Abstract
Chronic colonization of the human stomach by Helicobacter pylori, a Gram-negative bacterium, is the major cause of chronic gastritis, peptic ulcers and gastric cancer. Recent progress has elucidated important bacterial and host factors that are responsible for H. pylori-induced gastric inflammation and gastric malignancy. H. pylori cytotoxin-associated antigen A is the major oncogenic factor injected into host cells from bacteria and it disrupts epithelial cell functions. Together with H. pylori cag pathogenicity island, it causes general inflammatory stress within gastric mucosa and activates multiple oncogenic pathways in epithelial cells. A growing list of these pathways includes NF-κB, activator protein-1, PI3K, signal transducers and activators of transcription 3, Wnt/β-catenin and cyclooxygenase 2. H. pylori induces epigenetic alterations, such as DNA methylation and histone modification, which play critical roles in oncogenic transformation. In addition, investigations into gastric stem cell or progenitor cell biology have shed light on the mechanisms through which gastric cancer may originate. Continued investigation in these areas will yield novel insights and help to elucidate the mechanisms of bacteria-induced carcinogenesis.
Keywords: cancer, chromatin, epigenetics, gastric epithelial cell, Helicobacter pylori, histone, oncogene
The past 25 years have witnessed impressive progress in the study of the human stomach pathogen, Helicobacter pylori, a spiral-shaped Gram-negative bacterium, which infects half of the world's population and is the major cause of chronic gastritis, peptic ulcers and gastric malignancies, including gastric noncardia adenocarcinoma and mucosal-associated lymphoid tissue lymphoma [1]. H. pylori infection induces both acute and chronic gastritis, which present as superficial mucosal inflammation in the gastric mucosa. Although most infected individuals have no clinical presentation, approximately 10–20% will develop peptic ulcers and 1% will develop gastric cancer [2]. Gastric cancer continues to be the second leading cause of death among all cancers; in the year 2000, there were approximately 876,000 cases of primary gastric cancer, accounting for approximately 647,000 deaths worldwide, second only to lung cancer [3].
Upon infection, H. pylori activates multiple intracellular pathways in epithelial cells, such as MAPK, NF-κB, activator protein (AP)-1, Wnt/β-catenin, PI3K pathways and signal transducers and activators of transcription (STAT)3 [1,2,4-7]. These affect various cellular functions, leading to increased inflammatory cytokine production, altered apoptosis rate, epithelial cell proliferation and differentiation, and finally resulting in epithelial cell oncogenic transformation. Bacterial virulence factors, such as cytotoxin-associated antigen A (CagA), the cag pathogenicity island (PAI), vacuolating cytotoxin (VacA) and outer membrane proteins (OMPs), are responsible for many of these effects [8].
Effects of H. pylori CagA & cagPAI in gastric epithelial cells
The first H. pylori genome sequenced (strain 26695) is 1.67 million bp in size and has 1590 predicted coding sequences [9]. The cagPAI of H. pylori is an approximately 40-kbp region of the H. pylori genome that encodes approximately 30 genes, some of them form a type IV secretion system, which is important for pathogenesis and is responsible for delivery of CagA protein and peptidoglycan (PGN) into the host cells [10,11]. The type IV secretion system injects CagA protein by forming a syringe-like structure capable of penetrating into host cell. The CagL protein binds to and activates host cell integrins and is required for subsequent delivery of CagA across the host cell membrane [12]. Infection with cagA-positive, which is a marker for cagPAI-positive, H. pylori strains is linked to increased inflammation and gastric cancer risk [1,2].
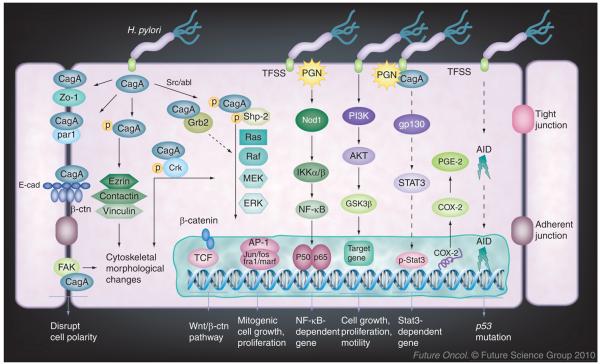
H. pylori CagA is encoded by the cagA gene within the cagPAI, has a molecular weight of approximately 120–145 kDa and profoundly affects host cell function (Figure 1) [13]. Following H. pylori's adherence to gastric epithelial cells, CagA is injected into the host cell cytoplasm. It is then tyrosine phosphorylated by Src family kinases or Abl kinases in its glutamic acid–proline–isoleucine–tyrosine–alanine (EPIYA) motif [14,15]. The phosphorylated CagA binds to and activates SHP-2 [16]. SHP-2 acts upstream of the RAS/RAF/MEK/ERK cascade and has important functions in regulating cell proliferation, morphogenesis and motility [13]. In addition, CagA interacts with and disrupts epithelial cell barrier function through dephosphorylation of cortactin and induction of actin rearrangement by Src inactivation [17], dephosphorylation of cell junction molecules, such as ezrin [18], and associates with the epithelial tight-junctional scaffolding protein, ZO-1 [19]. These effects together damage normal cell contact, cell polarity and epithelial cell permeability. Interaction of CagA with the c-Met receptor or phospholipase C-γ and Crk enhances motogenic responses [20]. Grb-2 is the key mediator of CagA activities via its binding to the EPIYA motif that stimulates growth factor-like signaling, leading to cell morphological changes and proliferation [21]. Thus, CagA disrupts normal cellular signaling pathways and confers the cell oncogenic potential that is critical for H. pylori pathogenesis.
Figure 1. Helicobacter pylori-induced host cell response and oncogenic signaling in gastric epithelial cells.
H. pylori cagPAI-positive strains, which possess TFSS, translocate the bacterial effectors CagA and PGN into the host cells; these effector molecules activate multiple epithelial cell signaling pathways and confer oncogenic potential to the epithelial cell. Once inside the cells, CagA is tyrosine phosphorylated by Src or Abl kinases and targets host proteins to induce the cell responses in a phosphorylation-dependent or -independent manner. The effects range from activation of oncogenic pathways to cytoskeletal rearrangements. PGN is also injected by TFSS and activates NF-κB, STAT3 and PI3K pathways. Persistent deregulation of these pathways provides an important molecular mechanism toward the H. pylori-induced carcinogenesis.
AP: Activator protein; CagA: Cytotoxin-associated antigen A; COX: Cyclooxygenase; PGN: Peptidoglycan; STAT: Signal transducers and activators of transcription; TFSS: Type IV secretion system.
CagA also interacts with VacA, another major virulence factor for H. pylori, to deregulate nuclear factor of activated T cell signaling [22]. VacA causes cellular vacuolization, inhibits cell proliferation and ulcer healing. Deregulation of nuclear factor of activated T cell signaling induces cell cycle gene p21 expression, affecting the cell cycle and differentiation [22]. More recently, CagA has been shown to disrupt cell polarity through its binding to the PAR1/MARK complex and inhibit its kinase activity [23]; interaction with E-cadherin deregulates β-catenin signaling, which induces expression of genes downstream of β-catenin, such as CDX1, and promotes cell intestinal transdifferentiation [24].
Previous studies indicated that cagPAI-dependent but cagA-independent host signaling was induced by H. pylori; of note is the activation of Rho-GTPase Rac1 and CDC42 that regulate host cell cytoskeleton rearrangement [25], introduction of PGN into the cytoplasm and activation of NF-κB by Nod1 signaling [11], which is especially important because NF-κB activation mediates expression of multiple genes involved in H. pylori-induced host responses. cagPAI is also shown to mediate c-Met-induced gastric cancer cell invasiveness, independent of cagA, during H. pylori infection in gastric epithelial cells [26]. These results provide a critical insight and the molecular basis for H. pylori-induced cellular dysfunction and potential for oncogenic transformation.
H. pylori activates multiple oncogenic pathways in host cells
In addition to causing inflammatory stress and disrupting the cell cycle and cell polarity, H. pylori activates multiple oncogenic pathways, such as PI3K/AKT/GSK3β, STAT3 and β-catenin pathways and induces aberrant expression of activation-induced cytidine deaminase (AID), all of which are important in promoting gastric oncogenesis (Figure 1) [4-7,27]. Several critical pathways, including MAPK, NF-κB and AP-1, which are well known for their role in these processes and are activated by H. pylori, will not be reviewed here.
The PI3K/AKT/GSK3β pathway regulates various cellular functions, including cell growth, proliferation, differentiation and motility. Its aberrant activation is associated with various types of cancers, including gastric cancer. AKT is activated as a result of PI3K activity and regulates its downstream targets, including GSK3β, which has important functions in regulating cell proliferation, inflammation, metabolism and apoptosis. H. pylori activates the PI3K pathway, including AKT and GSK3β, in a cagPAI-dependent manner. OMP outer inflammatory protein A (OipA) also activates AKT and induces GSK3β phosphorylation; mutation of the H. pylori cag-PAI or oipA gene decreases the effects on both AKT and GSK3β [4]. In addition, Nagy et al. reported that H. pylori activates PI3K and AKT in a Src- and EGF receptor-dependent manner, which is mediated by a functional cagPAI and PGN delivery; mutation of cagA does not affect the activation of AKT by H. pylori [5]. Therefore, constant PI3K activation induced by H. pylori may contribute to cellular transformation and the development of gastric cancer.
Signal transducers and activators of transcription 3, a transcription factor and a member of the STAT protein family, regulates cell growth, apoptosis and differentiation. Several inflammatory cytokines and growth factors, such as IL-6, interferon and EGF, trigger STAT3 activation, which has been related to carcino genesis [7]. H. pylori-induced STAT3 activation depends on a functional cagPAI and CagA, but not CagA phosphorylation. Pre-incubation of cells with an IL-6 receptor antagonist or inhibition of gp130 prevented H. pylori-mediated STAT3 activation. In addition, wild-type H. pylori but not a cagA-negative mutant activates STAT3 in gastric epithelial cells in a H. pylori gerbil infection model [7]. These results provide an additional mechanism by which H. pylori promotes the development of gastric cancer. In addition, STAT3 overexpression is also associated with cancer stages and poor prognosis of gastric cancer [28].
Recently, a clear relationship has emerged on the oncogenic mechanisms of Wnt/β-catenin, cyclooxygenase 2 (COX-2)/prostaglandin E2 (PGE2) and H. pylori-induced inflammation in gastric carcinogenesis. The Wnt/β-catenin pathway is implicated in multiple types of human malignancies, including gastric cancer [29], and overexpression of COX-2, which increases PGE2 production, is critical in gastric tumorigenesis. H. pylori infection activates both pathways [6,30].
Oshima et al. reported that the simultaneous overexpression of Wnt1 and COX-2 induces dysplastic gastric tumors in transgenic mice [29]. This process involves sequential changes from metaplasia and dysplasia to carcinoma in mice gastric mucosa. Wnt1 transgenic mice have increased β-catenin activity, suppressed epithelial differentiation and preneoplastic lesions, while the generation of tumors in these mice requires the cooperative activation of both Wnt and COX-2/PGE2 pathways. Furthermore, Oguma et al. demonstrated that macrophage-derived TNF-α promotes Wnt/β-catenin activation through the inhibition of GSK3b, which contributes to tumor development [31]. Helicobacter felis infection in Wnt1 transgenic mice induced increased gastric mucosal macro-phage infltration, which is the source of TNF-α and causes epithelium nuclear β-catenin accumulation. Since H. pylori infection both induces TNF-α production [32] and activates the GSK3b pathway [4], these results provide an important link between these signaling events.
In addition, the role of β-catenin in gastric carcinogenesis has been reported [6]. H. pylori strain 7.13 induces gastric dysplasia and adeno-carcinoma in a gerbil model. It selectively activates β-catenin in gerbil gastric epithelia, which is dependent on the translocation of CagA into host epithelial cells. β-catenin nuclear accumulation is also increased in gastric epithelium from H. pylori-infected gerbils and from people carrying cagPAI strains [6].
To further explore the molecular mechanisms of H. pylori CagA in regulating β-catenin activity, Kurashima et al. found that the CagA EPIYA-repeat region is required for β-catenin membranous translocalization [33]. Sequential mutational ana lysis reveals residues 1009–1086 of ABCCC type of Western CagA and residues 908–1012 of ABD type of East-Asian CagA, a 16-amino-acid CagA multimerization sequence, is required for this effect [33]. CagA also physically interacts with E-cadherin and disrupts E-cadherin and β-catenin complex formation, which causes cytoplasmic and nuclear accumulation of β-catenin. This subsequently activates downstream genes such as cdx1 and p21(WAF1/Cip1) and induces aberrant expression of the intestinal-differentiation marker, goblet-cell mucin (MUC2) [24]. Together, these results provide important insight into the mechanism of H. pylori, and in particular CagA, in the deregulation of the Wnt/β-catenin pathway and the promotion of gastric cancer.
The AID protein is a member of cytidine deaminase family that acts as a DNA- and RNA-editing enzyme. It was originally demonstrated to produce immune diversity in B cells by inducing somatic hypermutations and class-switch recombinations in human immunoglobulin genes [27]. The generation of somatic mutations in various host genes of non-lymphoid tissues contributes to tumorigenesis. Pathogenic factors, including H. pylori infection and proinflammatory cytokine stimulation, induce AID expression in epithelial cells. H. pylori-induced upregulation of AID depends on the cagPAI/NF-κB pathway. Activation of AID results in the accumulation of nucleotide alterations in the TP53 tumor suppressor gene, which may be an important mechanism of mutation accumulation in the gastric mucosa during H. pylori-associated gastric carcinogenesis [27].
Helicobacter-induced gastric cancer in animal models
Animal models provide critical insights to the mechanisms of H. pylori-induced oncogenesis. In a transgenic mouse model, Ohnishi et al. demonstrated that expression of CagA in mice induced multiple malignancies, including gastric epithelial hyperplasia, hyperplastic polyps, gastrointestinal carcinomas and hematological malignancies, such as myeloid leukemia and B-cell lymphoma [34]. The mice did not show any signs of gastritis or systemic inflammation and the cancer was cell autonomous. Since CagA alone is able to induce cancer without any inflammation, the experiment identifies its critical role in tumorigenesis as an oncoprotein.
Mongolian gerbils infected with wild-type H. pylori strain 7.13 or its cagA, vacA, oipA mutants all developed gastritis; inflammation was generally attenuated in animals infected with cagA mutant but not vacA- or oipA-negative strains [35]. Gastric dysplasia and cancer developed in more than 50% of the gerbils infected with either wild-type or vacA-negative strain, but none in those infected with cagA mutant strains. Inactivation of H. pylori oipA gene decreased β-catenin nuclear localization and reduced cancer incidence in gerbils. Eradication of H. pylori decreasesd the incidence and severity of lesions with carcinogenic potential, but the effectiveness of this eradication to prevent malignancy was dependent on the timing of intervention, resembling that seen in human clinical studies [36]. These results uncover a critical role of the major H. pylori virulence factors in the induction of gastric malignancies.
Using a primate oral carcinogen and H. pylori infection model (rhesus monkeys), Liu et al. found that the nitrosating carcinogen, ethyl-nitro-nitrosoguanidine and a virulent H. pylori strain (cagA, vacA and babA positive) together induced gastric cancer and neoplastic gene expression in non-neoplastic mucosa [37]. Transcriptional analysis of biopsies of infected animals revealed neoplasia-specific gene-expression profiles that were characterized by changes in multiple cancer-associated genes. The neoplastic profile was also evident in non-neoplastic mucosa, indicating that gene changes may represent early events preceding tumor formation and that consumption of carcinogen and H. pylori infection synergistically induces gastric neoplasia.
It has been known that chronic hypergastrinemia in insulin–gastrin transgenic mice synergizes with Helicobacter infection and contributes to eventual parietal cell loss and progression to gastric cancer [38]. Recently, this same group reported that stomach-specific expression of human IL-1β in transgenic mice lead to spontaneous gastric inflammation and cancer, which correlated with early recruitment of myeloid-derived suppressor cells to the stomach. Antagonism of IL-1 receptor inhibits the development of gastric pre-neoplasia and suppresses myeloid-derived suppressor cell mobilization [39]. Therefore, these results together suggest that, in addition to bacterial factors, host factors, including gastrin and inflammatory cytokines, participate in the gastric oncogenesis.
H. pylori-induced epigenetic changes: DNA methylation in gastric cancer
Epigenetic changes are generally categorized into four areas: DNA methylation, histone modification, chromatin remodeling and miRNAs [40]. Disruption of epigenetic mechanisms leads to abnormal development and malignant transformation. Both DNA methylation and histone modification have altered patterns of distribution in cancer cells; epigenetic alterations may occur at different stages of tumorogenesis and, thus, contribute to cancer development [40-42]. Connections between H. pylori infection, gastric cancer and epigenetic changes have been noted in just the past few years, and many unanswered questions remain to be explored, including the role of H. pylori virulence factors CagA, VacA, cagPAI and OMP, as well as their effects on stem cells. The results of future investigations will greatly expand our understanding in these areas.
DNA methylation is introduced by addition of a methyl group to the fifth carbon of a cytosine pyrimidine ring of DNA, which typically occurs in a CpG dinucleotide. CpG islands are genomic regions that contain a high frequency of CpG sites, usually near the 5′ transcription-start site of genes. In normal cells, approximately 80% of all CpGs are methylated. DNA of cancer cells is generally hypomethylated, while promoters of certain genes are hypermethylated, both of which are implicated in carcinogenesis. Promoter-specific increased methylation leads to silencing of the affected genes that may function as tumor suppressors and result in heritable transcriptional silence. Aging and chronic inflammation can induce methylation in CpG islands [40,41].
H. pylori infection induces aberrant methylation in a number of gene promoters in gastric mucosa, including cell growth-related genes p16(INK4a), p14(ARF) and APC; DNA-repair genes, hMLH1, BRCA1 and MGMT; the cell adherence gene E-cadherin; as well as LOX, FLNC, HRASLS, HAND1, THBD and p41ARC, which are known to be methylated in gastric cancer patients [43-46]. The gene methylations in H. pylori-infected individuals are increased and the methylations decrease after bacteria eradication, suggesting that the effects are induced by bacteria infection [43-46]. Gastric cancer tissue also shows higher levels of methylation than noncancerous tissues [44], providing a mechanistic explanation towards H. pylori-induced carcinogenesis.
There are a number of genes that are methylated in gastric cancer, but their correlation with H. pylori infection has not been reported, including those listed in Table 1. Their functions range from regulation of apoptosis and cell growth to tumor suppression. Treatment with a methyltransferase inhibitor, 5-aza-deoxycytidine, reverses the methylation status and usually restores gene expression. Therefore, aberrant methylation is regarded as major event during the early stages of malignant transformation as well as in the progression of cancer, and higher levels of methylations are found more frequently in late stages of gastric cancer [47].
Table 1.
Genes that are methylated in gastric cancer and their function.
| Gene name (abbreviation) | Protein function | Ref. |
|---|---|---|
| BCL2/adenovirus E1B-interacting protein 3 (BNIP3) | Proapoptotic Member of Bcl-2 family |
[48] |
| Harakiri, BCL2-interacting protein (HRK) | Proapoptotic | [49] |
| Death-associated protein (DAP) | Serine/threonine kinase Proapoptotic |
[50] |
| Solute carrier family 5 (iodide transporter), member 8 (SLC5A8) | Sodium cotransporter tumor suppressor |
[51] |
| Inhibitor of DNA binding 4 (ID4) | Transcriptional regulator | [52] |
| Helicase-like transcription factor (HLTF) | Tumor suppressor | [53] |
| Retinoblastoma-interacting zinc-finger protein 1 (RIZ1) | Zinc finger protein | [54] |
| Runt-related transcription factor 3 (RUNX3) | Tumor suppressor | [55] |
| Deleted in liver cancer 1 (DLC1) | Tumor suppressor | [56] |
| Retinoic acid receptor β (RARB) | Retinoid signaling | [57] |
| TSPY-like 5 (TSPYL5) | Cell growth | [58] |
| Ras association (RalGDS/AF-6) domain family member 1 (RASSF1A) | Putative tumor suppressor | [59] |
| XIAP-associated factor 1 (XAF1) | Putative tumor suppressor | [60] |
| ADAM metallopeptidase domain 23 (ADAM23) | Putative tumor suppressor | [61] |
| Lysyl oxidase (LOX) | Tumor suppressor | [62] |
| Checkpoint with fork head-associated (FHA) and ring finger (CHFR) |
Mitotic checkpoint protein | [63] |
Histone modification in gastric cancers & its induction by H. pylori
Recent advances also underscore the importance of histone modifications in the pathogenesis of gastric cancer. Histones are the basic unit of the nucleosome, consisting of two copies of each of the core histones, H2A, H2B, H3 and H4 [41,42]. H3 and H4 histones have long tails protruding from nucleosome that can be covalently modified. This allows regulatory proteins to access DNA and regulate transcription. Modifications of histone tails include methylation, acetylation, phosphorylation, ubiquitination, SUMOylation, citrullination and ADP-ribosylation. Histone modifications act in diverse biological processes, such as gene regulation, DNA repair and cell growth [42]. Disruption of certain histone modifications is associated with birth defects, age-related diseases and cancer [41,42].
The acetylation and deacetylation of key lysine residues of histone H3 and H4 are controlled by histone acetyltransferases and histone deacetylases (HDACs) [64]. Transcription becomes active when histones are acetylated, silenced when histones are deacetylated and silenced or activated when methylated by histone methyltransferases [41,42]. Both histone (H)3 lysine (K) 9 and H3K27 trimethylation (triMe) are associated with gene silencing and histone H3K27triMe causes gene silencing independent of promoter DNA methylation [65].
Global histone modification patterns are suggested to be an independent predictor for gastric cancer recurrence and survival. Park et al. evaluated the patterns of histone H3 and H4 acetylation and trimethylation in gastric cancer, including expression of acetylated H3K9, acetylated H4K16, H3K9triMe and H4K20triMe in gastric adenocarcinoma samples [66]. The results indicated that only H3K9triMe positively correlated with tumor stages, lymphovascular invasion and cancer recurrence. Higher levels of H3K9triMe expression correlated with a poor survival rate. Methylation dominance, which contains two trimethylated histone scores, is associated with lymphovascular invasion, cancer recurrence and poor survival rate.
Histone deacetylase overexpression results in histone hypoacetylation and is involved in multiple types of cancer, including gastric and breast cancers [67]. Weichert et al. investigated the expression status of class I HDAC isoforms 1, 2 and 3 in gastric cancer in a retrospective analysis [67]. They found that 32 of the 150 (21%) gastric tumors have nuclear expression of all three HDAC isoforms. General HDAC expression levels were higher when patients had lymph node metastases, and the 3-year survival rate decreased to 21% when HDAC1 was positive, 16% when HDAC2 was positive and 5% when all isoforms were positive. These data suggest that HDAC can be an independent prognostic marker for gastric cancer and an indication of the potential of HDAC inhibitors as therapeutics [67].
In certain cancer patients, overexpression of phosphorylated histone H3 Ser 10 (H3S10) has been reported to be an indicator of poor prognosis for gastric cancer. Takahashi et al. studied H3S10 expression and its relation to cancer progression [68]. In 30 out of 122 cancer cases, H3S10 showed overexpression (24.6%), while in the rest of the cases, the expression was low (75.4%). The cancer patients that showed overexpression of phosphorylated histone H3 had a poorer prognosis than those with low expression.
In addition to the general histone protein and HDAC expression, certain histone modifications are associated with specific gene promoters in gastric cancer and participate in the regulation of gene transcription. For example, DNA hypermethylation and histone hypoacetylation of the HLTF gene are linked to its reduced expression in gastric cancer [53]. HLTF is a homolog to the SWI/SNF genes, which encode chromatin-remodeling enzymes and serves as a tumor suppressor. In tumor cell lines, acetylation levels of histones H3 and H4 in the 5′ CpG islands of HLTF gene are inversely associated with DNA methylation status. Treatment with both DNA methytransferase and HDAC inhibitors, aza-2′-deoxycytidine and trichostatin A, restore HLTF mRNA expression, suggesting both mechanisms contribute to the loss of gene expression.
Histone H3 in the p21(WAF1/CIP1) promoter is hypoacetylated in gastric cancer [69]; this hypoacetylation is associated with reduced p21(WAF1/CIP1) expression in gastric cancer specimens. Treatment of gastric carcinoma cell lines with HDAC inhibitor, trichostatin A, increases the acetylation level and restores p21(WAF1/CIP1) expression. Aberrant DNA methylation and histone deacetylation are also linked to the silencing of the SLC5A8 gene in gastric cancer [51]. SLC5A8 is a sodium co-transporter, solute carrier family 5 member 8 gene and a putative tumor suppressor. Aberrant methylation of SLC5A8 gene is detected in both cell lines and in primary gastric cancers and acetylation of histone H3 correlates directly with SLC5A8 expression and inversely with DNA methylation.
The aforementioned results demonstrate that histone modification and DNA CpG island methylation are important epigenetic events in gastric cancer. Reduced histone acetylation correlates with the extent of tumor invasion and nodal metastasis of gastrointestinal cancers [70]. It has also been suggested that dysregulated epigenetic modifications, especially in early neoplastic development, may be just as significant as genetic mutations in driving cancer development and growth [40]. However, the role of H. pylori infection status during these processes is not clear, although it has been proven to be a crucial factor in triggering inflammation and cancer. We anticipate that future studies will advance our understanding in this important field.
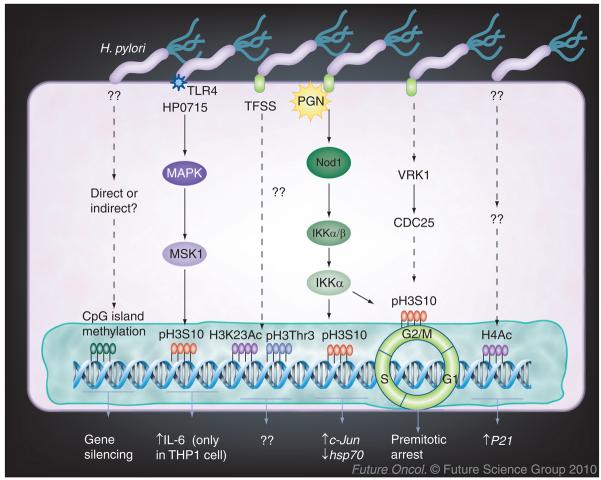
In fact, relatively little information is available about the effects of H. pylori infection on histone modifications (Figure 2). As expected, specific histone modification in host cells is cell type and promoter specific. In mouse macrophages, H. pylori peptidyl prolyl cis-, trans-isomerase (HP0175) induces H3S10 phosphorylation at the IL-6 promoter, and this is associated with increased IL-6 mRNA and protein expression [71]. Direct exposure of H. pylori to gastric epithelial cells causes upregulation of p21WAF1 protein expression in both the gastric epithelial cell line NCI-N87 and in primary gastric cells. The increased p21WAF1 expression is associated with increased HDAC1 recruitment from the p21WAF1 promoter and hyperacetylation of histone H4 [72].
Figure 2. Helicobacter pylori-induced epigenetic changes.
Several studies have investigated the effects of H. pylori on histone modifications. Infection with H. pylori causes acetylation of H4, and this is associated with p21 gene expression in gastric epithelial cells. H. pylori also induces cag pathogenicity island-dependent pH3S10, which is associated with c-Jun upregulation and hsp70 downregulation and cell cycle premitotic arrest. However, the function of H3Thr3 and H3K23, and whether H. pylori directly induces DNA methylation in gastric epithelial cells, remain to be studied.
Ac: Acetylation; H3K23: Histone 3 lysine 23; pH3S10: Phosphorylation of histone 3 serine 10; PGN: Peptidoglycan; pH3Thr3: Phosphorylation of histone 3 threonine 3; TLR: Toll-like receptor; TFSS: Type IV secretion system.
Our recent work [73], as well as the work by Fehri et al. [74], demonstrate that H. pylori cagPAI-dependently induces dephosphorylation of histone H3S10, H3 threonine 3 and deacetylation of H3K23 in gastric epithelial cells, but does not affect nine other distinct histone modifications. H. pylori-induced H3S10 dephosphorylation is associated with changes in host gene expression, including upregulation of the oncogene c-Jun and downregulation of hsp70. These results indicate novel mechanisms in H. pylori pathogenesis via histone modification. Future studies are required to clarify the correlation of H. pylori-induced histone modification in gastric cancer.
The origin of gastric cancer cells & future directions
Epithelial cells that line the gastric mucosa are formed by four major cell types: pit, parietal, neck and zymogenic cells, as well as their progenitor cells. Studies of the transcriptome of gastric epithelial cells from the stomach of H. pylori-infected mice demonstrate that gastric pit cells or mucus-producing cells are the major targets of H. pylori [75]. However, because these differentiated pit cells are regenerated so quickly, they may not carry genetic or epigenetic lesions for very long before they are replaced. Therefore, the cells located in the isthmus, the multipotent stem cells that are responsible for generating the four major cell types, may have the potential to carry molecular damage to the next generation of cells [76].
It has been reported that gastric cancers can originate from bone marrow-derived cells that repopulate the gastric mucosa, and H. felis infection induces gastric cancer in these cells [77]. Studies in a gnotobiotic mouse model of chronic atrophic gastritis have shown that loss of parietal cells results in the amplification of multi- and oligo-potential gastric stem cells that express sialylated glycan receptors, which are recognized by H. pylori adhesions, and H. pylori interacts with and resides within a subset of these progenitor cells [78].
Since sporadic gastric cancer has increased risk with age, one hypothesis is that stem or progenitor cells accumulate enough molecular lesions to evade homeostatic control, resulting in cancer. The damages that originate from genetic and/or epigenetic processes are progressively accumulated during aging and directly contribute to cell transformation [79]. H. pylori has been shown to invade epithelial cells, intracellularly, inter-cellularly and interstitially [80]. It is believed that H. pylori also interacts with gastric progenitor cells, therefore introducing the molecular damage to these cells. These results support the previous hypotheses that the mechanisms of carcinogenesis are based on atrophy (loss of differentiated cells) followed by redifferentiation of stem cells, which may be damaged or abnormal, therefore causing cancer [81].
Virtually all types of cancers require several critical steps during tumorigenesis. These include: self-sufficiency in growth signals, insensitivity to growth inhibition, evasion of apoptosis, immortalization, sustained angiogenesis and tissue invasion and metastasis [82]. H. pylori infection is able to activate several oncogenic pathways, as previously described, to fulfill these requirements. Since oncogenic transformation does not require activation of all the oncogenic pathways, nor are these pathways required for all stages of cancer development and progression, deregulation of a few relevant pathways may initiate transformation, while activation of other genes may contribute to progression or metastasis. As H. pylori has been observed to invade gastric progenitor cells [78], two interesting questions to answer in the future will be: does H. pylori induce oncogenic, genetic and epigenetic changes in progenitor cells, and how does this effect their differentiation? Furthermore, how are these alterations introduced into daughter cells, therefore accumulating toward oncogenesis? Future studies addressing these questions will be of great interests to expand our understanding of gastric cancer.
In summary, H. pylori infection and chronic inflammation linked to cancer is probably owing to the activation of multiple oncogenic pathways and facilitating tumorigenesis. In this context, inflammatory stress, activation of oncogenic proteins, epigenetic mechanisms, local micro-environment and host genetic susceptibility together determine and promote the tumorigenesis and cancer progression. Interactions of H. pylori with gastric stem cells or progenitor cells may, therefore, provide important clues to uncover the mechanism of carcinogenesis and, thus, are the targets of extensive investigations. Continued study of H. pylori-induced molecular pathogenesis will be critical to understand the basis and origin of gastric cancer and will also provide us with options for future prevention and intervention in combating this deadly disease.
Future perspective
Although a great deal of progress has been made over the past two decades in understanding the pathogenesis and carcinogenesis of H. pylori-induced gastric cancer, the detailed mechanisms still remain elusive. It is generally accepted that chronic inflammation induced by H. pylori is important for the promotion of oncogenesis. This general inflammatory micro-environment, which includes increased inflammatory cell infiltration, reactive oxygen species levels, inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IL-8, growth factors and hormones, such as gastrin, impacts on the epithelial cell and facilitates oncogenic transformation. It is important to consider the interaction of these factors and their net effects on host cells in future investigations, especially how these environmental factors might affect the local stem cells or progenitor cells and their differentiation.
Inside the host cell, various oncogenic pathways are activated by H. pylori or by its virulence factors, especially CagA and cagPAI. Deregulation of these pathways and disruption of normal cell–cell contact confer the epithelial cell oncogenic transformation potential. Therefore, study of the pathogen–host interaction from a platform of stomach stem cells or progenitor cells will greatly extend our understanding about the patho physiology of chronic inflammation and oncogenesis. This will be helpful to understand not only the H. pylori-induced inflammation, but also the pathogenesis of other chronic inflammatory diseases, such as chronic hepatitis and inflammatory bowel disease as well.
Epigenetic alternation, including DNA methylation and histone modification, during chronic inflammation or H. pylori infection represent another layer of gene transcription control; however, relatively little information is currently available, and we anticipate future studies addressing these important issues will uncover their effects in pathogenesis and carcinogenesis and, more importantly, provide possible options for prevention and intervention.
Executive summary.
■ Chronic infection with Helicobater pylori in the human stomach lasts decades and causes persistent inflammation. Most>H. pylori-infected patients have no clinical symptoms. Approximately 10–20% of H. pylori-infected patients will develop peptic ulcers and 1% will develop gastric cancer.
■ Upon infection, H. pylori activates multiple oncogenic pathways, such as activator protein-1, NF-kB, Wnt/β-catenin, signal transducers and activators of transcription 3, PI3K and cyclooxygenase-2, all of which have been shown to contribute to the oncogenic transformation processes.
■ Bacterial virulence factors, such as cytotoxin-associated antigen A and cag pathogenecity island, are critical for causing inflammation and the activation of oncogenic pathways. In addition, cytotoxin-associated antigen A protein also disrupts the epithelial cell normal contact and cell polarity, which confer the epithelial cell oncogenic potential.
■ H. pylori-induced epigenetic alternation, including DNA methylation and histone modification, as well as its interaction with gastric stem cells or progenitor cells are important topics that will greatly enhance our understanding of the bacteria-induced carcinogenesis; therefore, they are currently the target of extensive investigation.
Acknowledgments
This work was supported by NIH grants R01-AI51291 (Joanna B Goldberg) and Grant-in-Aid for Science Research from the Ministry of Education, Science, and Culture of Japan (Masanori Hatakeyama). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Footnotes
Financial & competing interests disclosure
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Peek RM, Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J. Pathol. 2006;208(2):233–248. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- 2.Ernst PB, Peura DA, Crowe SE. The translation of Helicobacter pylori basic research to patient care. Gastroenterology. 2006;130(1):188–206. doi: 10.1053/j.gastro.2005.06.032. quiz 212–213. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur. J. Cancer. 2001;37(Suppl. 8):S4–S66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 4■.Tabassam FH, Graham DY, Yamaoka Y. Helicobacter pylori activate epidermal growth factor receptor- and phosphatidylinositol 3-OH kinase-dependent AKT and glycogen synthase kinase 3β phosphorylation. Cell. Microbiol. 2009;11(1):70–82. doi: 10.1111/j.1462-5822.2008.01237.x. Confirms that Helicobacter pylori activates the PI3K pathway in gastric epithelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5■.Nagy TA, Frey MR, Yan F, Israel DA, Polk DB, Peek RM., Jr Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling. J. Infect. Dis. 2009;199(5):641–651. doi: 10.1086/596660. Confirms that H. pylori activates the PI3K pathway in gastric epithelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6■.Franco AT, Israel DA, Washington MK, et al. Activation of β-catenin by carcinogenic Helicobacter pylori. Proc. Natl Acad. Sci. USA. 2005;102(30):10646–10651. doi: 10.1073/pnas.0504927102. Reports that H. pylori activates the β-catenin pathway in gastric epithelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7■.Bronte-Tinkew DM, Terebiznik M, Franco A, et al. Helicobacter pylori cytotoxin-associated gene A activates the signal transducer and activator of transcription 3 pathway in vitro and in vivo. Cancer Res. 2009;69(2):632–639. doi: 10.1158/0008-5472.CAN-08-1191. Demonstrates that H. pylori activates signal transducers and activators of transcription 3, pathway in gastric epithelial cells depend on cytotoxin-associated antigen (Cag)A and cag pathogenecity island. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu H, Yamaoka Y, Graham DY. Helicobacter pylori virulence factors: facts and fantasies. Curr. Opin. Gastroenterol. 2005;21(6):653–659. doi: 10.1097/01.mog.0000181711.04529.d5. [DOI] [PubMed] [Google Scholar]
- 9■■.Tomb JF, White O, Kerlavage AR, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388(6642):539–547. doi: 10.1038/41483. Landmark work in elucidating the genomic sequence of H. pylori strain 26695. [DOI] [PubMed] [Google Scholar]
- 10.Segal ED, Cha J, Lo J, Falkow S, Tompkins LS. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl Acad. Sci. USA. 1999;96(25):14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11■■.Viala J, Chaput C, Boneca IG, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori Cag pathogenicity island. Nat. Immunol. 2004;5(11):1166–1174. doi: 10.1038/ni1131. Reveals the role of H. pylori peptidoglycan in the activation of NOD1 and NF-κB signaling in gastric epithelial cells. [DOI] [PubMed] [Google Scholar]
- 12■.Kwok T, Zabler D, Urman S, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449(7164):862–866. doi: 10.1038/nature06187. Indicates that H. pylori exploits the integrin system for the type four secretion system to introduce CagA into host cells. [DOI] [PubMed] [Google Scholar]
- 13.Hatakeyama M. Linking epithelial polarity and carcinogenesis by multitasking Helicobacter pylori virulence factor CagA. Oncogene. 2008;27(55):7047–7054. doi: 10.1038/onc.2008.353. [DOI] [PubMed] [Google Scholar]
- 14.Stein M, Bagnoli F, Halenbeck R, Rappuoli R, Fantl WJ, Covacci A. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol. Microbiol. 2002;43(4):971–980. doi: 10.1046/j.1365-2958.2002.02781.x. [DOI] [PubMed] [Google Scholar]
- 15■.Tammer I, Brandt S, Hartig R, Konig W, Backert S. Activation of Abl by Helicobacter pylori: a novel kinase for CagA and crucial mediator of host cell scattering. Gastroenterology. 2007;132(4):1309–1319. doi: 10.1053/j.gastro.2007.01.050. Reveals that the c-Src and Abl kinase are responsible for CagA protein phosphorylation in its glutamic acid–proline–isoleucine–tyrosine–alanine motif and for its function. [DOI] [PubMed] [Google Scholar]
- 16■■.Higashi H, Tsutsumi R, Muto S, et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295(5555):683–686. doi: 10.1126/science.1067147. Demonstration that SHP-2 tyrosine phosphatase is an intracellular target for H. pylori CagA protein. [DOI] [PubMed] [Google Scholar]
- 17.Selbach M, Moese S, Hurwitz R, Hauck CR, Meyer TF, Backert S. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 2003;22(3):515–528. doi: 10.1093/emboj/cdg050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selbach M, Moese S, Backert S, Jungblut PR, Meyer TF. The Helicobacter pylori CagA protein induces tyrosine dephosphorylation of ezrin. Proteomics. 2004;4(10):2961–2968. doi: 10.1002/pmic.200400915. [DOI] [PubMed] [Google Scholar]
- 19■.Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300(5624):1430–1434. doi: 10.1126/science.1081919. Demonstrates that H. pylori CagA associates with tight-junction scaffolding protein ZO-1 and disruption of the epithelial cell junction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki M, Mimuro H, Suzuki T, Park M, Yamamoto T, Sasakawa C. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J. Exp. Med. 2005;202(9):1235–1247. doi: 10.1084/jem.20051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mimuro H, Suzuki T, Tanaka J, Asahi M, Haas R, Sasakawa C. Grb2 is a key mediator of Helicobacter pylori CagA protein activities. Mol. Cell. 2002;10(4):745–755. doi: 10.1016/s1097-2765(02)00681-0. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama K, Higashi H, Ishikawa S, et al. Functional antagonism between Helicobacter pylori CagA and vacuolating toxin VacA in control of the NFAT signaling pathway in gastric epithelial cells. Proc. Natl Acad. Sci. USA. 2005;102(27):9661–9666. doi: 10.1073/pnas.0502529102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23■.Saadat I, Higashi H, Obuse C, et al. Helicobacter pylori CagA targets Par1/Mark kinase to disrupt epithelial cell polarity. Nature. 2007;447(7142):330–333. doi: 10.1038/nature05765. Provides evidence of H. pylori CagA disruption of cell polarity by targeting Par1/Mark kinase. [DOI] [PubMed] [Google Scholar]
- 24■.Murata-Kamiya N, Kurashima Y, Teishikata Y, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the β-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26(32):4617–4626. doi: 10.1038/sj.onc.1210251. Suggests that CagA promotes intestinal transdifferentiation in gastric epithelial cells. [DOI] [PubMed] [Google Scholar]
- 25.Churin Y, Kardalinou E, Meyer TF, Naumann M. Pathogenicity island-dependent activation of Rho GTPases Rac1 and Cdc42 in Helicobacter pylori infection. Mol. Microbiol. 2001;40(4):815–823. doi: 10.1046/j.1365-2958.2001.02443.x. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira MJ, Costa AC, Costa AM, et al. Helicobacter pylori induces gastric epithelial cell invasion in a c-Met and type IV secretion system-dependent manner. J. Biol. Chem. 2006;281(46):34888–34896. doi: 10.1074/jbc.M607067200. [DOI] [PubMed] [Google Scholar]
- 27■.Matsumoto Y, Marusawa H, Kinoshita K, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat. Med. 2007;13(4):470–476. doi: 10.1038/nm1566. Describes the role of AID in generating p53 mutation in gastric epithelial cells during H. pylori infection and reports that the effect is cag pathogenecity island dependent. [DOI] [PubMed] [Google Scholar]
- 28.Kim DY, Cha ST, Ahn DH, et al. STAT3 expression in gastric cancer indicates a poor prognosis. J. Gastroenterol. Hepatol. 2009;24(4):646–651. doi: 10.1111/j.1440-1746.2008.05671.x. [DOI] [PubMed] [Google Scholar]
- 29■.Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131(4):1086–1095. doi: 10.1053/j.gastro.2006.07.014. Presents evidence for the role of Wnt signaling and prostaglandin E2 in the development of gastric cancer. [DOI] [PubMed] [Google Scholar]
- 30.Chang YJ, Wu MS, Lin JT, et al. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol. Pharmacol. 2004;66(6):1465–1477. doi: 10.1124/mol.104.005199. [DOI] [PubMed] [Google Scholar]
- 31■.Oguma K, Oshima H, Aoki M, et al. Activated macrophages promote Wnt signalling through tumour necrosis factor-α in gastric tumour cells. EMBO J. 2008;27(12):1671–1681. doi: 10.1038/emboj.2008.105. Demonstrates that Wnt signaling and TNF-αare important in the development of gastric cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Gonzalez MA, Aisa MA, Strunk M, et al. Relevance of IL-1 and TNF gene polymorphisms on interleukin-1β and tumor necrosis factor-α gastric mucosal production. Hum. Immunol. 2009;70(11):935–945. doi: 10.1016/j.humimm.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Kurashima Y, Murata-Kamiya N, Kikuchi K, et al. Deregulation of β-catenin signal by Helicobacter pylori CagA requires the CagA-multimerization sequence. Int. J. Cancer. 2008;122(4):823–831. doi: 10.1002/ijc.23190. [DOI] [PubMed] [Google Scholar]
- 34■.Ohnishi N, Yuasa H, Tanaka S, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc. Natl Acad. Sci. USA. 2008;105(3):1003–1008. doi: 10.1073/pnas.0711183105. Initial report that CagA protein alone is able to induce multiple tumors in mice and that CagA is a bacterial oncoprotein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35■.Franco AT, Johnston E, Krishna U, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68(2):379–387. doi: 10.1158/0008-5472.CAN-07-0824. Indicates a critical role of H. pylori virulence factors in gastric tumorigenes is, especially CagA and outer inflammatory protein A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero-Gallo J, Harris EJ, Krishna U, Washington MK, Perez-Perez GI, Peek RM., Jr Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab. Invest. 2008;88(3):328–336. doi: 10.1038/labinvest.3700719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H, Merrell DS, Semino-Mora C, et al. Diet synergistically affects Helicobacter pylori-induced gastric carcinogenesis in non-human primates. Gastroenterology. 2009;137(4):1367–1379. doi: 10.1053/j.gastro.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang TC, Dangler CA, Chen D, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118(1):36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 39■.Tu S, Bhagat G, Cui G, et al. Overexpression of interleukin-1β induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14(5):408–419. doi: 10.1016/j.ccr.2008.10.011. Demonstrates that IL-1β overexpression induces gastric cancer in an animal model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esteller M. The necessity of a human epigenome project. Carcinogenesis. 2006;27(6):1121–1125. doi: 10.1093/carcin/bgl033. [DOI] [PubMed] [Google Scholar]
- 41.Kurdistani SK. Histone modifications as markers of cancer prognosis: a cellular view. Br. J. Cancer. 2007;97(1):1–5. doi: 10.1038/sj.bjc.6603844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15(18):2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- 43■.Maekita T, Nakazawa K, Mihara M, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin. Cancer Res. 2006;12(3 Pt 1):989–995. doi: 10.1158/1078-0432.CCR-05-2096. Initial report that H. pylori infection is associated with high levels of DNA methylation in gastric mucosa. [DOI] [PubMed] [Google Scholar]
- 44.Ushijima T, Nakajima T, Maekita T. DNA methylation as a marker for the past and future. J. Gastroenterol. 2006;41(5):401–407. doi: 10.1007/s00535-006-1846-6. [DOI] [PubMed] [Google Scholar]
- 45.Nardone G, Compare D, De Colibus P, de Nucci G, Rocco A. Helicobacter pylori and epigenetic mechanisms underlying gastric carcinogenesis. Dig. Dis. 2007;25(3):225–229. doi: 10.1159/000103890. [DOI] [PubMed] [Google Scholar]
- 46.Chan AO, Peng JZ, Lam SK, et al. Eradication of Helicobacter pylori infection reverses E-cadherin promoter hypermethylation. Gut. 2006;55(4):463–468. doi: 10.1136/gut.2005.077776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oue N, Mitani Y, Motoshita J, et al. Accumulation of DNA methylation is associated with tumor stage in gastric cancer. Cancer. 2006;106(6):1250–1259. doi: 10.1002/cncr.21754. [DOI] [PubMed] [Google Scholar]
- 48.Murai M, Toyota M, Suzuki H, et al. Aberrant methylation and silencing of the BNIP3 gene in colorectal and gastric cancer. Clin. Cancer Res. 2005;11(3):1021–1027. [PubMed] [Google Scholar]
- 49.Obata T, Toyota M, Satoh A, et al. Identification of HRK as a target of epigenetic inactivation in colorectal and gastric cancer. Clin. Cancer Res. 2003;9(17):6410–6418. [PubMed] [Google Scholar]
- 50.Satoh A, Toyota M, Itoh F, et al. DNA methylation and histone deacetylation associated with silencing DAP kinase gene expression in colorectal and gastric cancers. Br. J. Cancer. 2002;86(11):1817–1823. doi: 10.1038/sj.bjc.6600319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueno M, Toyota M, Akino K, et al. Aberrant methylation and histone deacetylation associated with silencing of SLC5A8 in gastric cancer. Tumour Biol. 2004;25(3):134–140. doi: 10.1159/000079145. [DOI] [PubMed] [Google Scholar]
- 52.Chan AS, Tsui WY, Chen X, et al. Downregulation of ID4 by promoter hypermethylation in gastric adenocarcinoma. Oncogene. 2003;22(44):6946–6953. doi: 10.1038/sj.onc.1206799. [DOI] [PubMed] [Google Scholar]
- 53.Hamai Y, Oue N, Mitani Y, et al. DNA hypermethylation and histone hypoacetylation of the HLTF gene are associated with reduced expression in gastric carcinoma. Cancer Sci. 2003;94(8):692–698. doi: 10.1111/j.1349-7006.2003.tb01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oshimo Y, Oue N, Mitani Y, et al. Frequent epigenetic inactivation of RIZ1 by promoter hypermethylation in human gastric carcinoma. Int. J. Cancer. 2004;110(2):212–218. doi: 10.1002/ijc.20090. [DOI] [PubMed] [Google Scholar]
- 55.Kim TY, Lee HJ, Hwang KS, et al. Methylation of RUNX3 in various types of human cancers and premalignant stages of gastric carcinoma. Lab. Invest. 2004;84(4):479–484. doi: 10.1038/labinvest.3700060. [DOI] [PubMed] [Google Scholar]
- 56.Kim TY, Jong HS, Song SH, et al. Transcriptional silencing of the DLC-1 tumor suppressor gene by epigenetic mechanism in gastric cancer cells. Oncogene. 2003;22(25):3943–3951. doi: 10.1038/sj.onc.1206573. [DOI] [PubMed] [Google Scholar]
- 57.Hayashi K, Yokozaki H, Goodison S, et al. Inactivation of retinoic acid receptor β by promoter CpG hypermethylation in gastric cancer. Differentiation. 2001;68(1):13–21. doi: 10.1046/j.1432-0436.2001.068001013.x. [DOI] [PubMed] [Google Scholar]
- 58.Jung Y, Park J, Bang YJ, Kim TY. Gene silencing of TSPYL5 mediated by aberrant promoter methylation in gastric cancers. Lab. Invest. 2008;88(2):153–160. doi: 10.1038/labinvest.3700706. [DOI] [PubMed] [Google Scholar]
- 59.Guo W, Dong Z, Chen Z, et al. Aberrant CpG island hypermethylation of RASSF1A in gastric cardia adenocarcinoma. Cancer Invest. 2009;27(4):459–465. doi: 10.1080/07357900802620828. [DOI] [PubMed] [Google Scholar]
- 60.Byun DS, Cho K, Ryu BK, et al. Hypermethylation of XIAP-associated factor 1, a putative tumor suppressor gene from the 17p13.2 locus, in human gastric adenocarcinomas. Cancer Res. 2003;63(21):7068–7075. [PubMed] [Google Scholar]
- 61.Takada H, Imoto I, Tsuda H, et al. ADAM23, a possible tumor suppressor gene, is frequently silenced in gastric cancers by homozygous deletion or aberrant promoter hypermethylation. Oncogene. 2005;24(54):8051–8060. doi: 10.1038/sj.onc.1208952. [DOI] [PubMed] [Google Scholar]
- 62.Kaneda A, Wakazono K, Tsukamoto T, et al. Lysyl oxidase is a tumor suppressor gene inactivated by methylation and loss of heterozygosity in human gastric cancers. Cancer Res. 2004;64(18):6410–6415. doi: 10.1158/0008-5472.CAN-04-1543. [DOI] [PubMed] [Google Scholar]
- 63.Satoh A, Toyota M, Itoh F, et al. Epigenetic inactivation of CHFR and sensitivity to microtubule inhibitors in gastric cancer. Cancer Res. 2003;63(24):8606–8613. [PubMed] [Google Scholar]
- 64.Fischle W, Wang Y, Allis CD. Histone and chromatin cross-talk. Curr. Opin. Cell Biol. 2003;15(2):172–183. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- 65.Kondo Y, Shen L, Cheng AS, et al. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat. Genet. 2008;40(6):741–750. doi: 10.1038/ng.159. [DOI] [PubMed] [Google Scholar]
- 66.Park YS, Jin MY, Kim YJ, Yook JH, Kim BS, Jang SJ. The global histone modification pattern correlates with cancer recurrence and overall survival in gastric adenocarcinoma. Ann. Surg. Oncol. 2008;15(7):1968–1976. doi: 10.1245/s10434-008-9927-9. [DOI] [PubMed] [Google Scholar]
- 67■.Weichert W, Roske A, Gekeler V, et al. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol. 2008;9(2):139–148. doi: 10.1016/S1470-2045(08)70004-4. Shows the correlation between histone deacetylase 1 expression and cancer progression in gastric cancer. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi H, Murai Y, Tsuneyama K, et al. Overexpression of phosphorylated histone H3 is an indicator of poor prognosis in gastric adenocarcinoma patients. Appl. Immunohistochem. Mol. Morphol. 2006;14(3):296–302. doi: 10.1097/00129039-200609000-00007. [DOI] [PubMed] [Google Scholar]
- 69.Mitani Y, Oue N, Hamai Y, et al. Histone H3 acetylation is associated with reduced p21(WAF1/CIP1) expression by gastric carcinoma. J. Pathol. 2005;205(1):65–73. doi: 10.1002/path.1684. [DOI] [PubMed] [Google Scholar]
- 70.Yasui W, Oue N, Ono S, Mitani Y, Ito R, Nakayama H. Histone acetylation and gastrointestinal carcinogenesis. Ann. NY Acad. Sci. 2003;983:220–231. doi: 10.1111/j.1749-6632.2003.tb05977.x. [DOI] [PubMed] [Google Scholar]
- 71.Pathak SK, Basu S, Bhattacharyya A, et al. TLR4-dependent NF-κB activation and mitogen- and stress-activated protein kinase 1-triggered phosphorylation events are central to Helicobacter pylori peptidyl prolyl cis-, trans-isomerase (HP0175)-mediated induction of IL-6 release from macrophages. J. Immunol. 2006;177(11):7950–7958. doi: 10.4049/jimmunol.177.11.7950. [DOI] [PubMed] [Google Scholar]
- 72.Xia G, Schneider-Stock R, Diestel A, et al. Helicobacter pylori regulates p21(WAF1) by histone H4 acetylation. Biochem. Biophys. Res. Commun. 2008;369(2):526–531. doi: 10.1016/j.bbrc.2008.02.073. [DOI] [PubMed] [Google Scholar]
- 73.Ding SZ, Fischer W, Kaparakis-Liaskos M, et al. Helicobacter pylori-induced histone modification, associated gene expression in gastric epithelial cells, and its implication in pathogenesis. PLoS ONE. 2010;5(4):E9875. doi: 10.1371/journal.pone.0009875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fehri LF, Rechner C, Janssen S, et al. Helicobacter pylori-induced modification of the histone H3 phosphorylation status in gastric epithelial cells reflects its impact on cell cycle regulation. Epigenetics. 2009;4(8):577–586. doi: 10.4161/epi.4.8.10217. [DOI] [PubMed] [Google Scholar]
- 75■.Mueller A, Merrell DS, Grimm J, Falkow S. Profiling of microdissected gastric epithelial cells reveals a cell type-specific response to Helicobacter pylori infection. Gastroenterology. 2004;127(5):1446–1462. doi: 10.1053/j.gastro.2004.08.054. Uncovered a H. pylori-induced gene expression profile in murine epithelial cells and indicated the gastric pit or mucus-producing cells are the target of H. pylori infection. [DOI] [PubMed] [Google Scholar]
- 76.Karam SM. Cellular origin of gastric cancer. Ann. NY Acad. Sci. 2008;1138:162–168. doi: 10.1196/annals.1414.023. [DOI] [PubMed] [Google Scholar]
- 77■■.Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306(5701):1568–1571. doi: 10.1126/science.1099513. Initial report that gastric cancer can originate from bone marrow-derived cells. [DOI] [PubMed] [Google Scholar]
- 78.Oh JD, Karam SM, Gordon JI. Intracellular Helicobacter pylori in gastric epithelial progenitors. Proc. Natl Acad. Sci. USA. 2005;102(14):5186–5191. doi: 10.1073/pnas.0407657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fraga MF, Agrelo R, Esteller M. Cross-talk between aging and cancer: the epigenetic language. Ann. NY Acad. Sci. 2007;1100:60–74. doi: 10.1196/annals.1395.005. [DOI] [PubMed] [Google Scholar]
- 80.Necchi V, Candusso ME, Tava F, et al. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology. 2007;132(3):1009–1023. doi: 10.1053/j.gastro.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 81.Kirchner T, Muller S, Hattori T, et al. Metaplasia, intraepithelial neoplasia and early cancer of the stomach are related to dedifferentiated epithelial cells defined by cytokeratin-7 expression in gastritis. Virchows Arch. 2001;439(4):512–522. doi: 10.1007/s004280100477. [DOI] [PubMed] [Google Scholar]
- 82.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]