SYNOPSIS
Objectives
We evaluated the association between intentional delay of vaccine administration and timely vaccination coverage.
Methods
We used data from 2,921 parents of 19- to 35-month-old children that included parents' reports of intentional delay of vaccine administration. Timely vaccination was defined as administration with ≥4 doses of diphtheria, tetanus, and pertussis; ≥3 doses of polio vaccine; ≥1 dose of measles, mumps, and rubella vaccine; ≥3 doses of Haemophilus influenzae type b vaccine; ≥3 doses of hepatitis B vaccine; and ≥1 dose of varicella vaccine by 19 months of age, as reported by vaccination providers.
Results
In all, 21.8% of parents reported intentionally delaying vaccinations for their children. Among parents who intentionally delayed, 44.8% did so because of concerns about vaccine safety or efficacy and 36.1% delayed because of an ill child. Children whose parents intentionally delayed were significantly less likely to receive all vaccines by 19 months of age than children whose parents did not delay (35.4% vs. 60.1%, p<0.05). Parents who intentionally delayed were significantly more likely to have heard or read unfavorable information about vaccines than parents who did not intentionally delay (87.6% vs. 71.9%, p<0.05). Compared with parents who intentionally delayed only because their child was ill, parents who intentionally delayed only because of vaccine safety or efficacy concerns were significantly more likely to seek additional information about their decision from the Internet (11.4% vs. 1.1%, p<0.05), and significantly less likely to seek information from a doctor (73.9% vs. 93.9%, p<0.05).
Conclusions
Intentionally delayed vaccine doses are not uncommon. Children whose parents delay vaccinations may be at increased risk of not receiving all recommended vaccine doses by 19 months of age and are more vulnerable to vaccine-preventable diseases. Providers should consider strategies such as educational materials that address parents' vaccine safety and efficacy concerns to encourage timely vaccination.
Today, the number of cases of most vaccine-preventable diseases (VPDs) is at an all-time low, and hospitalizations and deaths have shown striking decreases since the 1980s.1 This success has been accompanied by parents' concerns about vaccine safety. Although scientific evidence does not support an association between vaccines and autism,2–6 media coverage7,8 has aroused parents' concerns. Concerns were further aroused when elevated rates of intussusception were found to be associated with the first vaccine licensed for rotavirus,9,10 and when lawsuits against pharmaceutical companies and claims with the Vaccine Injury Compensation Program resulted in awards for injuries that were alleged to be caused by vaccines.11 As the number of recommended vaccines has increased, parents may ask providers to spread out the administration of vaccines so that fewer injections are administered at each visit.12 Concerns about vaccine safety and the increasing number of injections may explain why some parents delay the administration of selected vaccines for their children. However, children whose parents delay vaccinations may be at increased risk of not receiving all recommended vaccine doses and may be more vulnerable to VPDs.
We evaluated the association between intentional delay of vaccine administration and timely vaccination coverage. We also determined the reasons that parents give for delaying vaccines; investigated the association between a parental report of hearing or reading unfavorable information about vaccines and parents' decisions to delay vaccine administration; explored where parents seek further information in making their decision to delay vaccine administration; and identified child, maternal, and household characteristics that are associated with delay.
METHODS
We used data from the 2003 National Immunization Survey (NIS) for our study. Data are collected in the NIS in two phases: a telephone survey of households with landline telephones that have children aged 19 to 35 months, followed by a survey mailed to those children's vaccination providers. We used provider-reported vaccination histories to determine vaccination status in our study. In 2003, 30,909 households with 19- to 35-month-old children completed the NIS telephone interview; among those, we obtained provider-reported vaccination histories for 21,291 children. In 2003, the response rate of the telephone portion of the NIS was 70%, and the percentage of children with an adequate provider-reported vaccination history from the mailed survey was 78%. In 2003, 3,576 households with 19- to 35-month-old children were randomly selected to be administered a series of questions regarding parents' concerns about vaccine safety. Among those households, 3,403 completed that series of questions, and 2,921 19- to 35-month-old children had provider-reported vaccination histories returned from sampled children's vaccination providers.
In our study, we analyzed data from the 2,921 19- to 35-month-old children who had provider-reported vaccination histories returned in the mailed survey to their vaccination providers, and whose parents completed the NIS telephone interview and were administered questions about their vaccine safety concerns. Among the 2,921 completed telephone interviews that yielded provider-reported vaccination coverage histories for sampled children, 96% were conducted with the child's parent, nearly 3% were conducted with the child's grandparent, and a little more than 1% were conducted with another family member who was ≥18 years of age and knowledgeable about the child's vaccination history. For brevity, we refer to the respondent in the telephone portion of the NIS interview as the child's parent.
Because the vaccination schedule13 specifies that children are to be administered all recommended vaccine doses by 19 months of age, we defined “timely” vaccination coverage as vaccination coverage at 19 months of age and assessed coverage by a review of the provider-reported vaccination history of all sampled children. We defined “catch-up” vaccination coverage as vaccination coverage at 24 months of age and assessed this coverage by a review of the provider-reported vaccination histories of all sampled children ≥24 months of age. Children were defined to be up-to-date (UTD) at those milestone ages if their vaccination history indicated that they were administered ≥4 doses of diphtheria, tetanus, and pertussis (DTaP and/or DTP) vaccine; ≥3 doses of polio vaccine; ≥1 dose of measles, mumps, and rubella (MMR) vaccine; ≥3 doses of Haemophilus influenzae type b (Hib) vaccine; ≥3 doses of hepatitis B (Hep B) vaccine; and ≥1 dose of varicella vaccine. Children were defined to be 4:3:1:3:3:1 UTD at those milestone ages if they were UTD for DTaP/DTP, polio, MMR, Hib, Hep B, and varicella vaccines.
In the survey, parents were asked if they ever decided to delay a vaccine dose and if they ever decided to not allow the administration of a dose. In our study, parents who answered “yes” to either of these two questions were categorized as intentionally delaying vaccine administration. Parents who reported delaying administration were asked to provide one reason for the delay, and parents who reported deciding to not allow the administration of a dose were asked to provide one reason for the delay. Because of this, parents who were categorized in our study as intentionally delaying vaccine administration could provide up to two reasons for the delay.
To evaluate the association between a parental report of hearing or reading unfavorable information about vaccines and the parents' decision to intentionally delay vaccine administration, NIS interviewers asked parents if they had heard or read about vaccines sometimes not preventing disease, not being safe or having serious side effects, being opposed by groups for political or religious reasons, and being opposed by groups that oppose vaccines for health reasons.
We used SUDAAN® software14 in all of our statistical analyses to account for the NIS design and sampling weights. Estimated percentages are reported along with their 95% confidence intervals (CIs). We considered differences between estimated percentages to be significantly different if a z-score test used to compare the estimates had a p-value ≤0.05. Smith et al.15 provide a detailed description of the statistical methods used in the NIS. The NIS has been approved by the Centers for Disease Control and Prevention (CDC) Institutional Review Board.
RESULTS
Among all parents of children who were 19 to 35 months of age in 2003, 21.8% (95% CI 19.0, 23.6) of parents who had 19- to 35-month-old children intentionally delayed vaccine doses for their child. Among parents who intentionally delayed, 44.8% (95% CI 37.6, 52.0) delayed because of concerns about vaccine safety or efficacy, 36.1% (95% CI 29.1, 43.1) delayed because their child was ill, 7.7% (95% CI 4.4, 11.0) delayed because of a missed appointment, 5.6% (95% CI 2.3, 8.9) delayed because of cost, 8.5% (95% CI 4.1, 12.6) intentionally delayed for other reasons, and 5.9% (95% CI 2.2, 9.6) reported more than one reason for the delay.
Association between intentional delay of vaccine administration and vaccination coverage
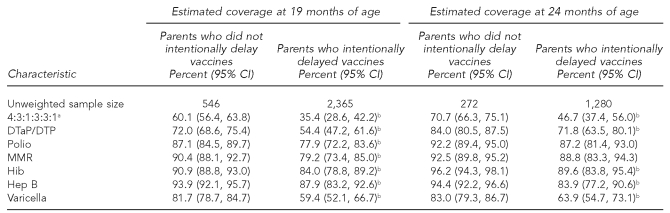
At 19 months of age, children whose parents intentionally delayed vaccine administration had significantly lower timely 4:3:1:3:3:1 vaccination coverage than children whose parents did not delay vaccination administration (35.4% vs. 60.1%, respectively, p<0.05). Also, estimated timely vaccination coverage at 19 months of age was significantly lower among parents who intentionally delayed for DTaP/DTP, polio, MMR, Hep B, Hib, and varicella vaccines.
At 24 months of age, children whose parents intentionally delayed vaccine administration continued to have significantly lower 4:3:1:3:3:1 vaccination coverage than children whose parents did not delay vaccination administration (46.7% vs. 70.7%, respectively, p<0.05). Also, catch-up vaccination coverage at 24 months of age was significantly lower among parents who intentionally delayed for DTaP/DTP, MMR, Hep B, and varicella vaccines (Table 1).
Table 1.
Estimated vaccination coverage of 19- to 35-month-old children at 19 and 24 months of age, by whether parents intentionally delayed vaccines: 2003 National Immunization Survey
aRefers to children who had completed ≥4 doses of DTaP and/or DTP vaccine; ≥3 doses of the polio vaccine; ≥1 dose of the MMR vaccine; ≥3 doses of the Hib vaccine; ≥3 doses of the Hep B vaccine; and ≥1 dose of the varicella vaccine.
bEstimated coverage among children whose parents intentionally delayed vaccines was significantly lower than among children whose parents did not intentionally delay vaccines at the specified month of age and the selected vaccine. CI = confidence interval
DTaP/DTP = diphtheria, tetanus, and pertussis
MMR = measles, mumps, and rubella
Hib = Haemophilus influenzae type b
Hep B = hepatitis B
Association between a parental report of hearing or reading unfavorable information about vaccines and the decision to intentionally delay vaccine administration
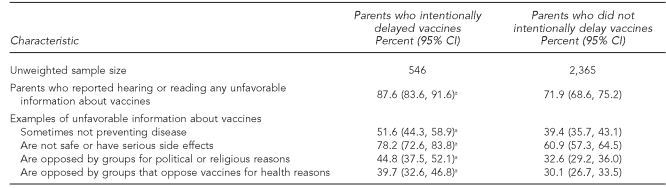
Parents who intentionally delayed vaccines were significantly more likely to have heard or read about some type of unfavorable information concerning vaccines than parents who did not intentionally delay (87.6% vs. 71.9%, respectively, p<0.05). In particular, compared with parents who did not delay vaccine administration, those who did were significantly more likely to report hearing or reading that vaccines sometimes do not prevent disease, are not safe or have serious side effects, are opposed by groups for political or religious reasons, and are opposed by groups that oppose vaccines for health reasons (Table 2).
Table 2.
Estimated percentage of parents who reported hearing or reading unfavorable information about vaccines, by whether they intentionally delayed vaccine administration for their child: 2003 National Immunization Survey
aEstimated percentage among parents who intentionally delayed vaccines who heard or read the unfavorable information about vaccines is significantly different from the estimated percentage among parents who did not delay vaccines.
CI = confidence interval
Where parents seek additional information when deciding to intentionally delay vaccine administration
Among parents who intentionally delayed only because their child was ill and not because of concerns about vaccine safety or efficacy, 64.3% (95% CI 52.8, 75.8) sought information in making their decision to delay vaccine administration. Among those who sought information, 93.9% (95% CI 86.7, 100.0) consulted a doctor. Compared with parents who intentionally delayed only because their child was ill, parents who intentionally delayed only because of vaccine safety or efficacy concerns were somewhat, although not significantly, more likely to seek information in making their decision (74.1% vs. 64.3%, respectively, p=0.11). Compared with parents who intentionally delayed vaccines only because their child was ill, parents who intentionally delayed vaccines only because of vaccine safety or efficacy concerns were significantly more likely to seek information from the Internet (11.4% vs. 1.1%, respectively, p<0.05), significantly more likely to seek information from the library or other media sources (10.8% vs. 1.0%, respectively, p<0.05), and significantly less likely to seek information from a doctor (73.9% vs. 93.9%, respectively, p<0.05). However, more than a majority (73.9%, 95% CI 60.2, 87.6) of parents who intentionally delayed because of safety or efficacy concerns sought information from a doctor in deciding whether or not to delay.
Child, maternal, and household characteristics associated with intentional delay
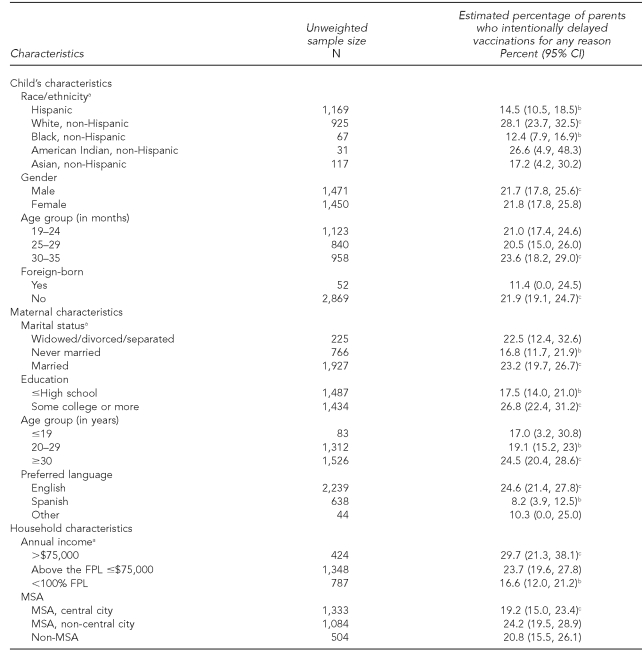
As shown in Table 3, the estimated percentage of parents who reported intentionally delaying vaccine administration was significantly higher among non-Hispanic white children compared with Hispanic and non-Hispanic black children, and significantly higher among children whose mothers were married vs. never married, had some college vs. a high school diploma or less, were at least 30 years of age vs. 20–29 years of age, or preferred to speak English rather than Spanish during the NIS telephone interview. Also, the estimated percentage of parents who reported intentionally delaying vaccine administration was significantly higher among children living in a household with an annual income that was >$75,000 compared with children living in a household with an annual income that was below 100% of the federal poverty level (FPL). The FPL is updated each year by the U.S. Census Bureau. The poverty thresholds are used mainly for statistical purposes—for instance, preparing estimates of the number of Americans in poverty each year. For any given child, the threshold depends on the number of adults and children ≤18 years of age in the household.
Table 3.
Estimated percentage of parents who intentionally delayed vaccinations for any reason, by child, maternal, and household characteristics: 2003 National Immunization Survey
aRace/ethnicity was missing for two case subjects, marital status was missing for three case subjects, and annual income was missing for 362 case subjects.
bEstimated percentage of reporting a delay is statistically different with p<0.05.
cReference category
CI = confidence interval
FPL = federal poverty level
MSA = metropolitan statistical area
DISCUSSION
New information provided by our study shows that children of parents who intentionally delayed the administration of vaccines were significantly less likely to be UTD at 19 and 24 months of age for the 4:3:1:3:3:1 vaccination series. As the number of recommended vaccines increases, parents may ask vaccination providers for an alternative vaccination schedule7 that spreads out vaccinations and requires fewer injections at each visit to their vaccination provider. Our findings suggest that if spreading out vaccinations entails delaying vaccinations, children are at significant risk of not receiving all recommended vaccine doses.
Delaying vaccination leaves young children vulnerable to disease. Recent outbreaks of VPDs among unvaccinated children in Pennsylvania,16,17 Washington State, Illinois,18 and California19 provide examples that illustrate that risk. Our national findings are consistent with an inner-city clinic-based study suggesting that if opportunities to administer vaccinations before 19 months of age are missed, catch-up doses may also be missed.20
We found that children whose parents intentionally delayed vaccine administration were significantly more likely to be non-Hispanic white children, to have a mother who had at least some college education, to live in a household with an annual income above the FPL, and to have concerns about vaccine safety. Other research has shown that these characteristics are associated with children who have not been administered any vaccine doses.21
Limitations
Because the NIS is a survey of 19- to 35-month-old children, we were unable to determine whether children with intentionally delayed vaccinations eventually became fully vaccinated or accumulate in the communities in which they live and become “pools” of partially vaccinated vulnerable children. However, other literature has demonstrated the significant association between the geographic clustering of children who take philosophical exemptions to state immunization laws that mandate vaccination before school entry and the geographic clustering of measles22 and pertussis.23 Therefore, accumulation of those vulnerable children within the communities in which they live seems likely. Also, statistics obtained from the NIS are weighted to be representative of all children aged 19 to 35 months in the U.S. Statistical adjustments are made to the survey weights to account for households without landline telephones and other effects that could bias estimates from the NIS.13
Because the NIS is a survey of children living in households with landline telephones only, our analyses did not contain data from children who lived in households with no telephone service or children who lived in households with cellular phone service only. When the data from our study were collected, the percentage of children who lived in a household with no telephone service was approximately 1.5%, and the percentage of households with cellular phone service only was approximately 3.0%. Because the percentage of children living in households with no phone service and in households with only cellular phone service is small (3.0% + 1.5%), the maximum bias in national estimates obtained from our analysis of data from the 2003 NIS that can be attributable to noncoverage of non-telephone and cellular-only households is expected to be small. Recent work suggests that bias in surveys that only sample households with landline telephones may be small.24,25
Also, between 2000 and 2003, there were shortages of the DTaP, MMR, and varicella vaccines.26,27 However, there were no shortages for polio and Hib vaccines, and we found significant gaps in vaccination coverage for those vaccines between children whose parents intentionally delayed and those that did not delay vaccinating their children. Because those gaps were approximately as large as we found for DTaP, MMR, and varicella, it seems unlikely that vaccine shortages could fully account for the gaps in vaccination coverage for DTaP, MMR, and varicella.
Finally, a strength of our study was that it relied on parents' reports of intentional delay of vaccines. Other research28 has measured delay as the duration of time between visits for vaccinations that exceeds the interval that is specified in the recommended vaccination schedule.13 We chose not to use those methods because when children in our study were being vaccinated, different manufacturers recommended different vaccination schedules for their own vaccine product29 and the NIS did not collect information on manufacturers at that time.
CONCLUSIONS
Our results showed that having an ill child was the second-most common reason for parents to intentionally delay vaccine administration. There is evidence that parents would be willing to accept vaccination at urgent care visits if their physicians strongly recommended it and reassured parents that vaccinating was safe when a child has a mild illness.30 Our findings indicated that parents who intentionally delay vaccine administration because of an ill child were likely to seek information about their decision to delay from their doctor. Because of this, providers who use their reminder/recall system to recall an ill child to their clinic and are effective in persuading parents to allow the administration of vaccines when a child is mildly ill may succeed in completing the recommended vaccination schedule for those children.
Our results showed that parents' concern about vaccine safety or efficacy is the most common reason associated with the delay of vaccine administration. Those results are concordant with other studies that have indicated vaccine safety concerns as a main reason for parents to delay vaccines for their children.31,32 These parents were approximately as likely to seek information about their decision as parents who intentionally delayed because of an ill child. However, parents with concerns about safety or efficacy who intentionally delayed were more likely to seek information from sources other than their child's doctor, such as the Internet, the library, or other media sources. Also, parents who intentionally delayed were more likely to have heard or read unfavorable information about vaccines compared with parents who did not delay. However, we found that a majority of parents who intentionally delayed vaccinations because of concerns about vaccine safety or efficacy seek additional information in making their decision to delay from a doctor. It is troubling that regardless of whether parents delay or not, the percentage who hear or read unfavorable information about vaccines is quite high.
Other research suggests that children of parents who feel that vaccines are not safe can have vaccination coverage that is as high as among children whose parents feel that vaccines are safe, provided that those parents' vaccination decisions are influenced by a doctor. Educational interventions,33 reading materials,34 and social marketing strategies35–37 that address safety concerns may encourage those parents to have their children vaccinated. Inadequate provider reimbursement for those types of non-vaccine-related costs are regarded as an important barrier to vaccinating children. To overcome this barrier, the Vaccine Financing Working Group of the National Vaccine Advisory Committee has recommended that Medicaid reimbursement amounts be updated to include all appropriate non-vaccine-related costs.38
Delay of vaccine doses is not uncommon. Children whose parents delay vaccinations may be at increased risk of not receiving all recommended vaccine doses by 19 months of age and are more vulnerable to VPDs. When parents delay vaccinations and their children fall behind the recommended schedule for administering vaccines, the recommended schedule does not need to be restarted.39 Catch-up vaccination schedules are available for children whose vaccinations have been delayed.40
Acknowledgments
The viewpoints expressed in this article are those of the authors and do not necessarily represent those of the Department of Health and Human Services or the Centers for Disease Control and Prevention.
Footnotes
Kirsten Vannice was supported in part by the National Institutes of Health International Maternal and Child Health Training Grant T32HD046405.
REFERENCES
- 1.Roush SW, Murphy TV Vaccine-Preventable Disease Table Working Group. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA. 2007;298:2155–63. doi: 10.1001/jama.298.18.2155. [DOI] [PubMed] [Google Scholar]
- 2.Immunization Safety Review Committee. Immunization safety review: vaccines and autism. Washington: National Academies Press; 2004. [PubMed] [Google Scholar]
- 3.Harris G. Deal in an autism case fuels debate on vaccine. New York Times. 2008. Mar 8, [cited 2008 Dec 17]. Also available from: URL: http://www.nytimes.com/2008/03/08/us/08vaccine.html.
- 4.Food and Drug Administration (US) Thimerosal in vaccines. [cited 2008 Dec 17]. Available from: URL: http://www.fda.gov/CbER/vaccine/thimerosal.htm#tox.
- 5.Verstraeten T, Davis RL, DeStefano F, Lieu TA, Rhodes PH, Black SB, et al. Safety of thimerosal-containing vaccines: a two-phased study of computerized health maintenance organization databases. Pediatrics. 2003;112:1039–48. [PubMed] [Google Scholar]
- 6.Andrews N, Miller E, Grant A, Stowe J, Osborne V, Taylor B. Thimerosal exposure in infants and developmental disorders: a retrospective cohort study in the United Kingdom does not support a causal association. Pediatrics. 2004;114:584–91. doi: 10.1542/peds.2003-1177-L. [DOI] [PubMed] [Google Scholar]
- 7.Speers T, Lewis J. Journalists and jabs: media coverage of the MMR vaccine. Commun Med. 2004;1:171–81. doi: 10.1515/come.2004.1.2.171. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy J. Mother warriors: a nation of parents healing autism against all odds. New York: Dutton Adult; 2008. [Google Scholar]
- 9.Suspension of rotavirus vaccine after reports of intussusception—United States, 1999. MMWR Morb Mortal Wkly Rep. 2004;53(34):786–9. published erratum appears in MMWR Morb Mortal Wkly Rep 2004 53(34):879. [PubMed] [Google Scholar]
- 10.Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–72. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 11.Offit PA. Vaccines and autism revisited—the Hannah Poling case. N Eng J Med. 2008;358:2089–91. doi: 10.1056/NEJMp0802904. [DOI] [PubMed] [Google Scholar]
- 12.Sears R. New York: Little, Brown and Company; 2007. The vaccine book: making the right decision for your child. Sears Parenting Library. [Google Scholar]
- 13.Recommended childhood and adolescent immunization schedule. MMWR Morb Mortal Wkly Rep. 2003;52(4):Q1–4. [PubMed] [Google Scholar]
- 14.Research Triangle Institute. SUDAAN®: Release 8.0. Research Triangle Park (NC): Research Triangle Institute; 2002. [Google Scholar]
- 15.PJ Smith, DC Hoaglin, MP Battaglia, M Khare, LE Barker. Statistical methodology of the National Immunization Survey, 1994–2002. [cited 2008 Dec 5];Vital Health Stat 2. 2005 (138) Also available from: URL: http://www.cdc.gov/nchs/data/series/sr_02/sr02_138.pdf. [PubMed] [Google Scholar]
- 16.Pennsylvania Department of Health. Health alert network 2009: health alert #152. Harrisburg (PA): Pennsylvania Department of Health; 2009. Three confirmed cases of measles in a Westmoreland County household. [Google Scholar]
- 17.Pennsylvania Department of Health. Health alert network 2009: health alert #153. Harrisburg (PA): Pennsylvania Department of Health; 2009. Management of measles exposure in Allegheny County. [Google Scholar]
- 18.Update: measles—United States, January–July 2008. MMWR Morb Mortal Wkly Rep. 2008;57(33):893–6. [PubMed] [Google Scholar]
- 19.Outbreak of measles—San Diego, California, January–February 2008. MMWR Morb Mortal Wkly Rep. 2008;57(8):203–6. [PubMed] [Google Scholar]
- 20.Sabnis SS, Pomeranz AJ, Lye PS, Amateau MM. Do missed opportunities stay missed? A 6-month follow-up of missed vaccine opportunities in inner city Milwaukee children. Pediatrics. 1998;101:e5. doi: 10.1542/peds.101.5.e5. [DOI] [PubMed] [Google Scholar]
- 21.Smith PJ, Chu SY, Barker LE. Children who have received no vaccines: who are they and where do they live? Pediatrics. 2004;114:187–95. doi: 10.1542/peds.114.1.187. [DOI] [PubMed] [Google Scholar]
- 22.Salmon DA, Haber M, Gangarosa EJ, Phillips L, Smith NJ, Chen RT. Health consequences of religious and philosophical exemptions from immunization laws: individual and societal risk of measles. JAMA. 1999;282:47–53. doi: 10.1001/jama.282.1.47. [DOI] [PubMed] [Google Scholar]
- 23.Omer SB, Enger SK, Moulton LH, Halsey NA, Stokley S, Salmon DA. Geographic clustering of nonmedical exemptions to school immunization requirements and associations with geographic clustering of pertussis. Am J Epidemiol. 2008;168:1389–96. doi: 10.1093/aje/kwn263. [DOI] [PubMed] [Google Scholar]
- 24.Blumberg SJ, Luke JV. Coverage bias in traditional telephone surveys of low-income and young adults. Public Opin Q. 2007;71:734–9. [Google Scholar]
- 25.Blumberg SJ, Luke JV. Reevaluating the need for concern regarding noncoverage bias in landline surveys. Am J Public Health. 2008;99:1806–10. doi: 10.2105/AJPH.2008.152835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Update on the supply of tetanus and diphtheria toxoids and of diphtheria and tetanus toxoids and acellular pertussis vaccine. MMWR Morb Mortal Wkly Rep. 2001;50(10):189–90. [PubMed] [Google Scholar]
- 27.Notice to readers: shortage of varicella and measles, mumps and rubella vaccines and interim recommendations from the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2002;51(9):190–7. [PubMed] [Google Scholar]
- 28.Dombkowski KJ, Lantz PM, Freed GL. Risk factors for delay in age-appropriate vaccination. Public Health Rep. 2004;119:144–55. doi: 10.1177/003335490411900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Notice to readers: recommended childhood immunization schedule—United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(2):31–3. [PubMed] [Google Scholar]
- 30.Udovic SL, Lieu TA, Black SB, Ray PM, Ray GT, Shinefield HR. Parent reports on willingness to accept childhood immunizations during urgent care visits. Pediatrics. 1998;102:e47. doi: 10.1542/peds.102.4.e47. [DOI] [PubMed] [Google Scholar]
- 31.Salmon DA, Moulton LH, Omer SB, DeHart MP, Stokley S, Halsey NA. Factors associated with refusal of childhood vaccines: a case-control study. Arch Pediatr Adolesc Med. 2005;159:470–6. doi: 10.1001/archpedi.159.5.470. [DOI] [PubMed] [Google Scholar]
- 32.Smith PJ, Kennedy AM, Wooten K, Gust DA, Pickering LK. Association between health care providers' influence on parents who have concerns about vaccine safety and vaccination coverage. Pediatrics. 2006;118:e1287–92. doi: 10.1542/peds.2006-0923. [DOI] [PubMed] [Google Scholar]
- 33.Briss PA, Rodewald LE, Hinman AR, Shefer AM, Strikas RA, Bernier RR, et al. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(1 Suppl):97–140. doi: 10.1016/s0749-3797(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (US) Vaccine safety information for parents. [cited 2008 Dec 17]. Available from: URL: http://www.cdc.gov/vaccinesafety/basic/parents.htm.
- 35.Opel DJ, Diekema DS, Lee NR, Marcuse EK. Social marketing as a strategy to increase immunization rates. Arch Pediatr Adolesc Med. 2009;163:432–7. doi: 10.1001/archpediatrics.2009.42. [DOI] [PubMed] [Google Scholar]
- 36.Findley SE, Sanchez M, Mejia M, Ferreira R, Pena O, Matos S, et al. Health Promot Pract. Suppl 2. Vol. 10. New York City: 2009. effective strategies for integrating immunization promotion into community programs; pp. S128–37. REACH 2010. [DOI] [PubMed] [Google Scholar]
- 36.Boom JA, Nelson CS, Kohrt AE, Kozinetz CA. Utilizing peer academic detailing to improve childhood immunization coverage levels. Health Promot Pract. 2008 Oct 27; doi: 10.1177/1524839908321487. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Department of Health and Human Services (US) Appendix 1: Children and adolescents vaccine financing recommendations adopted by NVAC—September 2008 (with approved editorial changes March 2, 2009) Available from: URL: http://www.hhs.gov/nvpo/nvac/cavfrecommendationssept08.html.
- 39.Kroger AT, Atkinson WL, Marcuse EK, Pickering LK, Advisory Committee on Immunization Practices (ACIP). General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-15):1–48. [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (US) 2010 child and adolescent immunization schedules for persons aged 0–6 years, 7–18 years, and “catch-up schedule”. [cited 2009 Sep 30]. Available from: URL: http://www.cdc.gov/vaccines/recs/schedules/child-schedule.htm.