Abstract
Trace mineral studies involving metal ion chelators have been conducted in investigating the response of gene and protein expressions of certain cell lines but a few had really focused on how these metal ion chelators could affect the availability of important trace minerals such as Zn, Mn, Fe and Cu. The aim of the present study was to investigate the availability of Zn for the treatment of MC3T3-E1 osteoblast-like cells and the availability of some trace minerals in the cell culture media components after using chelexing resin in the FBS and the addition of N,N,N',N'-tetrakis-(2-pyridylmethyl)ethylenediamine (TPEN, membrane-permeable chelator) and diethylenetriaminepentaacetic acid (DTPA, membrane-impermeable chelator) in the treatment medium. Components for the preparation of cell culture medium and Zn-treated medium have been tested for Zn, Mn, Fe and Cu contents by atomic absorption spectrophotometer or inductively coupled plasma spectrophotometer. Also, the expression of bone-related genes (ALP, Runx2, PTH-R, ProCOL I, OPN and OC) was measured on the cellular Zn depletion such as chelexing or TPEN treatment. Results have shown that using the chelexing resin in FBS would significantly decrease the available Zn (p<0.05) (39.4 ± 1.5 µM vs 0.61 ± 10.15 µM) and Mn (p<0.05) (0.74 ± 0.01 µM vs 0.12 ± 0.04 µM). However, levels of Fe and Cu in FBS were not changed by chelexing FBS. The use of TPEN and DTPA as Zn-chelators did not show significant difference on the final concentration of Zn in the treatment medium (0, 3, 6, 9, 12 µM) except for in the addition of higher 15 µM ZnCl2 which showed a significant increase of Zn level in DTPA-chelated treatment medium. Results have shown that both chelators gave the same pattern for the expression of the five bone-related genes between Zn- and Zn+, and TPEN-treated experiments, compared to chelex-treated experiment, showed lower bone-related gene expression, which may imply that TPEN would be a stronger chelator than chelex resin. This study showed that TPEN would be a stronger chelator compared to DTPA or chelex resin and TPEN and chelex resin exerted cellular zinc depletion to be enough for cell study for Zn depletion.
Keywords: Zn depletion, metal ion chelators, DTPA, TPEN, chelexing, MC3T3-E1 cells
Introduction
Trace element Zn generally participates in a wide variety of cellular processes as a cofactor of more than 300 enzymes, and these enzymes affect most major metabolic processes (Beyersmann & Haase, 2001). The bioavailability of Zn in vitro, participated in cell proliferation and differentiation, and the possible way that Zn generally functions in the regulation of cell proliferation is through the enzyme systems that influence cell division and proliferation (Tapiero & Tew, 2003). Zn and some bone-related trace minerals are important in the study of membrane transport mechanisms and for the investigation of protein and gene expressions. At present, metal ion chelators such as chelexing resins, DTPA (diethylenetriaminepentaacetic acid), TPEN [N,N,N',N'-tetrakis(2-pyridylmethyl) ethylenediamine], BAPTA [1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid] and its derivatives, EDTA (diaminoethanetetraacetic acid) and EGTA (ethyleneglycoltetraacetic acid) are being used for limiting the available metal ions contained in cellular medium (Aballay et al., 1995; Frederickson et al., 2002; Hyun et al.,2001; Kanekiyo et al., 2000; Libin et al., 2002). These metal ion chelators not only bind with the target mineral for certain studies but also bind to any possible metal ion present in the medium system. Chelating agents like TPEN and DTPA are often used in Zn treatment studies. The depletion treatment/method has a direct impact on both intra- and extracellular concentrations of Zn and other trace minerals thus affecting cellular metabolism (Frederickson et al., 2002). Depletion of Zn by TPEN induced apoptosis in hepatocytes, whereas depletion of cellular Zn by DTPA did not induce apoptosis (Nakatini et al., 2000).
Mouse calvariae MC3T3-E1 osteoblast-like cells have been studied for bone morphology, extracellular mineralization, growth factors and metabolism. Expression patterns for genes and proteins in response to trace minerals like Ca and Zn have been conducted using the MC3T3-E1 cells (Beyersmann & Haase, 2001). Osteoblasts produce osteoid and matrix vesicles to which Zn ions are either freely or loosely bound. The direct effect of Zn in osteoblasts is on the mineralization on the action of nucleation and mineral growth (Ovesen et al., 2001). It would be important to treat cells appropriately with the considerable amount of metal ions including Zn for each of different cell types. Zinc is known as to function on skeletal growth and bone metabolism. One of the major Zn deficiencies is characterized by growth retardation which would be affected by skeletal growth inhibition in vivo and decreased cell proliferation and differentiation (Avery & Bettger, 1992; Perry et al., 1997; Telford & Fraker, 1995). In the need to properly treat the osteoblastic cells in bone study with the considerable amount of Zn and other trace metal ions present in cell culture medium, this study was conducted to generally (1) evaluate the status of Zn and other trace elements (Mn, Fe and Cu) by chelexing FBS in osteogenic cell culture media and its components (2) compare the chelating effect of TPEN and DTPA in the status of Zn and Fe in osteogenic cell culture media and its components, (3) investigate the effect of Zn on bone-related gene expressions of osteoblastic MC3T3-E1 cells under mild Zn-deficiency (using chelexed FBS) and severe Zn-deficiency (using TPEN).
Materials and Methods
Chelation of Zn in MC3T3-E1 osteoblast-like cell medium. The availability of Zn in osteoblastic cell culture media was depleted by using Zn-chelexed fetal bovine serum (FBS), 5 µM N,N,N',N'-tetrakis(2-pyridylmethyl) ethylenediamine (TPEN) and diethylenetriaminepentaacetic acid (DTPA) as Zn chelators. Both chelex resins for chelexing of Zn in FBS and DTPA as extracellular Zn chelator can be used in mild Zn deficient treatments. TPEN as intracellular Zn chelator, on the other hand, can be used in severe Zn deficient treatments.
Mild Zn deficiency
The chelexing process of FBS was done as described in our previous paper (Cho et al., 2005). Chelex-100 exchange resin was purchased from Bio-Rad. ZnCl2 was purchased from Sigma and cell culture reagents such as FBS and basal media were purchased from GIBCO. Briefly, 5 g chelex-100 ion exchange resin was added to 100 mL FBS and stirred overnight at 4℃. The FBS supernatant was taken and was filter-sterilized into polyethylene centrifuge tubes after the removal of resin. At this stage, Zn which already combined to chelex resin can be discarded and Zn-eliminated FBS can be used for Zn-depleted culture media composition. The chelexed FBS was then added to the osteogenic differentiation medium with a final concentration of 10%. Different levels of Zn (0, 3, 6, 9, 12 and 15 µM ZnCl2) were added to the media prior to cell treatment.
Severe Zn deficiency
N,N,N,',N'-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN) and diethylenetriaminepentaacetic acid (DTPA) were purchased from Sigma. Exposure to TPEN and DTPA was accomplished by direct addition of the calculated volume based on the stock solution to the cell culture treatment media in a final concentration of 5 µM and 1.2 mM, respectively. The added TPEN (intracellular Zn chelator) and DTPA (extracellular Zn chelator) could bind with Zn and thus prevent cellular uptake of Zn bioavailability in cells. The various concentrations of Zn (0, 3, 6, 9, 12 and 15 µM ZnCl2) were then added to the culture treatment media.
Preparation of media samples and mineral analysis
Fresh media samples were collected and wet-digested in preparation for mineral analysis. Nitric acid (1 mL) was added to the fresh media samples (0.5 mL) and digested overnight on a hooded heating block. On the following day, distilled/deionized water was added to the digested samples. The solution obtained was used directly to determine the mineral content (Zn, Fe, Cu and Mn) using an atomic absorption spectrophotometer (SpectrAA 220 FS, Varian, Australia). The accuracy of the method for mineral analysis was evaluated using bovine liver standard reference material (SRM) obtained from the National Institute of Standards and Technology (NIST SRM 1577b, USA).
RT-PCR for bone-related gene expression
MC3T3-E1 cells were cultured in regular growth medium (α-MEM +10% FBS +100 units/mL penicillin +100 µg/mL streptomycin) until they reached 100% confluency. The regular growth medium was then replaced with an osteogenic differentiation media (growth medium +10 mM β-glycerophosphate +50 µg/mL ascorbic acid) and only two Zn levels: Zn- and Zn+ as described below for chelexing FBS or TPEN treatment. Two parallel treatments were done in the chelation of Zn: through addition of 5 µM TPEN and through addition of chelexed-FBS in the treatment medium. MC3T3-E1 cells were harvested after 5 days of treatment and subjected to gene expression analysis. The addition of Zn varied in concentration between chelexed FBS and TPEN-treated differentiation medium. Zn treatment using chelexed FBS used the Zn concentrations of 0 (Zn-) and 15 µM ZnCl2 (Zn+) while Zn treatment using TPEN used the Zn concentrations of 1 (Zn-) and 15 µM ZnCl2 (Zn+). Supplementation of Zn must be given with the TPEN-treated medium since cells often died within 5 days of treatment (data not shown). Osteoblastic bone marker gene expressions (ALP, Runx2, PTH receptor, procollagen type I, osteopontin, and osteocalcin) were measured by reverse-transcriptase-PCR. The primers for each gene were reported in the previous study (Cho et al., 2005). For first-strand cDNA synthesis, 100 ng of RNA from each sample was reverse transcribed using 20 U of AMV reverse transcriptase and Oligo-p (dT) 1 X random primers (Roche Diagnostics, USA). The resulting cDNAs were PCR-amplified by using a mixture of the corresponding sense and antisense primers. The PCR conditions were 95℃ for 2 min and then 30 cycles at 95℃ for 30 s, 54℃ for 45 s and 72℃ for 1 min and a final extension at 72℃ for 5 min. The PCR products were separated on 1.8% agarose gel.
Statistical analysis
All values were presented as mean ± SEM. Data were analyzed using SPSS program and differences were considered significant as p < 0.05. Statistical analysis of the data was performed by one-way ANOVA to test the effect of various Zn levels. Once significance was detected among the various Zn levels, Turkey's HSD test was used for comparison of the difference between groups.
Results
Effect of using chelexed FBS on the mineral (Zn, Mn, Fe and Cu) content of cell culture media components
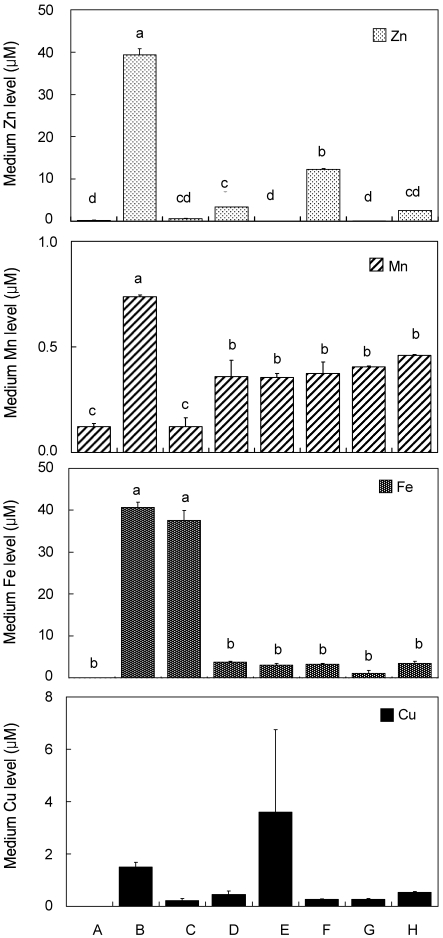
New cell culture media components were tested for trace mineral content - Zn, Mn, Fe and Cu - as shown in Figure 1. Zinc is an important trace mineral that is needed by many biological enzymes and was the target mineral of our study. Fresh α-MEM contained 0.23 ± 0.080 µM Zn which was considered to be absent in the basal media (Fig. 1). Fresh FBS was found to have a significantly higher amount of Zn, 39.37 ± 1.46 µM. However, chelexing FBS resulted in the decrease in the availability of Zn in fresh FBS which had an amount of 0.61 ± 0.15 µM Zn. The addition of 10% fresh FBS to α-MEM (as control medium) resulted in a diluted Zn content (3.36 ± 0.00 µM Zn). The addition of 10% chelexed FBS to α-MEM, on the other hand, resulted in a sudden decrease of Zn level in the fresh medium. This confirms that chelexing FBS decreases the available Zn in fresh medium. The addition of 15 µM Zn in a mixture of α-MEM and 10% chelexed FBS showed the concentration of 12.31 ± 0.23 µM Zn which was significantly higher than the control medium containing 10% fresh FBS in α-MEM. Similar test was done to the DMEM and DMEM +10% FBS, which showed a similar pattern with α-MEM and α-MEM +10% FBS.
Fig. 1.
Trace mineral (Zn, Mn, Fe and Cu) composition of various cell culture media components using chelexed FBS in different media (n=2)
Different letter superscripts mean significantly different between media composition at p<0.05 by Tukey, one-way ANOVA. [A, αMEM (fresh); B, FBS (fresh); C, chelexed FBS; D, αMEM +10% FBS; E, αMEM +10% chelexed FBS; F, αMEM +10% chelexed FBS +15 µM ZnCl2; G, DMEM; H, DMEM +10% FBS].
Manganese is also an important trace mineral which is affected by the chelaxing resin (Chelex 100) used in the experiment. Fresh α-MEM contained 0.12 ± 0.01 µM Mn and fresh FBS contained 0.74 ± 0.01 µM Mn. After the chelation of FBS, the Mn concentration was significantly decreased to 0.12 ± 0.04 µM. The addition of 10% FBS to α-MEM resulted in a final Mn concentration of 0.36 ± 0.08 µM which was not significantly different from Mn concentration of α-MEM +10% chelexed FBS and α-MEM +10% chelexed FBS +15 µM ZnCl2 which had values of 0.36 ± 0.02 and 0.37 ± 0.06 µM, respectively. Results suggest that the chelation of FBS will not significantly change the availability of Mn in the control and treatment media.
Another important trace mineral is Fe which also can be affected by the chelation of FBS with the chelexing resin. In α-MEM, Fe was found to be absent. Fresh FBS, however, contained a significantly higher amount of Fe, 40.65 ± 1.25 µM. Chelaxing FBS resulted in a decrease in concentration of 37.60 ± 2.33 µM but was found to be not significantly different from the fresh FBS. Addition of 10% fresh FBS to α-MEM resulted in a decreased amount of Fe which could be based on the dilution of the FBS to the Fe-free α-MEM. The Fe content of the mixtures of α-MEM +10% fresh FBS and α-MEM + chelexed FBS were not significantly different having 3.76 ± 0.18 µM and 3.04 ± 0.36 µM Fe, respectively. Moreover, the addition of 15 µM ZnCl2 to α-MEM + chelexed FBS, did not affect the Fe concentration either, as expected. Fresh DMEM, on the other hand, contained higher amount of Fe than with fresh α-MEM, even without significance. However, similar pattern was observed upon the addition of fresh 10% FBS to DMEM and 10% FBS to α-MEM.
Copper is found to have a significant relationship with Zn in several antioxidant proteins. Also, Cu is found to be affected by the chelating resin used in this study. There were no significant differences in the Cu content between the cell media components.
Effect of chelexed FBS on the mineral (Zn, Mn, Fe and Cu) content of Zn-treated medium
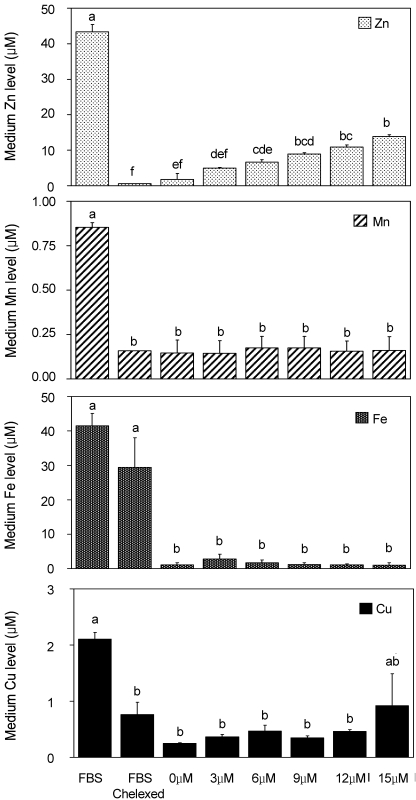
Since we confirmed the significant decrease in the availability of Zn in the culture media and its components, we therefore determined the mineral content of the treatment medium with various levels of Zn. Figure 2 shows the effect of using chelexed FBS on the contents of Zn, Mn, Fe, and Cu in the different Zn-treated medium. It must be noted that a new batch of FBS and chelexed FBS were used in this part of the experiment and that the range for values still fell at the same level. The trace mineral levels of FBS and chelexed FBS in Figure 1 may slightly vary from the values of FBS and chelexed FBS in Figure 2.
Fig. 2.
Trace mineral (Zn, Mn, Fe and Cu) composition of osteoblastic cell culture media with various Zn levels using chelexed FBS (n=2)
Zn-treated Media (0-15 µM Zn) were prepared as the addition of 0 to 15 µM Zn as ZnCl2 to a-MEM plus chelexed FBS. Different letter superscripts mean significantly different between Zn media treatments at p<0.05 by Tukey, one-way ANOVA.
The Zn level of the treatment medium resulted in an expected pattern. The Zn content of FBS was significantly dropped from 43.38 ± 0.04 µM to 0.562 ± 0.04 µM after chelexing the FBS. The addition of Zn (0, 3, 6, 9, 12, and 15 µM Zncl2) increased the availability of zinc in the treatment medium. The Mn level, on the other hand, showed no significant difference between the chelexed FBS and the different Zn-treated culture medium. Fe and Cu showed the same pattern as with the level of Mn with no significance between the chelexed FBS and the different Zn-treated culture medium.
Effect of TPEN and DTPA chelators on Zn-treated medium
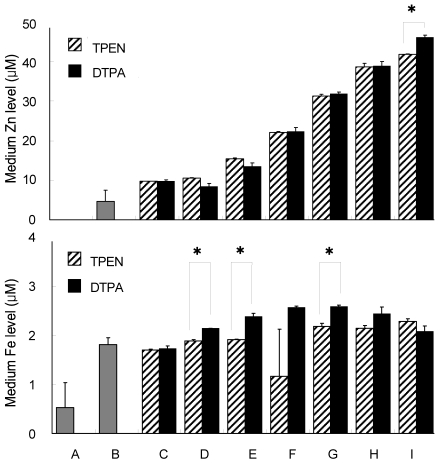
Unlike the chelexing resin, TPEN and DTPA are metal ion chelators that are being added directly to the treatment medium. The effect of TPEN as an intracellular (membrane-permeable) chelator of Zn would result in severe Zn-deficiency whereas DTPA as an extracellular (membrane-impermeable) chelator show relatively similar effect with FBS-chelaxing which would result in mild Zn-deficiency. In this part of the study, DTPA was used as a substitute for chelexed FBS. Figure 3. shows the mineral contents of different medium composition and Zn-treated medium with the addition of both TPEN and DTPA. Fresh α-MEM contained no Zn whereas growth media contained 2.33 ± 1.40 µM Zn. Upon the addition of TPEN and DTPA in the osteogenic differentiation media, Zn levels for both chelators were found to be at the same level with 4.88 ± 0.02 and 4.86± 0.18 µM Zn, respectively. Addition of 0, 3, 6, 9 and 12 µM ZnCl2 showed no significant difference between the TPEN- and DTPA-chelated Zn-treated medium. Addition of 15 µM ZnCl2, however, resulted in higher Zn level in DTPA-chelated medium than TPEN with Zn concentrations of 23.07 ± 0.28 µM and 20.91 ± 0.04 µM, respectively.
Fig. 3.
Zn and Fe level of osteoblastic cell culture media components using TPEN and DTPA as Zn chelators (n=2)
Five µM TPEN and 1.2 mM DTPA were the optimized concentration used to chelate Zn. Different letter superscripts mean significantly different between media composition at p<0.05 by one-way ANOVA. [A, αMEM; B, growth media; C, osteogenic differentiation media + TPEN or DTPA*; D, 0 µM ZnCl2 + TPEN or DTPA; E, 3 µM ZnCl2 + TPEN or DTPA; F, 6 µM ZnCl2 + TPEN or DTPA; G, 9 µM ZnCl2 + TPEN or DTPA; H, 12 µM ZnCl2 + TPEN or DTPA; I, 15 µM ZnCl2 + TPEN or DTPA].
Chelation of Fe through TPEN generally showed a lower value than with DTPA. However, there were significant differences in the Fe levels of Zn-treated medium of 0, 3 and 9 µM ZnCl2. Levels of Fe in 0, 3 and 9 µM ZnCl2-treated media were significantly lower in TPEN-chelated medium compared to DTPA-chelated medium with values of 1.88 ± 0.03, 1.90 ± 0.02 and 2.18 ± 0.07 µM vs 2.13 ± 0.00, 2.38 ± 0.06 and 2.58 ± 0.03 µM, respectively.
Response of osteoblastic MC3T3-E1 cells on bone-related gene expressions by TPEN- and chelexed FBS-treated Zn treatment
MC3T3-E1 cells were treated with Zn- and Zn+ treatments with the use of chelexed FBS and TPEN in the treatment medium. Note that at Zn-, the concentration of Zn added to the chelexed FBS and TPEN-treated differentiation medium differed with 0 and 1 µM ZnCl2, respectively. This was intentionally done since the medium with chelexed FBS contained considerable amount of Zn that could still be utilized by cells within 5 days of treatment. The TPEN-treated medium, however, needed to be supplemented with Zn since TPEN is known to chelate metal ions severely, thus chelating almost all Zn ions present in the treatment medium. MC3T3-E1 cell was found to negatively respond (die) to TPEN-treated medium with 0 µM ZnCl2.
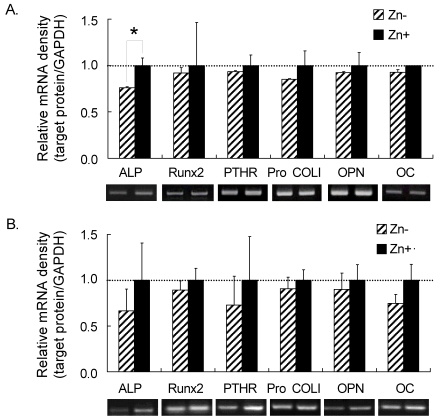
Five bone-related genes (ALP, Runx2, PTHR, Pro COL I, OPN and OC) were investigated on the effect of different Zn chelators, such as TPEN and chelexed FBS, in the treatment medium (Fig. 4). Treatments for both chelexed FBS and TPEN showed similar pattern for all the genes mentioned above. Treatment with Zn+ increased the bone-related gene expressions of MC3T3-E1 cells.
Fig. 4.
Bone-related gene expressions in osteoblastic MC3T3-E1 cells at 5 days of Zn treatment using A) chelexed FBS and B) TPEN in Zn-treated osteogenic differentiation medium
Asterisks (*) mean significantly different between Zn treatments (Zn- vs Zn+) at p<0.05 by one-way ANOVA.
Discussion
Cell culture studies involving the bioavailability of Zn as a varying factor in metabolism and apoptosis have become interesting subjects in these days. Recent studies have shown that Zn plays a catalytic, co-catalytic and/or structural role in the proper folding of proteins (Bray & Bettger, 1990). Zinc ions exist primarily in the form of complexes with proteins and nucleic acids and participate in all aspects of intermediary metabolism. Zn has been found to maintain metallothionein tissue concentrations, stabilize the cell membrane, contribute to the structure of antioxidants such as SOD and facilitate in the regulation of expression of genetic information, storage and synthesis. Thus, Zn deficiency may cause direct and indirect imbalances in the cellular system which could lead to apoptosis (Perry et al., 1997; Tapiero & Tew, 2003; Telford & Fraker, 1995).
Depletion of Zn prior to treatments in vitro is an important concern in cell culture study. Depletion of Zn in cell culture media can be achieved by using metal ion chelators such as chelexing resins, addition of metal ion chelators such as TPEN (membrane-permeable) and DTPA (membrane-impermeable), and other metal ion chelators which are not Zn-specific such as BAPTA (1,2-bis-(o-aminophenoxy)ethane-N,N,N',N-tetra-acetic acid), EDTA (ethylenediaminetetraacetic acid) and EGTA (ethylene glycol tetraacetic acid) (Aballay et al., 1995; Frederickson et al., 2002; Hyun et al., 2001; Kanekiyo et al., 2000; Libin et al., 2002). This study focused on the chelating effects of using chelexing resin, TPEN and DTPA on the bioavailability of Zn and other important trace minerals (Mn, Fe, and Cu) on the cell culture treatment media of osteoblastic MC3T3-E1 cells.
Chelexing resin, TPEN and DTPA are metal ion chelators known to have high affinities with Zn. The present study focused on Zn and three bone-related minerals, namely Mn, Fe and Cu. The first chelexing treatment done for this study was by using Chelex-100 (Bio-Rad). Chelex 100 resin is a styrene divinylbenzene copolymer containing paired iminodiacetate ions which act as chelating groups in binding polyvalent ions. It has high preference for Cu, Fe, and other heavy metals over monovalent cations such as Na and K (Swanson & Feigenson, 1990). The selectivity of cations to bind chelex resins varies depending on the solution's pH and composition. In an aqueous solution with pH 4, the selectivity for various cations is in the order of Cu2+ > Zn2+ > Fe2+ > Mn2+. In the presence of 1.5 M (NH4)2SO4 in aqueous solution with pH 9, the selectivity of cations applies only to copper and zinc (from the 4 trace minerals being studied) with an affinity of Cu2+ > Mn2+. In the presence of nitrate and chloride ions, chelex resin selectively binds to divalent cations with an order of selectivity of: Cu2+ > Fe3+ > Zn2+ > Fe2+ > Mn2+. This would be the basis of the affinity of metal ions in this study since α-MEM contains chloride (Cl-) ions from inorganic salts such as CaCl2, KCl and NaCl. Based on the data presented, Zn and Mn were significantly decreased after chelexing FBS with resin (Figure 1: column B for FBS and C for chelexed FBS). Zn, the main trace element being studied, has been shown to significantly decrease the concentration of Zn in FBS to chelexed FBS from 39.37 ± 1.46 to 0.61 ± 0.15 µM. Also, the addition of 10% chelexed FBS to α-MEM would give a 3-fold decrease in Zn concentration to 10% normal FBS and α-MEM. Addition of 15 µM ZnCl2 would result in approximately 12.31 ± 0.23 µM Zn in α-MEM and 10% chelexed FBS.
The addition of various levels of Zn to the growth media using 10% chelexed FBS was investigated in detail. Zn concentrations of 0, 3, 6, 9, 12 and 15 µM ZnCl2 were added to the growth media using 10% chelexed FBS. The resulting concentration of Zn turned out as expected with an increasing Zn level pattern as the addition of Zn increased. Interestingly, the level of Mn (which has the least affinity with chelex resin among the 4 trace minerals being studied) was not changed significantly upon the addition of ZnCl2. This suggests that chelexing resin still binds effectively to the metal ion even with the least affinity. Furthermore, levels of Fe and Cu also remained at one level upon the addition of Zn. Results has confirmed that using 10% chelexed FBS in the Zn treatment of cell culture media would change the levels of Zn but not the other trace minerals.
The use of heavy metal ion chelators, TPEN and DTPA, was tried as the second treatment of chelation. N,N,N,',N'-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN) is a cell and nuclear membrane permeable heavy metal chelator with high affinities for Zn2+ > Fe2+ > Mn2+. TPEN acts by chelating these ions in order of their affinities or combinations of these ions, thereby activating one or more metal-binding protein(s) (Shumaker, 1998). Diethylenetriaminepenta-acetic acid (DTPA), on the other hand, is a hydrophobic and membrane-impermeable chelator that accesses the extracellular space and binds iron as well as other ions from the extracellular pool (Aisen & Listowsky, 1980). DTPA is an elongated version of EDTA and has been used to detoxify humans who are contaminated with plutonium, americium and other actinides (Westin & Rasmuson, 2005). Cinatl et al. (1996) proposed that DTPA may directly interact with membrane associated proteins and lipids in in vitro study of virus for which Zn is a constituent of many proteins and enzymes. Previous studies have used DTPA in vitro in investigating the role of metal ions in cellular functions, particularly that of Zn (Chattopadhyay & Freake, 1998; Chesters et al., 1989; Lefebyre et al., 1998; Nakatani et al., 1998; Petrie et al., 1991).
TPEN was used in the cell culture media with a final concentration of 5 µM while DTPA with 1.2 mM. Chelation of trace minerals using TPEN and DTPA were not significantly different with regard to the final concentration of Zn (Figure 3). Levels for Cu and Mn were found to be chelated entirely by TPEN and DTPA (data not shown). The final concentration of Fe, however, showed a significant difference in 0, 3, and 9 µM ZnCl2. Lower levels of Fe in TPEN suggests that TPEN is a stronger chelator of metal ions than DTPA. With low levels of Fe and the absence of Mn and Cu, TPEN is better in chelating heavy metals than DTPA.
Finally, bone-related gene expressions were investigated in response to TPEN and chelexed resin. Since Zn level in TPEN showed no significant difference from that in DTPA, TPEN was used for the cell culture treatment because this chelator can penetrate through the cell membrane as well as the nuclear membrane. Thus, Zn chelation with TPEN would cause severe Zn-deficiency to MC3T3-E1 cells while Zn chelation with chelex resin would cause mild Zn-deficiency. Bone-related gene expressions of ALP, Runx2, PTHR, ProCOL I, OPN and OC were highly expressed in Zn+ for both chelexed FBS and TPEN-treated culture medium. Also, TPEN-treated experiments, compared to chelex-treated experiment, showed considerably lower bone-related gene expression, which may imply that TPEN would be a stronger chelator than chelex resin. In conclusion, this study showed that TPEN would be a stronger chelator compared to DTPA or chelex resin, and TPEN and chelexing resin can be used for the chelation of heavy metal ions especially Zn for osteogenic MC3T3-E1 cell culture condition.
Footnotes
This study was supported by Korea Science and Engineering Foundation (KOSEF) grant, No. 2004-0-220-008-2 and by the Scottish Executive Environment and Rural Affairs Department, Scotland, United Kingdom. We are also thankful to Phil-Young Yeum for her help on the cell study.
References
- 1.Aballay A, Sarrouf MN, Colombo MI, Stahl PD, Mayorga LS. Zn2+ depletion blocks endosome fusion. Biochem J. 1995;312:919–923. doi: 10.1042/bj3120919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aisen P, Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- 3.Avery RA, Bettger WJ. Zinc deficiency alters the protein composition of the membrane skeleton but not the extratability of oligomeric form of spectrin in rat erythrocyte membrane. J Nutr. 1992;122:428–434. doi: 10.1093/jn/122.3.428. [DOI] [PubMed] [Google Scholar]
- 4.Bray TM, Bettger WJ. The physiological role of zinc as an antioxidant. Free Radic Biol Med. 1990;8:281–291. doi: 10.1016/0891-5849(90)90076-u. [DOI] [PubMed] [Google Scholar]
- 5.Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14:331–341. doi: 10.1023/a:1012905406548. [DOI] [PubMed] [Google Scholar]
- 6.Chattopadhyay S, Freake HC. Zinc chelation enhances thyroid hormone induction of growth hormone mRNA in GH3 cells. Mol Cell Endocrinol. 1998;136:151–157. doi: 10.1016/s0303-7207(97)00228-1. [DOI] [PubMed] [Google Scholar]
- 7.Chesters JK, Petrie L, Vint H. Specificity and timing of the Zn2+ requirement for DNA synthesis by 3T3 cells. Exp Cell Res. 1989;184:499–508. doi: 10.1016/0014-4827(89)90347-9. [DOI] [PubMed] [Google Scholar]
- 8.Cho YE, Lomeda RAR, Kim YH, Ryu SH, Choi JY, Kim HJ, Beattie JH, Kwun IS. Zinc deficiency decreased alkaline phosphatase expression and bone matrix Ca deposits in osteoblast-like MC3T3-E1 cells. Nutritional Sciences. 2005;8:205–211. [Google Scholar]
- 9.Cinatl J, Jr, Hoffmann F, Cinatl J, Weber B, Scholz M, Rabenau H, Stieneker F, Kabickova H, Blasko M, Doerr HW. Invitro inhibition of human cytomegalovirus replication by calcium trinatrium diethylenetriaminepentaacetic acid. Antiviral Res. 1996;31:23–24. doi: 10.1016/0166-3542(95)00833-0. [DOI] [PubMed] [Google Scholar]
- 10.Frederickson CJ, Suh SW, Koh JY, Cha YK, Thompson RB, LaBuda CJ, Balaji RV, Cuajungco MP. Depletion of intracellular zinc from neurons by use of an extracellular chelator in vivo and in vitro. J Histochem Cytochem. 2002;50:1659–1662. doi: 10.1177/002215540205001210. [DOI] [PubMed] [Google Scholar]
- 11.Hyun HJ, Sohn JH, Ha DW, Ahn YH, Koh JY, Yoon YH. Depletion of intracellular zinc and copper with TPEN results in apoptosis of cutured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:460–465. [PubMed] [Google Scholar]
- 12.Kanekiyo M, Itoh N, Mano M, Kawasaki A, Tanaka J, Muto N, Tanaka K. Cellular zinc status regulates cytomegalovirus major immediate-early promoter. Antiviral Res. 2000;47:207–214. doi: 10.1016/s0166-3542(00)00108-x. [DOI] [PubMed] [Google Scholar]
- 13.Lefebvre D, Beckers F, Ketelslegers JM, Thissen JP. Zinc regulation of insulin-like growth factor-I (IGF-I), growth hormone receptor (GHR) and binding protein (GHBP) gene expression in rat cultured hepatocytes. Mol Cell Endocrinol. 1998;138:127–136. doi: 10.1016/s0303-7207(98)00012-4. [DOI] [PubMed] [Google Scholar]
- 14.Libin C, Schoene NW, Zhu L, Fanzo J, Alshatwi A, Lei KY. Zinc depletion reduced Egr-1 and HNF-3β expression and apolipoprotein A-I promoter activity in Hep G2 cells. Am J Physiol Cell Physiol. 2002;283:C623–C630. doi: 10.1152/ajpcell.00308.2001. [DOI] [PubMed] [Google Scholar]
- 15.Nakatini T, Kennedy DO, Murakami Y, Yano Y, Otani S, Matsui-Yuasa I. Restricted Zn2+ availability affects the antizyme-dependent onrithine decarboxylase degradation pathway in isolated primary cultured rat hepatocytes. Biochem Biophys Res Commun. 1998;243:797–800. doi: 10.1006/bbrc.1998.8168. [DOI] [PubMed] [Google Scholar]
- 16.Nakatini T, Tawaramoto M, Opare KD, Kojima A, Matsui-Yuasa I. Apoptosis induced by chelation of intracellular zinc is associated with depletion of cellular redubced glutathione level in rat hepatocytes. Chem Biol Interact. 2000;125:151–163. doi: 10.1016/s0009-2797(99)00166-0. [DOI] [PubMed] [Google Scholar]
- 17.Ovesen J, Moller-Madsen B, Thomsen JS, Danscher G, Mosekilde L. The positive effects of zinc on skeletal strength in growing rats. Bone. 2001;29:565–570. doi: 10.1016/s8756-3282(01)00616-0. [DOI] [PubMed] [Google Scholar]
- 18.Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG. Zinc a potent inhibitor of the apoptotic protease caspase-3: a novel target for zinc in the inhibition of apoptosis. J Biol Chem. 1997;272:18530–18533. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- 19.Petrie L, Chesters JK, Franklin M. Inhibition of myoblast differentiation by lack of zinc. Biochem J. 1991;276:109–111. doi: 10.1042/bj2760109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shumaker DK, Vann LR, Goldberg MW, Allen TD, Wilson K. TPEN, a Zn2+/Fe2+ chelator with low affinity for Ca2+, inhibits lamin assembly, destabilizes nuclear architecture and may independently protect nuclei from apoptosis in vitro. Cell Calcium. 1998;23:151–164. doi: 10.1016/s0143-4160(98)90114-2. [DOI] [PubMed] [Google Scholar]
- 21.Swanson JE, Feigenson GW. Thermodynamics of mixing of phosphatidylserine/phosphatidylcholine from measurements of high-affinity calcium binding. Biochemistry. 1990;29:8291–8297. doi: 10.1021/bi00488a013. [DOI] [PubMed] [Google Scholar]
- 22.Tapiero H, Tew KD. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother. 2003;57:399–411. doi: 10.1016/s0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 23.Telford W, Fraker P. Preferential induction of apoptosis in mouse CD4+CD8+TCR10CD310 thymocytes by zinc. J Cell Physiol. 1995;164:259–270. doi: 10.1002/jcp.1041640206. [DOI] [PubMed] [Google Scholar]
- 24.Westin KJ, Rasmuson AC. Nucleation of calcium carbonate in presence of citric acid, DTPA, EDTA and pyromellitic acid. J Colloid Interface Sci. 2005;282:370–379. doi: 10.1016/j.jcis.2004.09.071. [DOI] [PubMed] [Google Scholar]