Abstract
This study investigated the effect of physical training and oxidative stress on the antioxidative activity and on plasma lipid profile. Forty eight rats were given either a physical training or no training for 4 weeks and were then subdivided into 3 groups: before-exercise (BE); during-exercise (DE); after-exercise (AE). The antioxidative activity was evaluated with the activities of catalase in plasma and superoxide dismutase (SOD), the ratio of reduced glutathione/oxidized glutathione (GSH/GSSG) and the level of malondialdehyde (MDA) in liver. The plasma concentrations of triglyceride (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C)) were also compared. Compared to those of non-training group, catalase activities of training group were lower before exercise but higher during and after exercise. SOD activities were higher regardless of exercise. GSH/GSSG ratio was higher before exercise but was not significantly different during exercise and even lower after exercise. There were no differences between non-training group and training group in MDA levels regardless of exercise. Compared to those of non-training group, atherosclerotic index of training group was lower after exercise and there were no significant differences before and during exercise. There were no differences between non-training group and training group in HDL-C regardless of exercise. These results suggest that moderate physical training can activate antioxidant defenses and decrease the atherosclerotic index and this beneficial effect is evident under exercise-induced oxidative stress.
Keywords: Physical training, exercise induced-oxidative stress, antioxidative enzyme activity, lipid profile
Introduction
The beneficial effect of exercise on health has been known for centuries and it is generally accepted that regular physical activity is an important factor in the prevention and treatment of cardiovascular disease. Unfortunately, the frequency and magnitude of exercise necessary to achieve beneficial prognostic effect is still uncertain. Although health benefits of exercise have been reported extensively, not all models of exercise have beneficial effect on lipid profiles and antioxidant enzymes. Since exercise increases not only oxygen consumption but also the generation of reactive oxygen species, strenuous exercise induces an imbalance between free radical production and the body's antioxidant defense systems (Ji et al., 1999; Lovlin et al., 1991; Maxwell et al., 1993; Sahlin et al., 1991). The contribution of free radical damage to the development of atherosclerosis is also established (Schwenke, 1998). While acute and exhaustive exercise leave little time for cellular adaptation reactions, these adaptation reactions have shown to occur in moderate exercise training for several weeks (Fukai et al., 2000; Rush et al., 2003). It has been also reported that less the experience one has in training, higher the stress level was gotten (Powers & Hamilton, 1999). The increase in vascular oxidative stress initiated by exercise, even if this occurs only transiently during the acute bout of exercise, gives rise for serious concerns when exercise is prescribed to patients with cardiovascular disease. Therefore, the goals of this study were as follows; 1) to determine whether moderate physical training for 4 weeks has effects on antioxidant enzyme activities and lipid profile to mimic a regular moderate exercise 2) to determine whether these indices of trained animal can be altered when the exercise induced oxidative stress to mimic a acute strenuous exercise was given.
Materials and Methods
Experimental animals and exercise
Forty eight male Sprague-Dawley rats (Deahanbiolink Co., Korea) weighing 70g were fed a semisynthetic diet which met AIN-93 recommendation (Reeves, 1997) for 4 weeks. Food consumption and animal weight were measured daily. Rats were randomly divided into 2 groups each consisting of 24 rats, given either a physical training or no training for 4 weeks. Then they were subdivided into 3 groups: before-exercise (BE); during-exercise (DE); after-exercise (AE). Each group had 8 rats. For moderate physical training, animals were exercised on a treadmill (10°, 0.5-0.8 km/h) for 30 minutes everyday. The BE group did not exercise before being sacrificed. The DE group was exercised on treadmill for 1 hour just before being sacrificed. Animals of the AE group were allowed to take a rest for 1 hour after being exercised like the DE group.
Sample collection and biochemical analysis
At the respective time points, animals were sacrificed by decapitation under light anesthesia. Immediately following decapitation, blood was collected in heparinized tubes and centrifuged to separate the plasma. Liver was rapidly removed. The activity of plasma catalase was determined with a commercial kit based on the method of Zamocky (Bioxytech Catalase-520). The activity of superoxide dismutase (SOD), the ratio of reduced glutathione and oxidized glutathione (GSH/GSSG), the levels of malondialdehyde (MDA) were determined in liver cytosol. Liver was homogenized in cold Tris-KCl buffer (0.1M). The homogenized solution was centrifuged (8,000×g, 4℃, 30 min). The supernatant was then centrifuged (10,000×g, 4℃, 30 min). Again the supernatant was ultra-centrifuged (105,000×g, 4℃, 90 min) and separated the cytosol. SOD activity was determined with a commercial kit based on the method of Nebot (Bioxytech SOD-525). GSH/GSSG was determined with a commercial kit based on the method of Anderson (Bioxytech GSH/GSSG-412). The level of MDA was determined with a commercial kit based on the method of Gerard-Monnier (Bioxytech MDA-586). Plasma triglyceride (TG) was analyzed with a commercial kit based on the Trinder method (Youngdong Phamaceutical Co., Korea). Total cholesterol (TC) was analyzed with a commercial kit based on enzymatic method (Youngdong Phamaceutical Co., Korea). High-density lipoprotein-cholesterol (HDL-C) was analyzed with a commercial kit based on the same analytical method as total cholesterol after the precipitation of very low-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol and chylomicron with polyethyleneglycol (International Reagent Co., Japan). Atherosclerotic index was calculated as (TC - HDL-C)/HDL-C.
Statistical analysis
All data were subjected to an analysis of variance and tested for significant differences by Duncan's multiple range test (SAS Institute, Cary, NC). P value of<0.05 was considered to be significant. The significance of difference between non-trained group and trained group was tested using student t-test at p<0.05.
Results
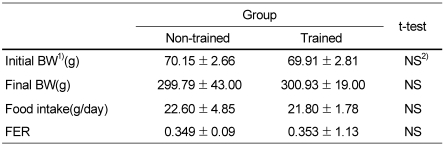
As shown in Table 1, there were no differences between non-trained group and trained group in body weight, FER(ratio of body weight change to food intake) and organ weights.
Table 1.
The effect of moderate physical training on body weight and feed efficiency ratio
1)BW: body weight, FER: feed efficiency ratio (ratio of body weight change to food intake).
2)No significant differences between non-trained group and trained group by student t-test (p<0.05).
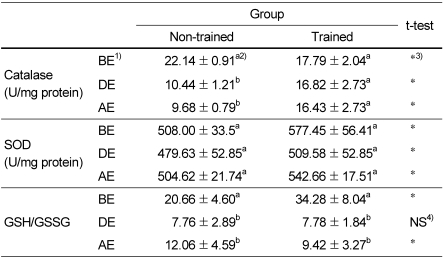
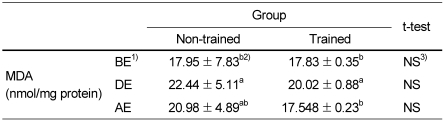
Table 2 shows the effects of moderate physical training on catalase activities. Catalase activities of trained group were significantly lower before exercise but higher during and after exercise than those of non-trained group. It was due to catalase activities of non-trained group were significantly decreased while those of trained group were not changed during and after exercise. SOD activities of trained group were significantly higher than those of non-trained group before as well as during and after exercise. SOD activities were not changed during and after exercise in both trained group and non-trained group. Table 2 also shows the effect of moderate physical training on GSH/GSSG ratio. Compared to those of non-trained group, GSH/GSSG ratios of trained group were significantly higher before exercise but were not significantly different during exercise and even lower after exercise because GSH/GSSG ratio of trained group was significantly decreased with exercise. Table 3 shows the effect of moderate physical training on liver MDA levels. There were no differences between non-trained group and trained group before as well as during and after exercise. MDA levels were increased during exercise and decreased after exercise and returned to the level of before exercise in both trained group and non-trained group.
Table 2.
The effect of moderate physical training on catalase and superoxide dismutase (SOD) activities and the ratio of reduced glutathione and oxidized glutathione (GSH/GSSG)
1)BE: before-exercise, DE: during-exercise, AE: after-exercise.
2)Data are means ± SD of eight rats. a,b: Different superscript letter mean significant difference among groups by Tukey Duncan's multiple range test after one-way ANOVA at α=0.05
3)*Significant differences between non-trained group and trained group by student t-test (p<0.05).
4)No significant differences between non-trained group and trained group by student t-test (p<0.05).
Table 3.
The effect of moderate physical training on malondialdehyde(MDA) levels
1)BE: before-exercise, DE: during-exercise, AE: after-exercise.
2)Data are means ± SD of eight rats. a,b: Different superscript letter mean significant difference among groups by Tukey Duncan's multiple range test after one-way ANOVA at α=0.05
3)No significant differences between non-trained group and trained group by student t-test (p<0.05).
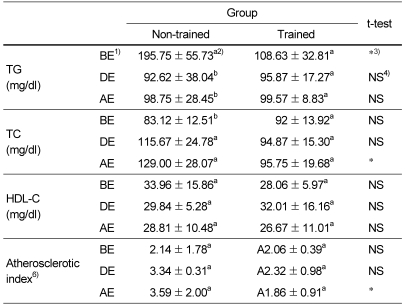
Table 4 shows the effect of moderate physical training on atherosclerotic index. Compared to those of non-trained group, TG level of trained group was lower before exercise and was not different during and after exercise. TC level of trained group was not different before exercise but was significantly lower during and after exercise. Atherosclerotic index of trained group was significantly lower after exercise. However, there were no significant differences before and during exercise because atherosclerotic index of trained group was not significantly changed with exercise but that of non-trained group tended to be increased during and after exercise. Table 4 also shows the effect of moderate physical training on HDL-C levels. There were no differences between non-trained group and trained group regardless of exercise and were not changed during and after exercise in both trained group and non-trained group.
Table 4.
The effect of moderate physical training on the levels of triglyceride, total cholesterol, HDL cholesterol and atherosclerotic index
1)BE, before-exercise; DE, during-exercise; AE, after-exercise; TG, triglyceride; TC; total cholesterol
2)Data are means ± SD of eight rats. a,b: Different superscript letter mean significant difference among groups by Tukey Duncan's multiple range test after one-way ANOVA at α=0.05
3)*Significant differences between non-trained group and trained group by student t-test (p<0.05).
4)No significant differences between non-trained group and trained group by student t-test (p<0.05).
5)Atherosclerotic index = (total cholesterol-HDL-C) /HDL-C
Discussion
This study showed that moderate physical training induced a favorable turn on the antioxidative status. The evidence of an alteration in the antioxidative status with oxidative stress is based on the change of antioxidant enzyme activities and lipid peroxidation with exercise induced oxidative stress. At various points during the study, the antioxidative status in rats was evaluated using the ratios of GSH/GSSG and the activities of catalase and SOD as a direct measure and the level of MDA and lipid profile as an indirect, long-term measure.
The hypothesis that moderate physical training can react effectively on exercise-induced oxidative stress and increase the activity of antioxidant enzymes was verified by results from two different studies. Catalase activities of trained group were significantly lower before exercise but higher during and after exercise than those of non-trained group because catalase activities of non-trained group were significantly decreased while those of trained group were not changed during and after exercise. Thus, it is suggested that exercise-induced oxidative stress did not affect the catalase activities in trained group while exercise-induced oxidative stress led a decrease in plasma catalase activities in non-trained group. SOD activities of trained group were significantly higher than those of non-trained group regardless of exercise. These tendencies were not changed during and after exercise in both trained group and non-trained group. Since superoxide barely traverses cell membrane and is rapidly converted by SOD, the resulting product hydrogen peroxide can diffuse through the vascular wall and is much more stable (Kojda & Harrison, 1999), and thus it is suggested that the SOD activity in trained group was increased and remained at higher levels and the antioxidative status of trained group can be remained as higher even under exercise-induced oxidative stress.
Compared to those of non-trained group, GSH/GSSG ratios of trained group were significantly higher before exercise but were not significantly different during exercise and even lower after exercise because GSH/GSSG ratio of trained group was significantly decreased with exercise induced oxidative stress. This result is consistent with the report that chronic exercise training appears to induce activities of antioxidant enzymes such as vascular eNOS and perhaps stimulate levels of reduced glutathione in body fluids (Banerjee et al., 2003). On the other hand, very hard exercise training for 4weeks induced an increase of plasma glutathione peroxidase activity and a decrease of plasma total antioxidant status under resting, pre-exercise conditions, and these effects were more pronounced under post-exercise conditions and accompanied by a decreased ratio of GSH/GSSG and an increase in plasma thiobarbituric acid reactive substances (TBARS) (Palazzetti et al., 2003). Previous studies (Tiidus et al., 1993; Viinikka et al., 1984) reported no increase in plasma lipid peroxidation with exercise in highly trained subjects, but a significant elevation in serum TBARS in less well-trained subjects submitted to a similar exercise protocol (Sumida et al., 1989). In this study, there were no differences of liver MDA levels between non-trained group and trained group regardless of exercise induced oxidative stress. MDA levels were increased during exercise and decreased after exercise and returned to the level of before exercise in both trained group and non-trained group. Thus, it is suggested that moderate physical training, repetition of short-term generation of increased vascular oxidative stress, induced an increase of antioxidative enzyme activity and the antioxidant status but did not seem to significantly contribute to the changes of live MDA levels.
Regular exercise has been associated with variable effects on the blood lipid profile. While many studies have shown benefits of exercise in reducing hypertension and dyslipidemic-lowering components (Bacon et al., 2004; Peters et al., 2006; Regina et al., 2006), but not all studies showed the same results (Blumenthal et al., 1991). Since elevated markers of oxidative stress have been associated with ventricular function (Vukasovic et al., 2005) and the decrease of these markers was observed in the trained group of this study, the change of lipid profile as well as atherosclerotic index as a result of moderate physical training was investigated. Compared to those of non-trained group, atherosclerotic indexes of trained group were significantly lower after exercise. However, there were no significant differences before and during exercise because atherosclerotic index of trained group was not significantly changed with exercise induced oxidative stress but that of non-trained group tended to be increased during and after exercise. Since there were no differences in HDL-C levels between non-trained group and trained group regardless of exercise, HDL-C levels were not changed during and after exercise in both groups. It is suggested that exercise-induced oxidative stress did not affect the HDL-C levels in both trained group and non-trained group. Moderate exercise-training has been reported to increase HDL-cholesterol (Schwartz, 1987; Sopko et al., 1985), however, the results of the present study did not support the predominant effect of exercise-training. These data suggest that a moderate physical training may be sufficient to increase the antioxidative status and decrease the atherosclerotic index but appears to have little further effect on HDL-cholesterol.
Therefore, despite many uncertainties regarding the mode of action, these results suggest that moderate physical training can activate antioxidant defenses and decrease the atherosclerotic index and this beneficial effects is evident under exercise induced oxidative stress. It is suggested that moderate physical training can be an effective antioxidant and antiatherogenic therapy at lower costs.
Footnotes
This study was supported by 2006 Research fund of Institute of Natural Science Research, Duksung Women's University
References
- 1.Bacon SL, Sherwood A, Hinderliter A, Blumenthal JA. Effects of exercise, diet and weight loss on high blood pressure. Sports Med. 2004;34:307–316. doi: 10.2165/00007256-200434050-00003. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee AK, Mandal A, Chanda D, Chakraborti S. Oxidant, antioxidant and physical exercise. Mol Cell Biochem. 2003;253:307–312. doi: 10.1023/a:1026032404105. [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal JA, Sigel WC, Applebaum M. Failure of exercise to reduce blood pressure in patients with mild hypertension. Results of a randomized controlled trial. JAMA. 1991;266:2098–2104. [PubMed] [Google Scholar]
- 4.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999;222:283–292. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- 6.Kojda G, Harrison DG. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 7.Lovlin R, Cottle W, Kavanagh M. Are indices of free radical damage related to exercise intensity? Eur J Appl Physiol Occup Physiol. 1991;56:313–316. doi: 10.1007/BF00690898. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell SRJ, Jakeman P, Thomason H. Changes in plasma antioxidant status during eccentric exercise and the effect of vitamin supplementation. Free Radical Research Communications. 1993;19:191–201. doi: 10.3109/10715769309111602. [DOI] [PubMed] [Google Scholar]
- 9.Palazzetti S, Richard MJ, Favier A, Margaritis I. Overloaded training increases exercise-induced oxidative stress and damage. Can J Appl Physiol. 2003;28:588–604. doi: 10.1139/h03-045. [DOI] [PubMed] [Google Scholar]
- 10.Peters PG, Alessio HM, Hagerman AE, Ashton T, Nagy Szilvia, Wiley RL. Short-term isometric exercise training reduces systolic blood pressure in hypertensive adults: Possible role of reactive oxygen species. Int J Cardiol. 2006;110:199–205. doi: 10.1016/j.ijcard.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Powers SK, Hamilton K. Antioxidants and exercise. Clin Sports Med. 1999;18:525–536. doi: 10.1016/s0278-5919(05)70166-6. [DOI] [PubMed] [Google Scholar]
- 12.Reeves PG. Components of the AIN-93 diets as im-provements in the AIN-76A diet. J Nutr. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 13.Regina CMB, Yeda SD, Criatinano MG, Hosana GR, Genova MXE, Luciane AF, Carlos RP, Antonio CC, Ethel LBN. Interaction of hyper caloric diet and physical exercise on lipid profile, oxidative stress and antioxidant defenses. Food Chem Toxicol. 2006;44:1167–1172. doi: 10.1016/j.fct.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol. 2003;284:H1378–H1387. doi: 10.1152/ajpheart.00190.2002. [DOI] [PubMed] [Google Scholar]
- 15.Sahlin K, Ekberg K, Cizinsky S. Changes in plasma hypoxanthine and free radical markers during exercise in man. Acta Physiol Scand. 1991;142:275–281. doi: 10.1111/j.1748-1716.1991.tb09157.x. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz RS. The independent effects of dietary weight loss and aerobic training on high density lipoproteins and apolipoprotein A-I concentrations in obese men. Metabolism. 1987;36:165–171. doi: 10.1016/0026-0495(87)90012-6. [DOI] [PubMed] [Google Scholar]
- 17.Schwenke DC. Antioxidants and atherogenesis. J Nutr Biochem. 1998;9:424–455. [Google Scholar]
- 18.Sopko G, Leon AS, Jacobs DR. The effects of exercise and weight loss on plasma level lipids in young obese men. Metabolism. 1985;34:227–236. doi: 10.1016/0026-0495(85)90005-8. [DOI] [PubMed] [Google Scholar]
- 19.Sumida S, Tanaka K, Kitao H, Nakadomo F. Exercise induced lipid peroxidation and leakage of enzymes before and after vitamin E supplementation. International Journal of Biochemistry. 1989;21:835–838. doi: 10.1016/0020-711x(89)90280-2. [DOI] [PubMed] [Google Scholar]
- 20.Tiidus PM, Behrens WA, Madere R, Kim JJ, Houston ME. Effects of vitamin E staus and exercise training on tissue lipid peroxidation based on two methods of assessment. Nutr Res. 1993;13:219–224. [Google Scholar]
- 21.Viinikka L, Vuori J, Ylikorkkala O. Lipid peroxides, prostacyclin, and thromboxane A2 in runners during acute exercise. Med Sci Sports Exerc. 1984;16:275–277. [PubMed] [Google Scholar]
- 22.Vukasovic JL, Moraga F, Diaz-Araya G, Turner E, Chiong M, Uriarte P, Florenzano F, Lavandero S. Oxidative stress in pericardial fluid and plasma and its association with ventricular function. Int J Cardiol. 2005;101:197–201. doi: 10.1016/j.ijcard.2004.03.013. [DOI] [PubMed] [Google Scholar]