Abstract
The role played by anatomic factors in ACL injury remains elusive. In this study, objective methods were used to characterize ACL volume, tibial slopes, and notch geometry from ACL-injured and matched control subjects. The study tested four hypotheses: 1) the medial tibial plateau slope is steeper posteriorly in the injured group compared to the non-injured group, 2) the lateral tibial plateau slope is steeper posteriorly in the injured group compared to the non-injured group, 3) the femoral intercondylar notch dimensions are smaller in the injured group compared to the non-injured group and 4) the ACL volume, tibial plateau slopes and intercondylar notch dimensions are all independent of each other. Fifty-four subjects were divided into two groups, those who had suffered a non-contact ACL injury and those who still had two healthy ACLs, matched to the injured subjects by gender, age, height and weight. The lateral tibial plateaus in the uninjured contralateral knees of the injured subjects had a significantly steeper posterior slope (1.8° vs. −0.3°), a factor that potentially contributed to the ACL injury in the opposite knee. The intercondylar notch dimensions were found to be smaller in the injured subjects, potentially putting the ACL at risk of impingement, and intercondylar notch volume was correlated to ACL volume (r=0.58). Discriminant analysis showed that the notch width at the inlet was the best single predictor of ACL injury.
Keywords: ACL, Tibial plateau, Intercondylar notch, Risk factors, Non-contact
Introduction
Both extrinsic and intrinsic factors of the human knee have been explored to determine if they could be potential risk factors for Anterior Cruciate Ligament (ACL) injuries. Extrinsic factors explored by previous research include training regimens (Engelhardt, Freiwald & Rittmeister 2002, Myer et al. 2008, Willson et al. 2005), skill level (Harmon, Dick 1998), quadriceps and hamstring muscle strength (Anderson et al. 2001, Draganich, Vahey 1988, Hashemi et al. 2007, McLean, Andrish & van den Bogert 2005, Myer, Ford & Hewett 2005, Shimokochi, Shultz 2008, Withrow et al. 2006), neuromuscular biomechanics (Hewett et al. 1999, Hewett, Myer & Ford 2005, Lephart, Abt & Ferris 2002, Myer et al. 2005, Myklebust et al. 2003) and proprioception (Barrack, Skinner & Buckley 1989, Mandelbaum et al. 2005). Intrinsic factors that have been studied previously include ACL volume, tibial plateau angles and femoral intercondylar notch width (Anderson et al. 2001, Chandrashekar, Slauterbeck & Hashemi 2005, Charlton et al. 2002, Davis, Shelbourne & Klootwyk 1999, Hashemi et al. 2008, Shelbourne, Davis & Klootwyk 1998, Stijak, Herzog & Schai 2008). Identifying these intrinsic variables as factors that promote ACL injuries could assist in the development of strategies that optimize extrinsic variables to counteract intrinsic limitations, thereby reducing the number of ACL injuries suffered by individuals.
The extant research shows that, compared to men, women have smaller ACL volumes (Anderson et al. 2001, Chandrashekar, Slauterbeck & Hashemi 2005, Charlton et al. 2002), steeper posterior slopes in the lateral tibial plateau (Hashemi et al. 2008, Stijak, Herzog & Schai 2008), and smaller intercondylar notches (Anderson et al. 2001, Charlton et al. 2002, Davis, Shelbourne & Klootwyk 1999, Shelbourne, Davis & Klootwyk 1998). All of these factors ha ve been hypothesized to account for the 2.4 – 9.5 times increased relative ACL injury risk for females as compared to males (Gwinn et al. 2000). However, a major limitation of the current literature is that these previous studies only compared females to males, and therefore they are unable to make general conclusions about intrinsic risk factors that put anyone at an increased risk of injuring their ACL. Another weakness of previous studies is the use of subjective methods, using landmarks that are difficult to justify clinically and duplicate. Finally, previous works have tended to examine individual variables from a single data set, thus limiting their generalizability and conclusions. The current study overcomes these limitations by comparing individuals who have suffered an ACL injury directly to those who have not, allowing for more accurate comparison. The current study also developed a set of criteria to measure these intrinsic variables that is both objective and easily repeatable. And lastly, this paper measures all of the intrinsic factors from one data set and is therefore able to examine the potential interrelationships between them.
Thus, the current study tests four hypotheses: 1) The medial tibial plateau of the injured subjects has a steeper posterior slope than those of the non-injured group 2) The lateral tibial plateau of the injured subjects has a steeper posterior slope than those of the non-injured group 3) The intercondylar notch widths at the inlet and outlet are narrower in the injured subjects compared to the non-injured subjects and 4) The ACL volume, tibial plateau angles and femoral intercondylar notch sizes are independent of each other.
Methods
Fifty-four subjects (34 males) participated after providing IRB-approved informed consent. They were divided into 2 equal groups, the injured group and the control group. The injured group contained the uninjured contralateral knees of subjects who had suffered a non-contact ACL injury, while the control group contained knees of subjects with no history of ACL injury. The control group was matched for gender, height, weight and age to the injured group. The average heights between the control and the injured subjects were within 1 cm (1.75m vs. 1.74m). Magnetic resonance imaging (1.5T, sagittal 3D-SPGR, voxel size 0.55mm × 0.55mm × 1.5mm) was performed on one randomly-selected knee in the control group and of the uninjured contralateral knee in the injured group.
ACL Volume
The ACL was segmented from the MRI dataset described above by a single rater under the guidance of a sports-fellowship-trained orthopedic surgeon. The method was previously validated using a porcine model, and both the method and the ACL volume results for this set of subjects have been previously reported (Chaudhari et al. 2009). After the manual identification of the ACL had been performed in each slice, the ACL volume was automatically calculated using a freely-available software package, Medical Image Processing, Analysis and Visualization (v2.7.45) (McAuliffe et al. 2001).
Tibial Long Axis
The most distal transverse slice available showing the tibial cortex from the MR dataset was used along with the most proximal transverse slice of the tibia just below the articular cartilage to calculate the long axis of the tibia. The outline of the tibia in each transverse slice was segmented in AMIRA software (v4.1.2, Visage Imaging, Carlsbad, CA) and exported to MATLAB (MathWorks, Natick, MA), where a custom script was used to calculate the centroid of that slice. A best-fit line was then fitted to the two centroids to define the long-axis of the tibia.
Tibial Plateau Slope
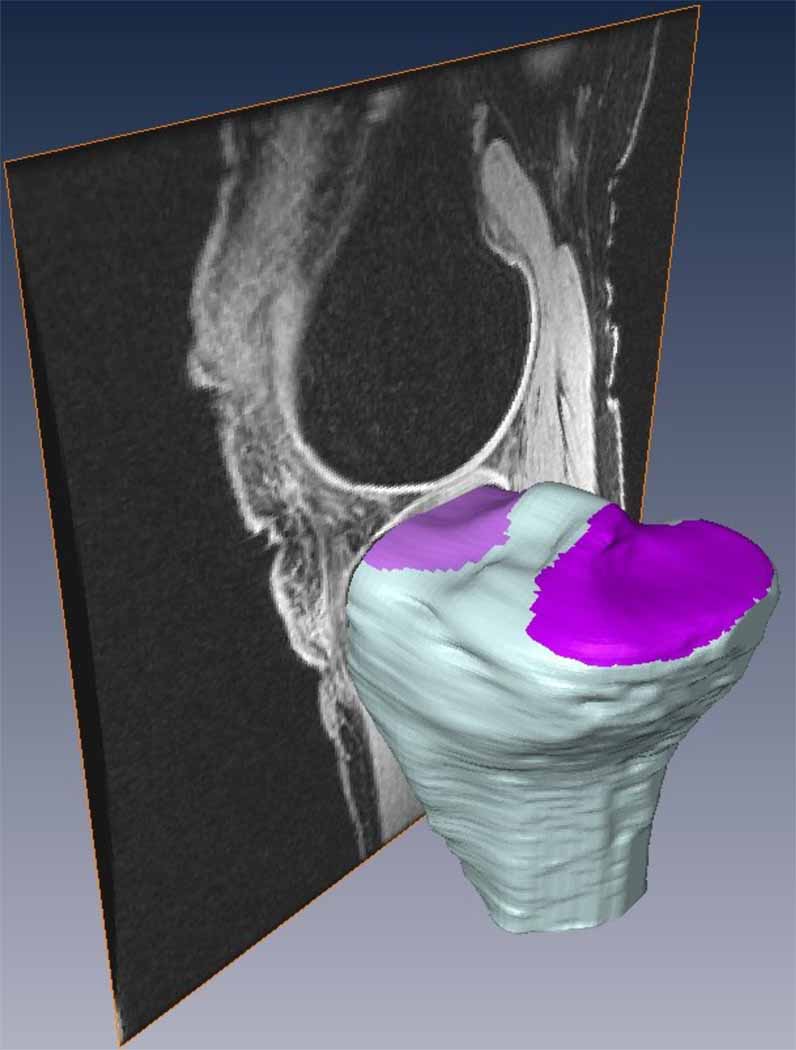
A modification of the methods from Hashemi et al. (2008) was used to determine the medial and lateral tibial plateau slopes in the sagittal plane. Three-dimensional models of the tibial bone were manually segmented from the MRIs using AMIRA. Since the MRI displayed strong contrast between bone and surrounding tissue, especially at the areas of interest, the “blow” tool was used (see Figure 1 for an example of the contrast between tissues). The blow tool is designed to allow interactive determination of the boundaries where a large image gradient exists. It expands from a seed point across homogeneous gray values as the mouse is dragged, but stops when it senses a large gradient, corresponding to the boundaries of the bones. Subsequent to segmentation of the bones, the original MRIs were used as a guide to identify the medial and lateral subchondral bone surfaces (Figure 1).
Figure 1.
Example of the segmented tibia with the medial and lateral tibial plateaus marked (purple sections). The sagittal MRI slices were used to identify the subchondral bone.
Once the tibial plateaus were determined, the principal axes of each subchondral surface were calculated from the eigenvectors of the set of surface points using MATLAB to choose an objective best-fit plane to the data points. The line intersection of this plane to the sagittal plane was calculated, and finally the tibial plateau slope for each tibiofemoral compartment was calculated as the angle of this intersection line relative to the long axis of the tibia (see above). An angle of zero indicates that the plateau is perpendicular to the long axis, and positive values correlate to posterior slopes.
Intercondylar Notch Methods
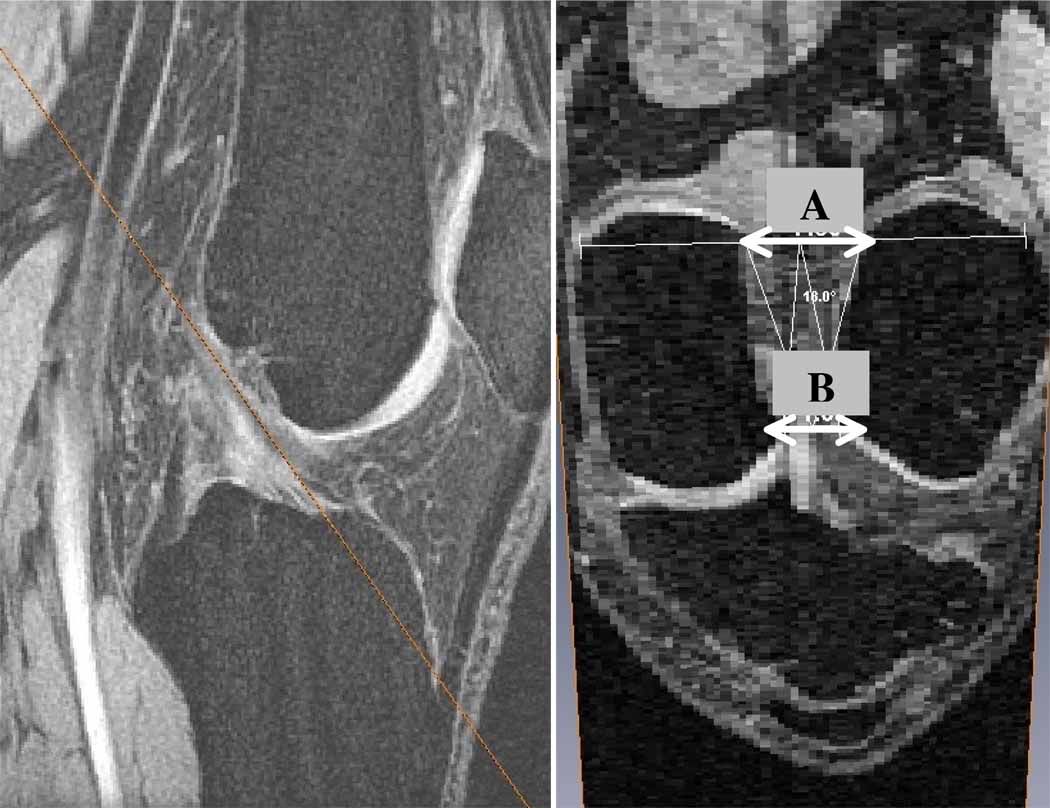
The MRI data was resampled in an oblique plane parallel to and containing the ACL and perpendicular to the sagittal plane using AMIRA. In this oblique plane, widths were measured at the intercondylar notch inlet and outlet (Figure 2). The inlet was defined where the ACL enters the notch posteriorly and the outlet is where the ACL leaves the notch anteriorly, using a proximal-to-distal direction convention for the ACL.
Figure 2.
Example of an oblique slice that is parallel to the ACL (orange line in left image). The right image describes where the measurements were made on the oblique slice (A = inlet; B = outlet).
Statistical Analysis
Two-sample t-tests (α=0.05) were performed to compare the injured and non-injured medial tibial plateau slopes, lateral tibial plateau slopes and intercondylar notch widths. To determine the strength of linear dependence between the variables, Pearson correlation coefficients were calculated with an inclusion cut-off of α=0.05. A discriminant analysis, using both linear and quadratic methods, was conducted to determine which variables could predict injury.
Results
The average slope of the medial tibial plateau for the injured subjects was −1.8° and for the non-injured group was −2.9°. This difference between the two groups was not significant (p = 0.20). However, the difference between the lateral tibial plateau slopes was significantly different (p = 0.02), with the injured group (1.8°) having a steeper posterior slope than the non-injured group (−0.3°) (Table 1).
Table 1.
Long axis adjusted medial and lateral tibial plateau slopes comparing the non-injured subjects to the injured subjects (mean±SD). Positive numbers indicate posterior slopes.
| Medial Tibial Plateau Slope | Lateral Tibial Plateau Slope | |
|---|---|---|
| Non-injured | −2.9° ± 2.8° | −0.3° ± 3.6° |
| Injured | −1.8° ± 3.7° | 1.8° ± 3.2° |
| p-value | 0.20 | 0.02 |
The intercondylar notch inlet was significantly different between the two groups, having an average width of 13.3 mm for the injured group and 15.6 for the non-injured group (p = 0.003). The intercondylar notch widths at the outlet for the injured and non-injured groups were 21.0 mm and 22.6 mm, respectively, which were also significantly different (p = 0.02) (Table 2).
Table 2.
Comparison of intercondylar notch (ICN) dimensions between injured and non-injured subjects (mean±SD). ICN widths were taken at both the inlet and the outlet. The volume of the ICN was also calculated.
| ICN Width – Inlet (mm) |
ICN Width – Outlet (mm) |
ICN Volume (mm3) |
|
|---|---|---|---|
| Non-injured | 15.6 ± 2.9 | 22.6 ± 2.5 | 11.2 ± 2.6 |
| Injured | 13.3 ± 2.6 | 21.0 ± 2.5 | 10.3 ± 2.5 |
| p-value | 0.003 | 0.02 | 0.24 |
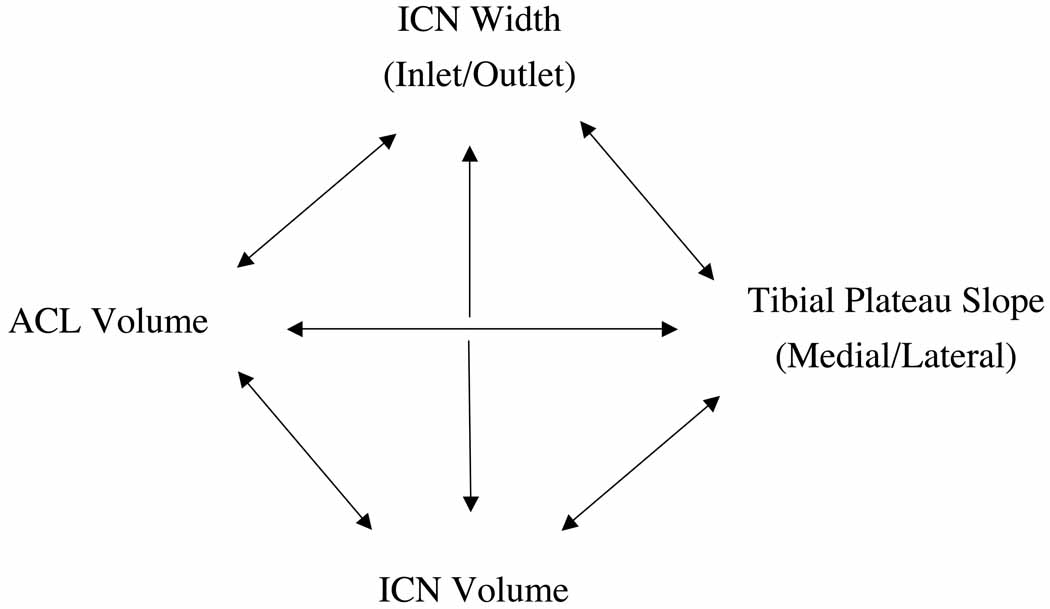
The final aim to determine independence of the variables was analyzed using the Pearson correlation. The lateral tibial plateau slope was negatively correlated to the intercondylar notch width at the inlet (−0.30). The ACL volume was positively correlated to the intercondylar notch width at the outlet (0.42) and the intercondylar notch volume (0.58). None of the other relationships had a statistically significant correlation (Table 3).
Table 3.
Pearson correlations between variables of interest. Highlighted correlations are significant (p < 0.05).
| Notch WidthOUTLET |
Notch Volume |
Lateral Plateau Slope |
ACL Volume |
|
|---|---|---|---|---|
| Notch WidthINLET |
0.29* | 0.20 | −0.30* | 0.22 |
| Notch WidthOUTLET |
− | 0.59* | −0.10 | 0.42* |
| Notch Volume |
− | −0.24 | 0.58* | |
| Lateral Plateau Slope |
− | −0.08 |
A discriminant analysis was performed to determine the predictive power of the variables. When the intercondylar notch width at the inlet was used alone it predicted 70% of the subjects correctly with a sensitivity and specificity of 74% and 67%, respectively and positive and negative predictive values of 69% and 72%, respectively. When the ACL volume, lateral tibial plateau slope and intercondylar notch width at the inlet were used, only 69% of the subjects were predicted correctly with the same specificity as before, but a lower sensitivity of 70%, and positive and negative predictive values of 68% and 69%, respectively (Table 4).
Table 4.
Sensitivity and specificity of predicted ACL injuries based on intercondylar notch (ICN) width at the inlet only and of the combined model (ACL volume, lateral plateau slope, and ICN inlet width). PPV = Positive Predictive Value; NPV = Negative Predictive Value
|
Intercondylar Notch Width Inlet Only |
Injured (Actual) |
Non-Injured (Actual) |
|
| Injured (Predicted) |
20 | 9 | PPV = 69% |
| Non-Injured (Predicted) |
7 | 18 | NPV = 72% |
| Sensitivity = 74% |
Specificity = 67% |
||
|
ACL Vol, Lat Plat, ICN Inlet |
Injured (Actual) |
Non-Injured (Actual) |
|
| Injured (Predicted) |
19 | 9 | PPV = 68% |
| Non-Injured (Predicted) |
8 | 18 | NPV = 69% |
| Sensitivity = 70% |
Specificity = 67% |
Discussion
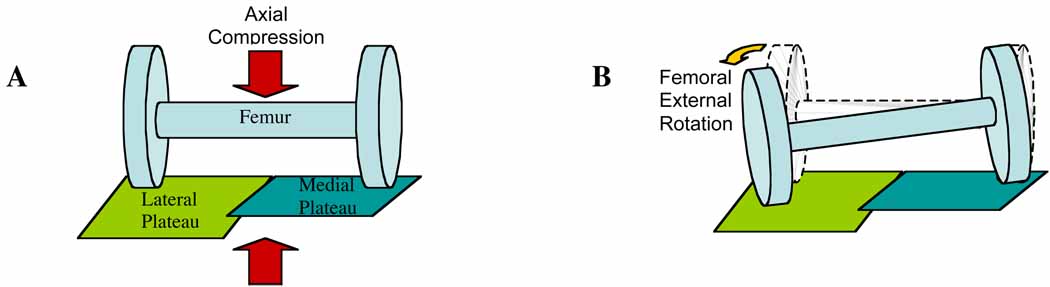
Our data suppor t the previously stated hypotheses on the importance of medial and lateral tibial plateau slopes (Hashemi et al. 2008, Stijak, Herzog & Schai 2008). Those who suffered an ACL injury had medial tibial plateau slopes that were not significantly different than those in the uninjured group. The lateral tibial plateaus however, had a steeper posterior slope in the injured subjects compared to the noninjured subjects. Under these conditions, an axial loading force would be more likely to cause the lateral side of the femur in the injured subjects to slide posteriorly off of the lateral tibial plateau, using the medial tibial plateau as a pivot point (Figure 4). An external rotation of the femur would result, which has been shown to put excess strain on the ACL (Shimokochi & Shultz 2008, Fleming et al. 2001, Markolf et al. 1995). This suggests that under the same axial force, the ACLs in the injured group would be under higher strain than ACLs of the uninjured group, putting them at an increased risk of rupturing their ACL.
Figure 4.
Diagram of axial loading force on a flat medial plateau and a posteriorly sloped lateral plateau (A: Before loading B: After loading)
The ultimate goal of this line of research is to determine if one or more risk factors could predict the chances of a person injuring their ACL and apply it to practical use. Since all of these anatomic risk factors were examined using the same data set we were able to examine relative predictive value, and we found that the intercondylar notch width at the inlet was the best predictor of ACL injury, with a sensitivity of 74% and a specificity of 67%. This result, combined with the positive predictive value of 69%, means that measurement of the intercondylar notch width at the inlet potentially could be used as a screening tool to determine whether a patient requires special training and exercises to reduce the chances of an ACL rupture. If so, this would offer the obvious advantage of using a single measurement (notch width) to predict potential for ACL rupture. A larger, prospective study will be required to test this observation.
One possible explanation for the apparent interdependence of the ACL volume, tibial slope, and notch dimension measurements in our correlational analysis is that these parameters may influence one another. Some previous studies have examined notch width as a risk factor for ACL injury under the hypothesis that it could be used as a surrogate for ACL size (Davis, Shelbourne & Klootwyk 1999, Muneta, Takakuda & Yamamoto 1997). Other studies have examined notch width under the hypothesis that a smaller notch presents a larger risk of ACL rupture due to impingement (Anderson et al. 1987, Fung et al. 2007, Houseworth et al. 1987, Ireland et al. 2001, Shelbourne, Facibene & Hunt 1997, Souryal, Moore & Evans 1988). Those with a narrower notch may also have a coronal ACL orientation that lies more vertical, which could potentially influence the loads the ACL experiences, although this has not been investigated to our knowledge. Given the evidence that ligaments and bone do respond to their loading environments it is possible that a steeper tibial slope could lead to greater ACL strain and thereby to ACL hypertrophy (Amiel et al. 1982, Cowin 1983, Kazarian 1975, Maffulli, King 1992, Newton et al. 1995, Noyes et al. 1974, Rasch et al. 1960, Tipton et al. 1970, Yasuda, Hayashi 1999). Moreover, repeated impingement of the ACL against the intercondylar notch could potentially lead to notch stenosis (Everhart et al. 2010, in press). It remains unknown whether these factors do influence each other either during skeletal development or after skeletal maturity is achieved, but the apparent interdependence observed in this study suggests that further research in this area may be warranted.
One of the advances in this study is the objective determination of tibial slope from MRI. In past studies, a single sagittal slice was chosen both to determine the long axis of the tibia and the orientation of the tibial plateau. To ensure the objectivity of our results, the long-axis of each tibia was determined by automated calculation of the centroid of the bone from several transverse slices, and the tibial plateau slope by automated calculation of the principal axes of the entire subchondral surface of the plateau. Using this approach, we avoided potential subjectivity and inaccuracy due to choosing an inappropriate sagittal slice, incorrectly drawing the lines for the slope or long axis, or inability to ensure that the knee was in full extension during scanning.
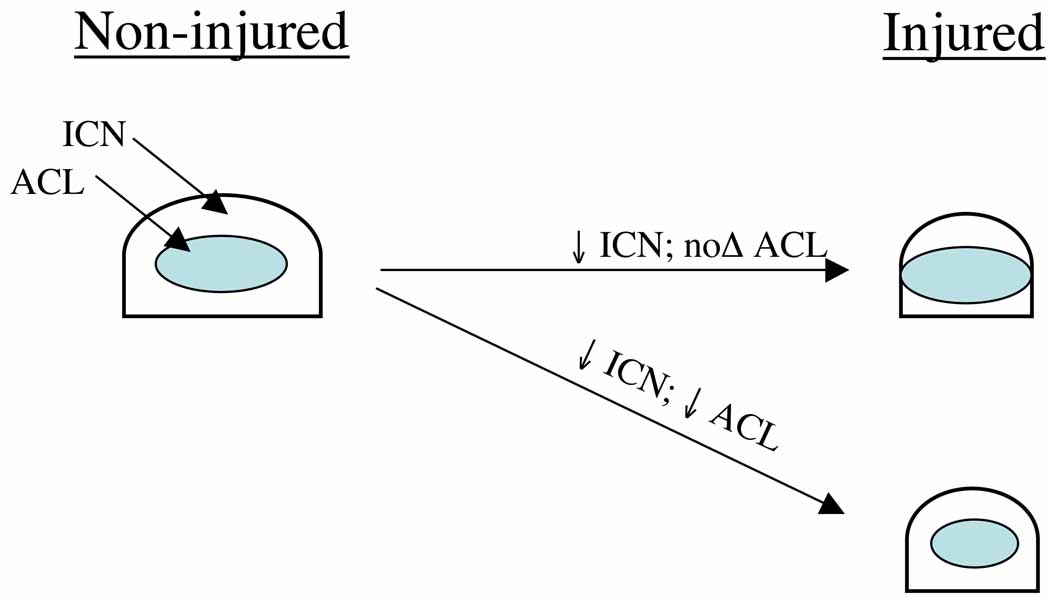
In order to make the intercondylar notch measurements objective, reproducible and relevant, they were measured along the oblique path of the ACL. The ACL is well demarcated in the MRI modality (Moore 2002), making it easy to identify a plane perpendicular to the sagittal plane that contains the ACL. In addition, any potential contact between the ACL and the intercondylar notch will most likely occur along this plane. The positive correlation between the notch volume and the ACL volume suggests that as the ACL gets smaller, as in the injured group, so does the intercondylar notch volume. The intercondylar notch width at the outlet is also positively correlated with the ACL volume, whereas the intercondylar notch width at the inlet is not correlated at all. Since the width at the inlet and the ACL volume do not get smaller at the same rate, the potential exists for a “mismatch” between the inlet and the ACL in some subjects, which could lead either to impingement that could weaken or endanger the ACL (Figure 5) or altered coronal orientation of the ACL that may lead to altered tensions in the ACL. As mentioned above, these possible relationships between notch dimensions and ACL loading deserve further examination.
Figure 5.
In injured subjects, if the ICN is reduced in size, but the ACL is not, rubbing is more likely to occur. If both the ICN and the ACL are reduced by the same factor though, it is no different than the non-injured subjects and will not contribute to the risk of injuring the ACL.
One of the limitations of this study is that in our effort to make objective measurements of the tibial plateaus, we used the subchondral bone surfaces rather than the articular surfaces, which are much more difficult to visualize in MRI. We felt that this measurement was the most clinically relevant, given the desire clinically to measure tibial slope from x-ray or CT scan rather than the more expensive MRI. When using the overlying cartilage to define the subchondral surface, however, the boundary of the tibial plateau extended up onto the tibial spine as well as down around some of the medial and lateral edges. However, since the proportion of the tibial plateau area at the edges is extremely small relative to the bulk of the articulating surface, the inclusion of these edges did not alter our data significantly or our conclusions.
Another limitation of the study is the use of the contralateral knees of the injured subjects to represent the knee with the ruptured ACL. Contralateral ACL volume has been previously shown to be an appropriate surrogate measure for volume of an injured ACL (Jamison et al. 2009), and Teitz et al. (1997) showed that the intercondylar notch dimensions are symmetrical, but side-to-side variability of tibial slope remains unknown.
While many of the observations made in this study agree with previously-reported observations for both tibial plateau slope and intercondylar notch width, these results must be considered in light of the facts that (1) novel objective methods were used to make the measurements rather than the subjective measurements used previously, and (2) all measurements were made on the same data set containing both injured and matched-control knees. The objectivity of these methods should make them more repeatable without extensive training of the person making the measurements, which is especially important for making these measurements in a clinical setting as a screening tool. Making all the measurements from a single data set is especially valuable because it allowed us to determine whether the predictor variables are independent of each other, and whether they are all individual risk factors. Also, since the data directly compares injured to non-injured subjects, as opposed to other studies that examined only gender differences, we are able to conclude that these risk factors are more likely to be present in someone at risk of injuring their ACL, independent of whether that person is male or female. In the future, further development of the described methods to make them as quick and inexpensive as possible while maintaining objectivity would allow for large-scale prospective screening studies to determine the proportional odds of an ACL rupture as tibial plateau slope, notch widths, and ACL volume vary. This further development would also enable physicians to use these tools in clinical practice, identifying individuals who stand to benefit most from targeted interventions to prevent ACL injuries.
Figure 3.
Summary of comparisons made between anatomical variables.
Acknowledgements
The authors gratefully acknowledge Tom Andriacchi, Sean Scanlan, and Chris Dyrby, of Stanford University, for providing the human magnetic resonance images and subject demographic data analyzed in this study.
Partial financial support came from NIH CTSA grant UL1RR025755.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All of the authors were fully involved in the study and preparation of the manuscript and that the material within has not been and will not be submitted for publication elsewhere.
References
- Amiel D, Woo SL, Harwood FL, Akeson WH. The effect of immobilization on collagen turnover in connective tissue: a biochemical-biomechanical correlation. Acta Orthopaedica Scandinavica. 1982;vol. 53(no. 3):325–332. doi: 10.3109/17453678208992224. [DOI] [PubMed] [Google Scholar]
- Anderson AF, Dome DC, Gautam S, Awh MH, Rennirt GW. Correlation of Anthropometric Measurements, Strength, Anterior Cruciate Ligament Size, and Intercondylar Notch Characteristics to Sex Differences in Anterior Cruciate Ligament Tear Rates. American Journal of Sports Medicine. 2001;vol. 29(no. 1):58–66. doi: 10.1177/03635465010290011501. [DOI] [PubMed] [Google Scholar]
- Anderson AF, Lipscomb AB, Liudahl KJ, Addlestone RB. Analysis of the intercondylar notch by computed tomography. The American Journal of Sports Medicine. 1987;vol. 15(no. 6):547–552. doi: 10.1177/036354658701500605. [DOI] [PubMed] [Google Scholar]
- Barrack RL, Skinner HB, Buckley SL. Proprioception in the anterior cruciate deficient knee. American Journal of Sports Medicine. 1989;vol. 17(no. 1):1–6. doi: 10.1177/036354658901700101. [DOI] [PubMed] [Google Scholar]
- Chandrashekar N, Slauterbeck J, Hashemi J. Sex-based differences in the anthropometric characteristics of the anterior cruciate ligament and its relation to intercondylar notch geometry: a cadaveric study. American Journal of Sports Medicine. 2005;vol. 33(no. 10):1492–1498. doi: 10.1177/0363546504274149. [DOI] [PubMed] [Google Scholar]
- Charlton WPH, St. John TA, Ciccotti MG, Harrison N, Schweitzer M. Differences in Femoral Notch Anatomy between Men and Women : A Magnetic Resonance Imaging Study. American Journal of Sports Medicine. 2002;vol. 30(no. 3):329–333. doi: 10.1177/03635465020300030501. [DOI] [PubMed] [Google Scholar]
- Chaudhari AM, Zelman EA, Flanigan DC, Kaeding CC, Nagaraja HN. Anterior Cruciate Ligament-Injured Subjects Have Smaller Anterior Cruciate Ligaments Than Matched Controls: A Magnetic Resonance Imaging Study. The American Journal of Sports Medicine. 2009 doi: 10.1177/0363546509332256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin SC. The mechanical and stress adaptive properties of bone. Annals of Biomedical Engineering. 1983;vol. 11(no. 3–4):263–295. doi: 10.1007/BF02363288. [DOI] [PubMed] [Google Scholar]
- Davis TJ, Shelbourne KD, Klootwyk TE. Correlation of the intercondylar notch width of the femur to the width of the anterior and posterior cruciate ligaments. Knee Surgery, Sports Traumatology, Arthroscopy. 1999;vol. 7(no. 4):209–214. doi: 10.1007/s001670050150. [DOI] [PubMed] [Google Scholar]
- Draganich LF, Vahey JW. An in-vitro study of anterior cruciate ligament strain induced by quadriceps and hamstring forces. Trans ORS. 1988;vol. 13:203. doi: 10.1002/jor.1100080107. [DOI] [PubMed] [Google Scholar]
- Engelhardt M, Freiwald J, Rittmeister M. [Rehabilitation after anterior cruciate ligament reconstruction] Orthopäde. 2002;vol. 31(no. 8):791–798. doi: 10.1007/s00132-002-0337-6. [DOI] [PubMed] [Google Scholar]
- Everhart J, Flanigan D, Simon R, Chaudhari A. Association of non-contact ACL with presence and thickness of a bony ridge on the anteromedial aspect of the femoral intercondylar notch. American Journal of Sports Medicine. doi: 10.1177/0363546510367424. in press. [DOI] [PubMed] [Google Scholar]
- Fleming BC, Renstrom PA, Beynnon BD, Engstrom B, Peura GD, Badger GJ, Johnson RJ. The effect of weightbearing and external loading on anterior cruciate ligament strain. Journal of Biomechanics. 2001;vol. 34(no. 2):163–170. doi: 10.1016/s0021-9290(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Fung DT, Hendrix RW, Koh JL, Zhang LQ. ACL impingement prediction based on MRI scans of individual knees. Clinical orthopaedics and related research. 2007;vol. 460:210–218. doi: 10.1097/BLO.0b013e31804d2339. [DOI] [PubMed] [Google Scholar]
- Fung DT, Zhang LQ. Modeling of ACL impingement against the intercondylar notch. Clin.Biomech. 2003;vol. 18(no. 10):933–941. doi: 10.1016/s0268-0033(03)00174-8. [DOI] [PubMed] [Google Scholar]
- Gwinn DE, Wilckens JH, McDevitt ER, et al. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. The American Journal of Sports Medicine. vol.28(no. 1):98–102. doi: 10.1177/03635465000280012901. [DOI] [PubMed] [Google Scholar]
- Harmon KG, Dick R. The relationship of skill level to anterior cruciate ligament injury. Clinical journal of sport medicine. 1998;vol. 8(no. 4):260–265. doi: 10.1097/00042752-199810000-00002. [DOI] [PubMed] [Google Scholar]
- Hashemi J, Chandrashekar N, Gill B, Beynnon BD, Slauterbeck JR, Schutt RC, Jr, Mansouri H, Dabezies E. The geometry of the tibial plateau and its influence on the biomechanics of the tibiofemoral joint. The Journal of bone and joint surgery.American volume. 2008;vol. 90(no. 12):2724–2734. doi: 10.2106/JBJS.G.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi J, Chandrashekar N, Jang T, Karpat F, Oseto M, Ekwaro-Osire S. An Alternative Mechanism of Non-contact Anterior Cruciate Ligament Injury During Jump-landing: In-vitro Simulation. Experimental Mechanics. 2007;vol. 47(no. 3):347–354. [Google Scholar]
- Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes : A prospective study. American Journal of Sports Medicine. 1999;vol. 27(no. 6):699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR. Reducing knee and anterior cruciate ligament injuries among female athletes: a systematic review of neuromuscular training interventions. J.Knee Surg. 2005;vol. 18(no. 1):82–88. doi: 10.1055/s-0030-1248163. [DOI] [PubMed] [Google Scholar]
- Houseworth SW, Mauro VJ, Mellon BA, Kieffer DA. The intercondylar notch in acute tears of the anterior cruciate ligament: a computer graphics study. American Journal of Sports Medicine. 1987;vol. 15(no. 3):221–224. doi: 10.1177/036354658701500305. [DOI] [PubMed] [Google Scholar]
- Ireland ML, Ballantyne BT, Little K, McClay IS. A radiographic analysis of the relationship between the size and shape of the intercondylar notch and anterior cruciate ligament injury. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2001;vol. 9(no. 4):200–205. doi: 10.1007/s001670100197. [DOI] [PubMed] [Google Scholar]
- Jamison ST, Flanigan DC, Nagaraja HN, Chaudhari AM. Side-to-Side Differences in Anterior Cruciate Ligament Volume in Healthy Subjects. Transactions of the 55th Orthopaedic Research Society Annual Meeting; American Academy of Orthopaedic Surgeons; 2009. p. 1453. [Google Scholar]
- Kazarian LE. Creep characteristics of the human spinal column. The Orthopedic clinics of North America. 1975;vol. 6(no. 1):3–18. [PubMed] [Google Scholar]
- Lephart SM, Abt JP, Ferris CM. Neuromuscular contributions to anterior cruciate ligament injuries in females. Current opinion in rheumatology. 2002;vol. 14(no. 2):168–173. doi: 10.1097/00002281-200203000-00014. Copyright 2001 BIOSIS. [DOI] [PubMed] [Google Scholar]
- Maffulli N, King JB. Effects of physical activity on some components of the skeletal system. Sports medicine (Auckland, N.Z.) 1992;vol. 13(no. 6):393–407. doi: 10.2165/00007256-199213060-00003. [DOI] [PubMed] [Google Scholar]
- Mandelbaum BR, Silvers HJ, Watanabe DS, Knarr JF, Thomas SD, Griffin LY, Kirkendall DT, W G., Jr Effectiveness of a Neuromuscular and Proprioceptive Training Program in Preventing Anterior Cruciate Ligament Injuries in Female Athletes: 2-Year Follow-up. American Journal of Sports Medicine. 2005;vol. 33(no. 7):1003–1010. doi: 10.1177/0363546504272261. [DOI] [PubMed] [Google Scholar]
- Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GAM, Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. Journal of Orthopaedic Research. 1995;vol. 13(no. 6):930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- McAuliffe MJ, Lalonde FM, McGarry D, Gandler W, Csaky K, Trus BL. Medical Image Processing, Analysis & Visualization in Clinical Research; 14th IEEE Symposium on Computer-Based Medical Systems (CMBS '01); 2001. p. 381. IEEE. [Google Scholar]
- McLean SG, Andrish JT, van den Bogert AJ. Aggressive quadriceps loading can induce noncontact anterior cruciate ligament injury. The American Journal of Sports Medicine. 2005;vol. 33(no. 7):1106. doi: 10.1177/0363546505278247. author reply 1106-7. [DOI] [PubMed] [Google Scholar]
- Moore SL. Imaging the anterior cruciate ligament. The Orthopedic Clinics of North America. 2002;vol. 33(no. 4):663. doi: 10.1016/s0030-5898(02)00022-6. [DOI] [PubMed] [Google Scholar]
- Muneta T, Takakuda K, Yamamoto H. Intercondylar Notch Width and Its Relation to the Configuration and Cross-Sectional Area of the Anterior Cruciate Ligament: A Cadaveric Knee Study. Am J Sports Med. 1997;vol. 25(no. 1):69–72. doi: 10.1177/036354659702500113. [DOI] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Hewett TE. The effects of gender on quadriceps muscle activation strategies during a maneuver that mimics a high ACL injury risk position. Journal of electromyography and kinesiology : official journal of the International Society of Electrophysiological Kinesiology. 2005;vol. 15(no. 2):181–189. doi: 10.1016/j.jelekin.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Palumbo JP, Hewett TE. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. J.Strength Cond Res. 2005;vol. 19(no. 1):51–60. doi: 10.1519/13643.1. [DOI] [PubMed] [Google Scholar]
- Myer GD, Paterno MV, Ford KR, Hewett TE. Neuromuscular training techniques to target deficits before return to sport after anterior cruciate ligament reconstruction. J Strength Cond Res. 2008;vol. 22(no. 3):987–1014. doi: 10.1519/JSC.0b013e31816a86cd. [DOI] [PubMed] [Google Scholar]
- Myklebust G, Engebretsen L, Braekken IH, Skjolberg A, Olsen OE, Bahr R. Prevention of anterior cruciate ligament injuries in female team handball players: a prospective intervention study over three seasons. Clinical journal of sport medicine. 2003;vol. 13(no. 2):71–78. doi: 10.1097/00042752-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Newton PO, Woo SL, MacKenna DA, Akeson WH. Immobilization of the knee joint alters the mechanical and ultrastructural properties of the rabbit anterior cruciate ligament. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1995;vol. 13(no. 2):191–200. doi: 10.1002/jor.1100130207. [DOI] [PubMed] [Google Scholar]
- Noyes FR, Torvik PJ, Hyde WB, DeLucas JL. Biomechanics of ligament failure. II. An analysis of immobilization, exercise, and reconditioning effects in primates. The Journal of bone and joint surgery.American volume. 1974;vol. 56(no. 7):1406–1418. [PubMed] [Google Scholar]
- Rasch PJ, Maniscalco R, Pierson WR, Logan GA. Effect of exercise, immobilization and intermittent stretching on strength of knee ligaments of albino rats. Journal of applied physiology. 1960;vol. 15:289–290. doi: 10.1152/jappl.1960.15.2.289. [DOI] [PubMed] [Google Scholar]
- Shelbourne KD, Davis TJ, Klootwyk TE. The relationship between intercondylar notch width of the femur and the incidence of anterior cruciate ligament tears. A prospective study. American Journal of Sports Medicine. 1998;vol. 26(no. 3):402–408. doi: 10.1177/03635465980260031001. [DOI] [PubMed] [Google Scholar]
- Shelbourne KD, Facibene WA, Hunt JJ. Radiographic and intraoperative intercondylar notch width measurements in men and women with unilateral and bilateral anterior cruciate ligament tears. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 1997;vol. 5(no. 4):229–233. doi: 10.1007/s001670050055. [DOI] [PubMed] [Google Scholar]
- Shimokochi Y, Shultz SJ. Mechanisms of noncontact anterior cruciate ligament injury. Journal of athletic training. 2008;vol. 43(no. 4):396–408. doi: 10.4085/1062-6050-43.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souryal TO, Moore HA, Evans JP. Bilaterality in anterior cruciate ligament injuries: associated intercondylar notch stenosis. American Journal of Sports Medicine. 1988;vol. 16(no. 5):449–454. doi: 10.1177/036354658801600504. [DOI] [PubMed] [Google Scholar]
- Stijak L, Herzog RF, Schai P. Is there an influence of the tibial slope of the lateral condyle on the ACL lesion? A case-control study. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2008;vol. 16(no. 2):112–117. doi: 10.1007/s00167-007-0438-1. [DOI] [PubMed] [Google Scholar]
- Teitz CC, Lind BK, Sacks BM. Symmetry of the femoral notch width index. American Journal of Sports Medicine. 1997;vol. 25(no. 5):687–690. doi: 10.1177/036354659702500517. [DOI] [PubMed] [Google Scholar]
- Tipton CM, James SL, Mergner W, Tcheng TK. Influence of exercise on strength of medial collateral knee ligaments of dogs. The American Journal of Physiology. 1970;vol. 218(no. 3):894–902. doi: 10.1152/ajplegacy.1970.218.3.894. [DOI] [PubMed] [Google Scholar]
- Willson JD, Dougherty CP, Ireland ML, Davis IM. Core stability and its relationship to lower extremity function and injury. Journal of the American Academy of Orthopaedic Surgeons. 2005;vol. 13(no. 5):316–325. doi: 10.5435/00124635-200509000-00005. [DOI] [PubMed] [Google Scholar]
- Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The relationship between quadriceps muscle force, knee flexion, and anterior cruciate ligament strain in an in vitro simulated jump landing. American Journal of Sports Medicine. 2006;vol. 34(no. 2):269–274. doi: 10.1177/0363546505280906. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Hayashi K. Changes in biomechanical properties of tendons and ligaments from joint disuse. Osteoarthritis and Cartilage. 1999;vol. 7(no. 1):122. doi: 10.1053/joca.1998.0167. [DOI] [PubMed] [Google Scholar]