airway afferent nerve subtypes likely contributes to the misconception that RARs regulate coughing. The term RAR is used to describe a variety of afferent nerve subtypes that differ substantially in peripheral termination sites, sensitivity to mechanical stimuli and in the reflexes initiated upon their activation. The term ‘rapidly adapting’, when used as a means of differentiating airway afferent nerve subtypes, should refer only to the response of afferent nerves to sustained lung inflation. Indeed, RARs adapt only modestly to smooth muscle contraction/ bronchoconstriction or lung deflation [1, 51]. By contrast, most bronchopulmonary afferent nerves adapt rapidly to punctate mechanical stimuli. Studies in guinea pigs and a reappraisal of Widdicombe’s work carried out in cats suggests that vagal afferent nerves that are distinct from RARs but possessing RAR-like characteristics likely regulate the coughing studied in anesthetized animals [49, 51].
Cough Receptors
Coughing can be initiated in anesthetized animals by mechanically probing the laryngeal, tracheal or bronchial mucosa or by acid applied topically to the mucosa of these airways [22, 36, 49-52]. C-fiber selective stimuli (e.g. capsaicin and bradykinin) consistently fail to initiate coughing, as does sustained lung inflation (which activates SARs) or the bronchoconstrictors histamine, neurokinin A and methacholine. Sustained or dynamic increases or decreases in intraluminal pressure in an isolated segment of the extrathoracic trachea also fails to initiate coughing, while acid applied topically to this tracheal segment readily initiates cough. Electrophysiological analyses combined with these physiological studies of cough suggest that a vagal afferent nerve subtype distinct from RARs, SARs and C-fibers plays an essential role in the initiation of cough [22, 26, 49, 51].
The “cough receptors” are myelinated as evidenced by their action potential conduction velocity (~5 m/ sec) and terminate almost exclusively in the larynx, trachea and extrapulmonary bronchi. These afferents are insensitive to airway smooth muscle contraction or changes in intraluminal pressure, but are exquisitely sensitive to punctate mechanical stimuli. The cough receptors are also activated by acid and by the voltage-gated K+ channel blocker 4-aminopyridine [22, 49].
Cough receptors terminate peripherally in the submucosa of the laryngeal, tracheal and bronchial mucosa [22, 49, 53, 54]. The terminations of these afferents nerves branch extensively in a circumferential arbor, adhered the subepithelial matrix and largely uncoupled to the underlying smooth muscle. Their structure and sites of termination give the appearance of a spider adhered to its web, sensing mechanical stimuli transduced through the intricate structure of the extracellular matrix. Their sites of termination and sensitivity to acid and to punctate mechanical stimuli render the cough receptors ideally suited to protect the airways from aspiration and to facilitate clearance of accumulated secretions.
Physiological and pharmacological studies have identified several mechanisms regulating cough receptor excitability [22, 49, 54, 55]. The cough receptors are insensitive to capsaicin and bradykinin and not surprisingly, therefore, do not express the capsaicin receptor TRPV1. Acid activates the cough receptors, perhaps through gating of Acid-Sensing Ion Channels (ASICs). Other regulatory mechanisms identified on the peripheral terminals of the cough receptors include unique isozymes of Na+-K+-ATPase and the Na+-K+-2Cl- transporter as well as voltage-gated sodium and chloride channels. Centrally, the cough receptors utilize glutamate acting via NMDA and nonNMDA receptors to initiate coughing [1, 56].
Afferent Nerve Interactions in Cough and Mechanisms of Cough in Disease
There is considerable evidence for vagal afferent nerve convergence onto subpopulations of relay neurons in the nucleus of the solitary tract (nTS). Such convergence may account for the imprecise nature of visceral reflexes, whereby vagal afferent nerve activation in one organ reflexively initiates changes in autonomic outflow to other organs [57-59]. Similar interactions likely regulate the cough reflex and may explain the extrapulmonary origins of cough in some patients.
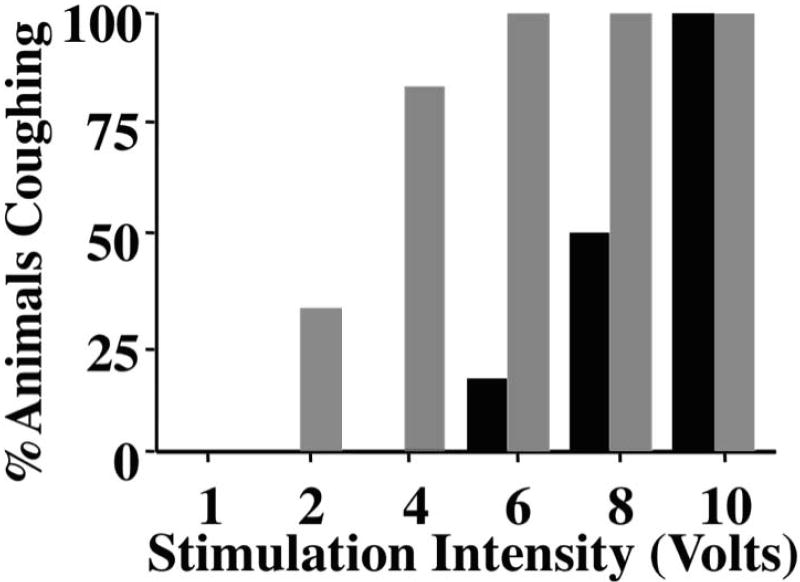
Studies carried out in guinea pigs suggest that bronchopulmonary C-fibers and cough receptors may act synergistically to regulate coughing [43]. As discussed previously, C-fiber activation is consistently ineffective at initiating cough in anesthetized animals. But C-fiber activation coincident with cough receptor stimulation produces a heightened sensitivity to tussive challenge (Figure 3). This sensitizing effect of C-fiber activation is similar to the central sensitization attributed to somatic C-fibers in models of pain [60]. Like central sensitization in somatic tissues, neurokinin receptor antagonists prevent the sensitizing effects of bronchopulmonary C-fiber activation in cough.
Figure 3.
Cough reflex sensitivity can be enhanced by coincident activation of airway afferent nerve subtypes. Coughing was evoked electrically from the tracheal mucosa of anesthetized guinea pigs. Optimal stimulation frequencies (16 Hz) and pulse duration were maintained during 10 second stimuli delivered at varying stimulation voltages. The percentage of animals coughing at various voltages was determined in animals inhaling saline (black bars) or the C-fiber stimulant bradykinin (1mg/ mL; grey bars).
This graph is reproduced with permission from Mazzone et al. (43).
Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol 2005;569:559-73.
Vagal afferent nerve interactions may account for the coughing attributed to gastroesophageal reflux disease. Refluxate or acid in the esophagus are very ineffective at initiating cough in animals or in human subjects and refluxate rarely reaches the airways or even the pharynx. But acid or capsaicin infusion into the esophageal lumen markedly enhances airway sensitivity to tussive stimuli [61, 62]. This sensitizing effect of acid or capsaicin in the esophagus on subsequently evoked cough suggests that in patients presenting with GERD cough, in addition to the sensitizing refluxate in the esophagus, some tussive stimuli or condition within the airways ultimately initiates coughing. Evidence for airway inflammation in GERD has been presented [63-66]. A similar sensitizing effect on cough may also account for the coughing associated with upper airways diseases [67].
Synopsis
The cough reflex is initiated by activation of bronchopulmonary C-fibers and the mechanically-sensitive cough receptors. Stimuli initiating cough through activation of one or both of these vagal afferent nerves include capsaicin, acid, bradykinin, cinnamaldehyde, cigarette smoke and non-isotonic aerosols. Multiple ion channels and cell surface receptors regulating the response to these tussive stimuli have been identified. The cough receptors and C-fibers may interact centrally to produce a heightened sensitivity to challenge. Interactions of afferent pathways innervating the esophagus and upper airways may contribute to the heightened cough sensitivity in chronic diseases such as GERD, asthma and upper airways disorders.
Acknowledgments
Funding for the authors research is provided by a grant from the National Institutes of Health (HL083192)
Footnotes
Financial disclosure: The author’s research is funded by grants from the National Institutes of Health as well as by grants from Eisai, Glaxo-Smithkline, Merck and Sanofi-Aventis. The author has no other financial interests relevant to the contents of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol. 2006;152(3):223–42. doi: 10.1016/j.resp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Karlsson JA, Fuller RW. Pharmacological regulation of the cough reflex--from experimental models to antitussive effects in Man. Pulm Pharmacol Ther. 1999;12:215–228. doi: 10.1006/pupt.1999.0207. [DOI] [PubMed] [Google Scholar]
- 3.Laude EA, Higgins KS, Morice AH. A comparative study of the effects of citric acid, capsaicin and resiniferatoxin on the cough challenge in guinea-pig and man. Pulm Pharmacol. 1993;6:171–175. doi: 10.1006/pulp.1993.1023. [DOI] [PubMed] [Google Scholar]
- 4.Maher SA, Belvisi MG. Prostanoids and the Cough Reflex. Lung. 2009 doi: 10.1007/s00408-009-9190-2. In press. [DOI] [PubMed] [Google Scholar]
- 5.Bolser DC, Aziz SM, Chapman RW. Ruthenium red decreases capsaicin and citric acid-induced cough in guinea pigs. Neurosci Lett. 1991;126:131–133. doi: 10.1016/0304-3940(91)90536-3. [DOI] [PubMed] [Google Scholar]
- 6.Jia Y, McLeod RL, Wang X, et al. Anandamide induces cough in conscious guinea-pigs through VR1 receptors. Br J Pharmacol. 2002;137:831–836. doi: 10.1038/sj.bjp.0704950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu Q, Lee LY. Characterization of acid signaling in rat vagal pulmonary sensory neurons. Am J Physiol Regul Integr Comp Physiol. 2006;291(1):L58–L65. doi: 10.1152/ajplung.00517.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol. 2002;543:591–600. doi: 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalloo UG, Fox AJ, Belvisi MG, et al. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol. 1995;79:1082–1087. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]
- 10.Leung SY, Niimi A, Williams AS, et al. Inhibition of citric acid- and capsaicin-induced cough by novel TRPV-1 antagonist, V112220, in guinea-pig. Cough. 2007;3:10. doi: 10.1186/1745-9974-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin YS, Lee LY. Stimulation of pulmonary vagal C-fibres by anandamide in anaesthetized rats: role of vanilloid type 1 receptors. J Physiol. 2002;539(Pt 3):947–55. doi: 10.1113/jphysiol.2001.013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trevisani M, Milan A, Gatti R, et al. Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax. 2004;59:769–72. doi: 10.1136/thx.2003.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr MJ, Kollarik M, Meeker SN, Undem BJ. A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther. 2003;304(3):1275–9. doi: 10.1124/jpet.102.043422. [DOI] [PubMed] [Google Scholar]
- 14.Kollarik M, Undem BJ. Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild-type and TRPV1-/- mice. J Physiol. 2004;555(Pt 1):115–23. doi: 10.1113/jphysiol.2003.054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnizler K, Shutov LP, Van Kanegan MJ, et al. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci. 2008;28(19):4904–17. doi: 10.1523/JNEUROSCI.0233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho CY, Gu Q, Hong JL, Lee LY. Prostaglandin E(2) enhances chemical and mechanical sensitivities of pulmonary C fibers in the rat. Am J Respir Crit Care Med. 2000;162(2 Pt 1):528–33. doi: 10.1164/ajrccm.162.2.9910059. [DOI] [PubMed] [Google Scholar]
- 17.Andrè E, Gatti R, Trevisani M, et al. Transient receptor potential ankyrin receptor 1 is a novel target for pro-tussive agents. Br J Pharmacol. 2009 doi: 10.1111/j.1476-5381.2009.00438.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birrell MA, Belvisi MG, Grace M, et al. TRPA1 Agonists Evoke Coughing in Guinea-pig and Human Volunteers. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200905-0665OC. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor-Clark TE, Nassenstein C, McAlexander MA, Undem BJ. TRPA1: a potential target for anti-tussive therapy. Pulm Pharmacol Ther. 2009;22(2):71–4. doi: 10.1016/j.pupt.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Irwin RS, Baumann MH, Bolser DC, et al. American College of Chest Physicians (ACCP). Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):1S–23S. doi: 10.1378/chest.129.1_suppl.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morice AH, Fontana GA, Sovijarvi AR, et al. ERS Task Force. The diagnosis and management of chronic cough. Eur Respir J. 2004;24(3):481–92. doi: 10.1183/09031936.04.00027804. [DOI] [PubMed] [Google Scholar]
- 22.Canning BJ, Farmer DG, Mori N. Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2006;291:R454–63. doi: 10.1152/ajpregu.00862.2005. [DOI] [PubMed] [Google Scholar]
- 23.Ing AJ, Ngu MC, Breslin AB. Pathogenesis of chronic persistent cough associated with gastroesophageal reflux. Am J Respir Crit Care Med. 1994;149(1):160–7. doi: 10.1164/ajrccm.149.1.8111576. [DOI] [PubMed] [Google Scholar]
- 24.Irwin RS, French CL, Curley FJ, et al. Chronic cough due to gastroesophageal reflux. Clinical, diagnostic, and pathogenetic aspects. Chest. 1993;104(5):1511–7. doi: 10.1378/chest.104.5.1511. [DOI] [PubMed] [Google Scholar]
- 25.Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol. 2006;101(3):971–85. doi: 10.1152/japplphysiol.00313.2006. [DOI] [PubMed] [Google Scholar]
- 26.Chou YL, Scarupa MD, Mori N, Canning BJ. Differential effects of airway afferent nerve subtypes on cough and respiration in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1572–84. doi: 10.1152/ajpregu.90382.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irwin RS, French CL, Curley FJ, et al. Chronic cough due to gastroesophageal reflux. Clinical, diagnostic, and pathogenetic aspects. Chest. 1993;104(5):1511–7. doi: 10.1378/chest.104.5.1511. [DOI] [PubMed] [Google Scholar]
- 28.Mansfield LE, Stein MR. Gastroesophageal reflux and asthma: a possible reflex mechanism. Ann Allergy. 1978;41(4):224–6. [PubMed] [Google Scholar]
- 29.Schan CA, Harding SM, Haile JM, et al. Gastroesophageal reflux-induced bronchoconstriction. An intraesophageal acid infusion study using state-of-the-art technology. Chest. 1994;106(3):731–7. doi: 10.1378/chest.106.3.731. [DOI] [PubMed] [Google Scholar]
- 30.Mazzone SB, Canning BJ. Evidence for differential reflex regulation of cholinergic and noncholinergic parasympathetic nerves innervating the airways. Am J Respir Crit Care Med. 2002;165(8):1076–83. doi: 10.1164/ajrccm.165.8.2001121270c. [DOI] [PubMed] [Google Scholar]
- 31.Spaulding HS, Jr, Mansfield LE, Stein MR, et al. Further investigation of the association between gastroesophageal reflux and bronchoconstriction. J Allergy Clin Immunol. 1982;69(6):516–21. doi: 10.1016/0091-6749(82)90176-2. [DOI] [PubMed] [Google Scholar]
- 32.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- 33.Kollarik M, Dinh QT, Fischer A, Undem BJ. Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol. 2003;551:869–879. doi: 10.1113/jphysiol.2003.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Undem BJ, Chuaychoo B, Lee MG, et al. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–17. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forsberg K, Karlsson JA, Theodorsson E, et al. Cough and bronchoconstriction mediated by capsaicin-sensitive sensory neurons in the guinea-pig. Pulm Pharmacol. 1988;1:33–9. doi: 10.1016/0952-0600(88)90008-7. [DOI] [PubMed] [Google Scholar]
- 36.Bolser DC, DeGennaro FC, O’Reilly S, et al. Central antitussive activity of the NK1 and NK2 tachykinin receptor antagonists, CP-99,994 and SR 48968, in the guinea-pig and cat. Br J Pharmacol. 1997;121:165–70. doi: 10.1038/sj.bjp.0701111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daoui S, Cognon C, Naline E, et al. Involvement of tachykinin NK3 receptors in citric acid-induced cough and bronchial responses in guinea pigs. Am J Respir Crit Care Med. 1998;158:42–8. doi: 10.1164/ajrccm.158.1.9705052. [DOI] [PubMed] [Google Scholar]
- 38.Girard V, Naline E, Vilain P, et al. Effect of the two tachykinin antagonists, SR 48968 and SR 140333, on cough induced by citric acid in the unanaesthetized guinea pig. Eur Respir J. 1995;8:1110–4. doi: 10.1183/09031936.95.08071110. [DOI] [PubMed] [Google Scholar]
- 39.Ujiie Y, Sekizawa K, Aikawa T, Sasaki H. Evidence for substance P as an endogenous substance causing cough in guinea pigs. Am Rev Respir Dis. 1993;148(6 Pt 1):1628–32. doi: 10.1164/ajrccm/148.6_Pt_1.1628. [DOI] [PubMed] [Google Scholar]
- 40.Tatar M, Webber SE, Widdicombe JG. Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol. 1988;402:411–20. doi: 10.1113/jphysiol.1988.sp017212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tatar M, Sant’Ambrogio G, Sant’Ambrogio FB. Laryngeal and tracheobronchial cough in anesthetized dogs. J Appl Physiol. 1994;76:2672–79. doi: 10.1152/jappl.1994.76.6.2672. [DOI] [PubMed] [Google Scholar]
- 42.Canning BJ, Chou YL. Cough sensors. I. Physiological and pharmacological properties of the afferent nerves regulating cough. Handb Exp Pharmacol. 2009;187:23–47. doi: 10.1007/978-3-540-79842-2_2. [DOI] [PubMed] [Google Scholar]
- 43.Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol. 2005;569:559–73. doi: 10.1113/jphysiol.2005.093153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stone RA, Worsdell YM, Fuller RW, Barnes PJ. Effects of 5-hydroxytryptamine and 5-hydroxytryptophan infusion on the human cough reflex. J Appl Physiol. 1993;74:396–401. doi: 10.1152/jappl.1993.74.1.396. [DOI] [PubMed] [Google Scholar]
- 45.Winning AJ, Hamilton RD, Shea SA, Guz A. Respiratory and cardiovascular effects of central and peripheral intravenous injections of capsaicin in man: evidence for pulmonary chemosensitivity. Clin Sci (Lond) 1986;71(5):519–26. doi: 10.1042/cs0710519. [DOI] [PubMed] [Google Scholar]
- 46.Burki NK, Dale WJ, Lee LY. Intravenous adenosine and dyspnea in humans. J Appl Physiol. 2005;98:180–5. doi: 10.1152/japplphysiol.00913.2004. [DOI] [PubMed] [Google Scholar]
- 47.Widdicombe JG. Receptors in the trachea and bronchi of the cat. J Physiol. 1954;123:71–104. doi: 10.1113/jphysiol.1954.sp005034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol. 2001;127:113–24. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- 49.Canning BJ, Mazzone SB, Meeker SN, et al. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557:543–58. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Widdicombe JG. Respiratory reflexes from the trachea and bronchi of the cat. J Physiol. 1954;123:55–70. doi: 10.1113/jphysiol.1954.sp005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canning BJ, Chou YL. Cough sensors. I. Physiological and pharmacological properties of the afferent nerves regulating cough. Handb Exp Pharmacol. 2009;187:23–47. doi: 10.1007/978-3-540-79842-2_2. [DOI] [PubMed] [Google Scholar]
- 52.House A, Celly C, Skeans S, et al. Cough reflex in allergic dogs. Eur J Pharmacol. 2004;492:251–8. doi: 10.1016/j.ejphar.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 53.Hunter DD, Undem BJ. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med. 1999;159:1943–8. doi: 10.1164/ajrccm.159.6.9808078. [DOI] [PubMed] [Google Scholar]
- 54.Mazzone SB, Reynolds SR, Mori N, et al. Selective expression of a sodium pump isozyme by cough receptors and evidence for its essential role in regulating cough. J Neurosci. 2009;29(43):13662–71. doi: 10.1523/JNEUROSCI.4354-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazzone SB, McGovern AE. Na+-K+-2Cl- cotransporters and Cl- channels regulate citric acid cough in guinea pigs. J Appl Physiol. 2006;101(2):635–43. doi: 10.1152/japplphysiol.00106.2006. [DOI] [PubMed] [Google Scholar]
- 56.Canning BJ. Central regulation of the cough reflex: therapeutic implications. Pulm Pharmacol Ther. 2009;22(2):75–81. doi: 10.1016/j.pupt.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paton JF. Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J Neurophysiol. 1998;79(5):2365–73. doi: 10.1152/jn.1998.79.5.2365. [DOI] [PubMed] [Google Scholar]
- 58.Silva-Carvalho L, Paton JF, Rocha I, et al. Convergence properties of solitary tract neurons responsive to cardiac receptor stimulation in the anesthetized cat. J Neurophysiol. 1998;79(5):2374–82. doi: 10.1152/jn.1998.79.5.2374. [DOI] [PubMed] [Google Scholar]
- 59.Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol. 2005;569:559–73. doi: 10.1113/jphysiol.2005.093153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Javorkova N, Varechova S, Pecova R, et al. Acidification of the oesophagus acutely increases the cough sensitivity in patients with gastro-oesophageal reflux and chronic cough. Neurogastroenterol Motil. 2008;20(2):119–24. doi: 10.1111/j.1365-2982.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 62.Wu DN, Yamauchi K, Kobayashi H, et al. Effects of esophageal acid perfusion on cough responsiveness in patients with bronchial asthma. Chest. 2002;122(2):505–9. doi: 10.1378/chest.122.2.505. [DOI] [PubMed] [Google Scholar]
- 63.Patterson RN, Johnston BT, Ardill JE, et al. Increased tachykinin levels in induced sputum from asthmatic and cough patients with acid reflux. Thorax. 2007;62(6):491–5. doi: 10.1136/thx.2006.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sacco O, Silvestri M, Sabatini F, et al. IL-8 and airway neutrophilia in children with gastroesophageal reflux and asthma-like symptoms. Respir Med. 2006;100(2):307–15. doi: 10.1016/j.rmed.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 65.Chang AB, Gibson PG, Ardill J, McGarvey LP. Calcitonin gene-related peptide relates to cough sensitivity in children with chronic cough. Eur Respir J. 2007;30(1):66–72. doi: 10.1183/09031936.00150006. [DOI] [PubMed] [Google Scholar]
- 66.Groneberg DA, Niimi A, Dinh QT, et al. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med. 2004;170(12):1276–80. doi: 10.1164/rccm.200402-174OC. [DOI] [PubMed] [Google Scholar]
- 67.Plevkova J, Kollarik M, Brozmanova M, et al. Modulation of experimentally-induced cough by stimulation of nasal mucosa in cats and guinea pigs. Respir Physiol Neurobiol. 2004;142(2-3):225–35. doi: 10.1016/j.resp.2004.06.006. [DOI] [PubMed] [Google Scholar]