SYNOPSIS
Objectives
To better understand disparities in pregnancy outcomes, we analyzed data from North Carolina to determine how the pattern of maternal hypertensive disorders differs among non-Hispanic white (NHW), non-Hispanic black (NHB), and Hispanic women across the range of maternal ages. In addition, we explored whether rates of poor birth outcomes, including low birthweight (LBW) and preterm birth (PTB), among hypertensive women differed by race.
Methods
We restricted our analyses to births occurring between 1994 and 2003, constructing six five-year maternal age categories: 15–19 years, 20–24 years, 25–29 years, 30–34 years, 35–39 years, and 40–44 years. We used logistic regression to determine the relative contribution of race and age to incidence of maternal hypertension. All analyses controlled for the standard covariates of maternal education, marital status, and tobacco use. To assess the impact of maternal hypertension on birth outcomes, we limited the dataset to women with any hypertensive disorder and used linear regression to determine how particular race-age combinations affected outcomes. We also used logistic regression to find out how particular race-age combinations affected the likelihood of LBW and PTB.
Results
The risk of hypertension differed by race, with NHB women exhibiting the highest risk and Hispanic women the lowest risk. Further, rates of hypertension increased with age. Among hypertensive women, pregnancy outcomes differed by race and age, with NHB women having the poorest outcomes (i.e., LBW and PTB) and age exhibiting a dose-response relationship in PTB and very PTB.
Conclusions
Patterns of maternal hypertension and subsequent outcomes are important contributors to persistent disparities in pregnancy outcomes.
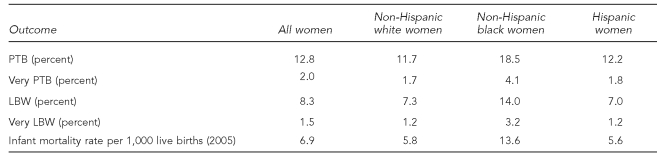
One of the most persistent disparities in American health status is the pronounced difference in pregnancy outcomes for non-Hispanic black (NHB) compared with non-Hispanic white (NHW) women (Table 1).1–3 In 2006, compared with NHW infants, NHB infants were nearly twice as likely to be born low birthweight (LBW), defined as <2,500 grams (14.0% vs. 7.3%, respectively), and almost three times as likely to be very LBW, defined as <1,500 grams (3.2% vs. 1.2%, respectively). The incidence of LBW and very LBW is, respectively, 7.0% and 1.2% among Hispanic women. Approximately 18.5% of NHB infants were preterm births (PTB) (born at <37 completed weeks of gestation; very PTB refers to those born at <34 completed weeks of gestation), as compared with 11.7% for NHW women and 12.2% for Hispanic women.4 In 2005, NHB infants were more than twice as likely to die before their first birthday; the infant mortality rate for NHB infants was 13.63 per 1,000 live births compared with 5.76 for NHW infants. While congenital anomalies were the leading cause of infant mortality in the United States, for infants of NHB women, disorders related to short gestation and LBW were the leading cause of infant mortality.5 The relative differences have remained fairly constant during the past several decades, narrowing only slightly due to an increase in multiple gestation in NHW women.6
Table 1.
Pregnancy outcome rates in the United States, 2006a,b
aMartin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: final data for 2006. Natl Vital Stat Rep 2009 Jan 7;57: 1-104.
bMathews TJ, MacDorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. Natl Vital Stat Rep 2008 Jul 30;57:1-32.
PTB = preterm birth
LBW = low birthweight
While numerous studies have found that indicators of socioeconomic status are correlated with birth outcomes,7–10 these are not the only factors. If that were the case, NHB and Hispanic women would have similar rates of poor birth outcomes given their comparable socioeconomic standing. In fact, Hispanic women present with rates of LBW and PTB that are very similar to NHW women.
One potential explanation for differences in pregnancy outcomes may relate to differences in presentation of maternal medical complications. In particular, hypertensive disorders complicate approximately 12% to 22% of all pregnancies.11 Maternal hypertension, including preeclampsia, gestational hypertension, and chronic hypertension, is a major driver of adverse pregnancy outcomes, including reduced birthweight and gestation.12,13 Infants of hypertensive mothers compared with normotensive mothers have been found to have higher rates of being small for gestational age.12,14,15 Such infants are more likely to be admitted to the neonatal intensive care unit.15 Although rates of fetal and maternal mortality have been found to be similar between hypertensive and normotensive groups, morbidity is greater in hypertensive mothers and their neonates.13 Chronic hypertension results in increased risk for preeclampsia, which, in turn, can cause many morbidities and increased mortality.16,17 Understanding who is most likely to present with hypertension and what the possible associated outcomes might be is critical to developing interventions aimed at improving maternal health.
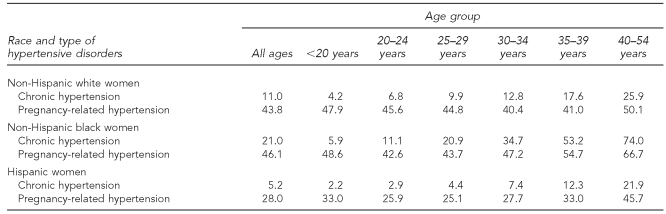
In 2006, chronic hypertension among NHB mothers of all ages was twice as prevalent as that among NHW mothers (21.0 per 1,000 births for NHB mothers and 11.0 per 1,000 births for NHW mothers). Chronic hypertension rates among Hispanic women were very low (5.2 per 1,000 births).4 Disparities in the rate of chronic hypertension between NHW and NHB women increased in a linear fashion with advancing maternal age. The rate of chronic hypertension among NHW mothers increased from 4.2 per 1,000 births among teenage mothers to 25.9 per 1,000 births among mothers aged 40–54 years. For NHB mothers in these two age categories, the increase in prevalence was more dramatic: rising from 5.9 per 1,000 births among women younger than 20 years of age to 74.0 per 1,000 births among women aged 40–54 years. For Hispanic mothers, the rate of increase was much slower, from 2.2 to 21.9 per 1,000 births. Rates of pregnancy-related hypertension were more similar between NHB and NHW mothers (46.1 and 43.8 per 1,000 births for NHB and NHW mothers of all ages, respectively) and lower for Hispanic mothers (28.0 per 1,000 births for Hispanic mothers of all ages); however, race- and age-specific rates began to diverge by age 30 (Table 2).4
Table 2.
Incidence of hypertensive disorders per 1,000 live births by maternal race, U.S., 2006a
aMartin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: final data for 2006. Natl Vital Stat Rep 2009 Jan 7;57: 1-104.
We analyzed data on pregnancy outcomes from North Carolina to determine whether the pattern of maternal hypertensive disorders differed among NHW, NHB, and Hispanic women across the range of maternal ages. In addition, we explored whether rates of poor birth outcomes (e.g., LBW and PTB) among hypertensive women differed by race category.
METHODS
Data
The North Carolina Detailed Birth Record (NCDBR) database contains extensive information on every documented live birth in North Carolina, including maternal age, birthweight, clinical estimate of gestation, plurality, maternal complications, congenital anomalies, maternal tobacco use, and a variety of maternal and paternal demographic variables. For this analysis, we focused on North Carolina births occurring between 1994 and 2003. Access to the data, as well as methods for receiving, storing, linking, and analyzing data and presenting results related to this study, were all governed by a research protocol approved by Duke University's Institutional Review Board.
The NCDBR contains three medical history variables related to hypertension: pregnancy-related hypertension, chronic hypertension, and eclampsia. We combined all three hypertension designations into a single category of “any hypertensive disorder.” Because we have access to clinical data on a subset of women in the NCDBR, we are aware that women who might more reasonably be coded as undiagnosed chronic hypertension when presenting in the obstetrics clinic are, in fact, coded as pregnancy-related hypertension within the NCDBR. Thus, the distinctions made in the NCDBR may not always be medically accurate. This potential limitation was considered in the interpretation of our study results.
We restricted the analysis to singleton births with no documented congenital anomalies. To focus specifically on the presentation and impact of hypertensive disorders, we excluded women with any other maternal medical complications (i.e., women with diabetes or women with chronic hypertension and diabetes were excluded from the analysis). We used self-declared maternal race/ethnicity to establish three distinct subgroups: NHW, NHB, and Hispanic women. We excluded all other maternal races from the analysis.
Maternal age is documented in integer years ranging from 10 to 55. We restricted our analyses to mothers between 15 and 44 years of age because of the small cell sizes at very young or older age categories, and then constructed six five-year age categories: 15–19, 20–24, 25–29, 30–34, 35–39, and 40–44 years of age. Birthweight was coded in pounds (0–14) and ounces (0–15), which we subsequently converted to grams, resulting in 240 possible integer values for the variable. The clinical estimate of gestation was given as number of completed weeks and ranged from 0 to 45 as estimated by the attending health-care provider. Again, because we have access to clinical data on a subset of women in the NCDBR, we are aware that the clinical estimate of gestation, as compared with the last menstrual period estimate of gestational age, more reliably matches up with a clinical estimate based on the first trimester ultrasound.
We restricted analyses to live births ≥24 weeks of gestation and birthweight ≥400 grams. Finally, we restricted analyses to first births only, because a first birth accompanied by maternal hypertension may decrease the willingness of women to become pregnant again (Unpublished report, Marie Lynn Miranda, Duke University, 2009), making the population of women with higher parity selectively biased. Observations without complete covariate data (1,039 observations) were also omitted. The resulting dataset included 350,717 birth records.
We used logistic regression modeling to determine the relative contribution of race and maternal age to incidence of maternal hypertensive disorders, controlling for the standard covariates of maternal education, marital status, and tobacco use. These covariates were chosen based on their predominance in the extant literature on adverse pregnancy outcomes and maternal hypertension.2,12,18,19 This analysis allowed us to assess whether certain race-age combinations were more prone to present with maternal hypertensive disorders.
Because we were also interested in the impact of maternal hypertension on birth outcomes, in additional analyses we limited the dataset to those women with any hypertensive disorder and used linear regression to see how particular race-age combinations among these women affected birthweight. We also used logistic regression to find out how particular race-age combinations affected the likelihood of LBW (<2,500 grams) and PTB (<37 weeks gestation). All analyses controlled for the standard covariates of infant sex, maternal education, marital status, and tobacco use. In the birthweight and LBW models, we also controlled for gestational age. We conducted analyses using SAS® version 9.1.20
RESULTS
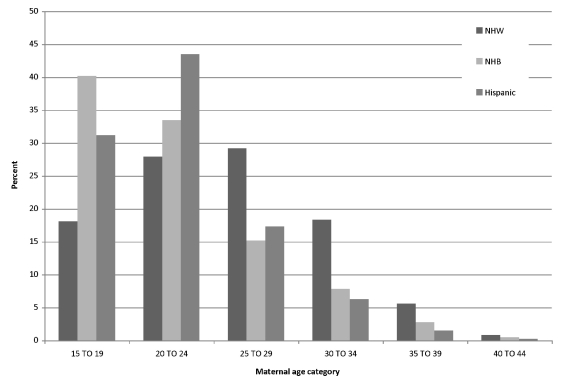
Figure 1 shows the age distribution of women included in the analysis, stratified by age and race/ethnicity, and reveals noticeable differences across subgroups: compared with NHW women, a greater proportion of births to NHB and Hispanic women occurred among the younger age groups (15–19 and 20–24 years of age).
Figure 1.
Maternal age distribution by race and age in North Carolina: 1994–2003 singleton nulliparous births
NHW = non-Hispanic white
NHB = non-Hispanic black
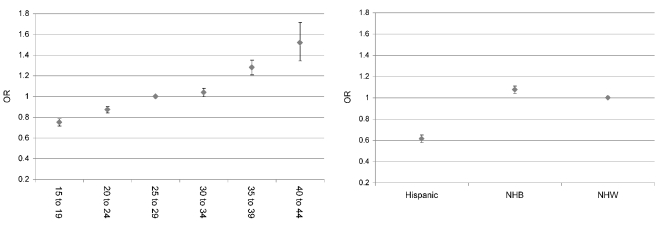
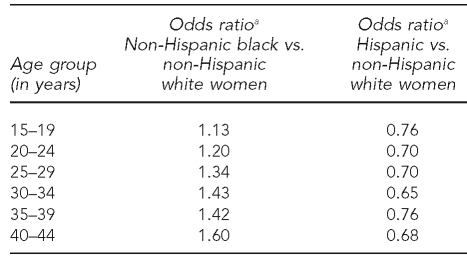
Using logistic regression, we estimated the likelihood of presenting with some form of hypertension as a function of maternal race (referent group = NHW women) and age (referent group = 25–29 years of age), controlling for marital status, tobacco use during pregnancy, and maternal education. In our sample, the proportion of the population presenting with any hypertensive disorder was 8.18% for all, 8.36% for NHW, 8.65% for NHB, and 5.47% for Hispanic women. Figure 2 shows the odds ratios for maternal age and race/ethnicity. The left panel of Figure 2 clearly demonstrates an increasing risk of hypertension as women age, and the right panel demonstrates a reduced risk for Hispanic women and increased risk of hypertension for NHB women, both relative to NHW women.
Figure 2.
Covariate-adjusted odds ratios for hypertension by maternal age (left) and maternal race (right) in North Carolina: 1994–2003 singleton nulliparous births
OR = odds ratio
NHB = non-Hispanic black
NHW = non-Hispanic white
We repeated the aforementioned analyses, adding race × age interactions. The results are summarized in Table 3. Compared with NHW women, NHB women were at an elevated risk for maternal hypertension in every age group, with the racial gap widening with increasing age. In contrast with NHB women, Hispanic women were at decreased risk of hypertension compared with NHW women, with the difference in risk basically stable across age categories.
Table 3.
Covariate-adjusted odds ratios for hypertension, including race X age interactions, in North Carolina: 1994–2003 singleton nulliparous births
aAll odds ratios are statistically significant.
In addition to considering the contribution of maternal race and age to the likelihood of presenting with hypertension, we conducted analyses on all NHW, NHB, and Hispanic women in the restricted NCDBR dataset used in the aforementioned logistic analyses who were coded as being hypertensive (n=28,697) to identify differential effects on birth outcomes. The age distribution of women in this sample was very similar to that shown in Figure 1, with a greater proportion of births to NHB and Hispanic women occurring among the younger age groups (15–19 and 20–24 years).
In analyses restricted to women with hypertension, maternal race and age were individually significant predictors of birthweight, LBW, gestational age, and PTB, but no race × age interactions were significant. We estimated birthweight as a function of maternal race (referent group = NHW women) and age (referent group = 25–29 years of age), controlling for infant sex, marital status, tobacco use during pregnancy, and maternal education. Among women with hypertension, NHB and Hispanic women had infants weighing a mean of 186 grams and 121 grams less (both p<0.0001) compared with NHW women. Note that while Hispanic women were less likely than NHW women to present with hypertension, among hypertensives, Hispanic birthweights were significantly lower than those for NHW women. In analysis of weeks of gestation, NHB women had gestational lengths with a mean of a half-week shorter (p<0.0001) than NHW women. No significant differences were observed between NHW and Hispanic women.
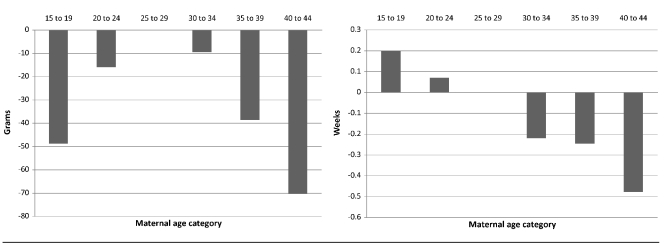
Among hypertensive women, Figure 3 (left panel) shows the U-shaped relationship between maternal age and birthweight, with the best outcomes accruing to women aged 25–29 years. Again, among hypertensive women, younger maternal age (15–19 and 20–24 years) tended to result in longer gestations, and older maternal age (30–34, 35–39, and 40–44 years) tended to result in shorter gestations (Figure 3, right panel).
Figure 3.
Relationship between maternal age and birthweight (left) and gestational age (right) among hypertensive women in North Carolina: 1994–2003 singleton nulliparous births
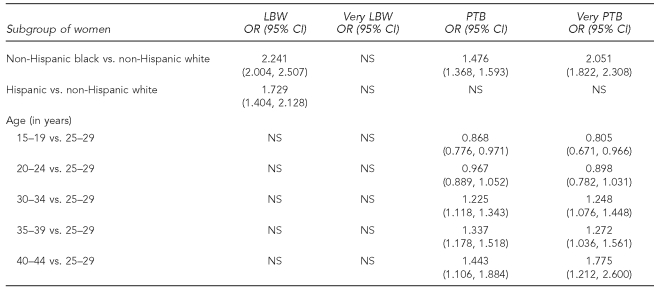
Table 4 summarizes the results for logistic regression on LBW, very LBW, PTB, and very PTB. (Because none of the race × age interactions were significant in these models, they were not included in the final analyses.) Neither race nor age resulted in significant odds ratios for very LBW. Maternal race, particularly NHB, was a significant predictor of poorer outcomes for LBW, PTB, and very PTB. Maternal age was not significant in the LBW and very LBW model, but followed a dose-response relationship in the PTB and very PTB models.
Table 4.
Covariate-adjusted odds ratios demonstrating effect of race and age on LBW, very LBW, PTB, and very PTB in North Carolina: 1994–2003 singleton nulliparous births
LBW = low birthweight
PTB = preterm birth
OR = odds ratio
CI = confidence interval
NS = not significant
DISCUSSION
We used the pattern of maternal hypertensive disorders as a way to explain disparities in pregnancy outcomes among NHW, NHB, and Hispanic women. We attempted to explain two important issues: (1) who is more likely to present with maternal hypertension and (2) among hypertensive women, who is more likely to have a poor birth outcome? We conducted all analyses using NCDBR data from 1994–2003, which provided a large population sample for analysis.
We found a clear association between race and age (and their interaction) and the likelihood of hypertension among pregnant women. The presentation of hypertension clearly differs by race, with NHB women demonstrating the highest rates, followed by NHW women, and then Hispanic women. In addition, rates of hypertension increase with age. Our analysis highlights significant race × age interactions: as NHB women age, their likelihood of presenting with hypertension increases more quickly compared with NHW women.
As stated previously, we defined hypertension during pregnancy as any hypertensive disorder, which includes both pregnancy-related and chronic hypertension. While there are clear clinical and epidemiologic differences between chronic and pregnancy-related hypertension, the disease incidence for both conditions follows the same pattern with regard to racial disparities (i.e., NHB mothers have higher rates of both conditions as compared with NHW and Hispanic mothers4). Furthermore, both diagnoses carry significant risk for adverse pregnancy outcomes, including LBW and PTB. Grouping the two hypertensive conditions together eliminates our ability to determine if one is driving the disparities in adverse pregnancy outcomes more than the other; however, it should not have affected our overall findings regarding the contributions of race and age.
Among hypertensive pregnant women, we did not note any race × age interactions; however, the main effects were quite substantial. Among hypertensive pregnant women, race was associated with birthweight, with NHW infants being significantly larger than Hispanic infants, and with Hispanic infants being significantly larger than NHB infants. The parameter estimates on the age categories demonstrated a U-shaped curve, with the highest birthweights in the 25–29 and 30–34 age categories. The risk for LBW among hypertensives followed a similar pattern, with NHB women having the highest risk for LBW infants, followed by Hispanic and then NHW women. Age was not a significant predictor in these models. Neither race nor age was a significant predictor in the model for very LBW infants.
Finally, among hypertensives, NHB women were at greater risk for early deliveries, as assessed by weeks of gestation, PTB, or very PTB. Hispanic women were not significantly different from NHW women on any of these three measures. Age appears to have a dose-response effect on the three measures of early deliveries, with younger maternal age (15–19 and 20–24 years) tending to result in longer gestations, and older maternal age (30–34, 35–39, and 40–44 years) tending to result in shorter gestations.
To understand the complex etiology of black birth outcomes relative to white birth outcomes, Geronimus proposed the “weathering hypothesis,”21 which postulates that poor birth outcomes of African Americans are in part due to the cumulative and interactive effects over time of negative material and psychosocial stressors on the physical health of black women.22–25 Thus, the weathering hypothesis posits that chronic and persistent stressors lead to accelerated biological aging of women, which may, in turn, account for excess infant mortality and adverse pregnancy outcomes among certain subpopulations.
Geronimus derived the weathering hypothesis after noting that NHB women have their best birth outcomes (lowest PTB and infant mortality rates) as teenagers, but birth outcomes for this group begin to deteriorate once they enter their 20s. For NHW women, the pattern is reversed: birth outcomes are poorest for teen mothers and best for women in their 20s.20,26,27 The weathering hypothesis argues that cumulative insults to the physical and emotional health of NHB women, beginning in early childhood, accelerate their biological aging (as evidenced by an earlier onset of chronic degenerative health problems such as hypertension, diabetes, and heart disease), thus compromising their ability to carry their fetuses to term.21,22
Our analysis can be used to determine whether the pattern of maternal hypertensive disorders is consistent with previous research on the weathering hypothesis. In particular, the weathering hypothesis suggests that presentation of hypertension among NHB women will be disproportionately higher in older age categories compared with NHW women. In addition, among women with hypertension, we expect the impact on birth outcomes to be more significant for NHB women compared with NHW women. Both of these hypothesized relationships held in our data sample. In particular, the widening gap between NHB and NHW women as we moved from the youngest to the oldest age categories was especially consistent with the notion of accelerated biological aging.
Despite high rates of socioeconomic disadvantage in North Carolina, Hispanic women tend to have birth outcomes that are equivalent to those of NHW women. This “Latina paradox” has been attributed to healthy migrant effects, cultural protective factors, and strong social support networks.27 The erosion of these protective factors appears to be associated with length of residence in the United States, with U.S.-born Hispanic women having poorer birth outcomes. We were unable to analyze this possibility, as the vast majority of Hispanic women in North Carolina are recent migrants. Preliminary analysis does support the notion that U.S.-born Hispanic women in North Carolina have worse pregnancy outcomes compared with foreign-born Hispanic women (e.g., the PTB rate is 7.81% among U.S.-born Hispanic women and 6.36% among foreign-born Hispanic women). As additional data become available, we will pursue this line of research.
Limitations
This study had a number of limitations. The analysis was based on the NCDBR. As a vital records database, it has the advantage of being a population sample, but also may not accurately reflect the clinical record on all counts. Additional relevant details, such as whether a PTB was spontaneous or medically indicated, are also not available. Ideally, in the PTB analyses, the clinical estimate of gestation would be based on reconciliation of menstrual dating and early ultrasound estimates—a measure that is again not available in the NCDBR. The analysis was also limited to births occurring in North Carolina, which may not be fully representative of the United States as a whole.
CONCLUSIONS
Persistent disparities in pregnancy outcomes have significant implications for population health. Survivors of LBW and PTB are at significant risk for both short-term neonatal morbidity as well as long-term disabilities.28 Of similar importance is the impact of LBW on increased risk of diabetes, obesity, cardiovascular disease, and other health problems in adulthood.29–31 Thus, understanding, and eventually intervening to prevent, these adverse birth outcomes is of critical importance to the overall health of the nation. Our analysis indicated that maternal hypertension is an important contributor to disparities in pregnancy outcomes. Direct management of hypertensive disorders, even preconceptionally, holds the potential to improve maternal and child health outcomes.
Footnotes
This research was supported by a grant from the U.S. Environmental Protection Agency (RD-83329301-0).
REFERENCES
- 1.Johnston RB, Jr, Williams MA, Hogue CJ, Mattison DR. Overview: new perspectives on the stubborn challenge of preterm birth. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):3–6. doi: 10.1046/j.1365-3016.2001.00003.x. [DOI] [PubMed] [Google Scholar]
- 2.Singh GK, Yu SM. Infant mortality in the United States: trends, differentials, and projections, 1950 through 2010. Am J Public Health. 1995;85:957–64. doi: 10.2105/ajph.85.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wise PH, Kotelchuck M, Wilson ML, Mills M. Racial and socioeconomic disparities in childhood mortality in Boston. N Engl J Med. 1985;313:360–6. doi: 10.1056/NEJM198508083130605. [DOI] [PubMed] [Google Scholar]
- 4.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: final data for 2006. Natl Vital Stat Rep. 2009 Jan 7;57:1–104. [PubMed] [Google Scholar]
- 5.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. Natl Vital Stat Rep. 2008 Jul 30;57:1–32. [PubMed] [Google Scholar]
- 6.MacDorman MF, Mathews TJ. Recent trends in infant mortality in the United States. Hyattsville (MD): National Center for Health Statistics (US); 2008. [PubMed] [Google Scholar]
- 7.Lynch JW, Everson SA, Kaplan GA, Salonen R, Salonen JT. Does low socioeconomic status potentiate the effects of heightened cardiovascular responses to stress on the progression of carotid atherosclerosis? Am J Public Health. 1998;88:389–94. doi: 10.2105/ajph.88.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch J, Smith GD, Hillemeier M, Shaw M, Raghunathan T, Kaplan G. Income inequality, the psychosocial environment, and health: comparisons of wealthy nations. Lancet. 2001;358:194–200. doi: 10.1016/S0140-6736(01)05407-1. [DOI] [PubMed] [Google Scholar]
- 9.Lynch J, Smith GD, Harper S, Hillemeier M, Ross N, Kaplan GA, et al. Is income inequality a determinant of population health? Part 1. A systematic review. Milbank Q. 2004;82:5–99. doi: 10.1111/j.0887-378X.2004.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finch BK. Early origins of the gradient: the relationship between socioeconomic status and infant mortality in the United States. Demography. 2003;40:675–99. doi: 10.1353/dem.2003.0033. [DOI] [PubMed] [Google Scholar]
- 11.ACOG Committee on Obstetric Practice. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 12.Allen VM, Joseph KS, Murphy KE, Magee LA, Ohlsson A. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: a population based study. BMC Pregnancy Childbirth. 2004;4:17. doi: 10.1186/1471-2393-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauth JC, Ewell MG, Levine RJ, Esterlitz JR, Sibai B, Curet LB, et al. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95:24–8. doi: 10.1016/s0029-7844(99)00462-7. [DOI] [PubMed] [Google Scholar]
- 14.Buchbinder A, Sibai BM, Caritis S, Macpherson C, Hauth J, Lindheimer MD, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186:66–71. doi: 10.1067/mob.2002.120080. [DOI] [PubMed] [Google Scholar]
- 15.Habli M, Levine RJ, Qian C, Sibai B. Neonatal outcomes in pregnancies with preeclampsia or gestational hypertension and in normotensive pregnancies that delivered at 35, 36, or 37 weeks of gestation. Am J Obstet Gynecol. 2007;197:406.e1–7. doi: 10.1016/j.ajog.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 16.Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369–77. doi: 10.1016/s0029-7844(02)02128-2. [DOI] [PubMed] [Google Scholar]
- 17.Sibai BM, Lindheimer M, Hauth J, Caritis S, VanDorsten P, Klebanoff M, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;339:667–71. doi: 10.1056/NEJM199809033391004. [DOI] [PubMed] [Google Scholar]
- 18.Ros HS, Cnattingius S, Lipworth L. Comparison of risk factors for preeclampsia and gestational hypertension in a population-based cohort study. Am J Epidemiol. 1998;147:1062–70. doi: 10.1093/oxfordjournals.aje.a009400. [DOI] [PubMed] [Google Scholar]
- 19.Luo ZC, Wilkins R, Kramer MS, Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System Effect of neighbourhood income and maternal education on birth outcomes: a population-based study. CMAJ. 2006;174:1415–2. doi: 10.1503/cmaj.051096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2:207–21. [PubMed] [Google Scholar]
- 21.Geronimus AT. Black/white differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Soc Sci Med. 1996;42:589–97. doi: 10.1016/0277-9536(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 22.Geronimus AT. Understanding and eliminating racial inequalities in women's health in the United States: the role of the weathering conceptual framework. J Am Med Womens Assoc. 2001;56(133-6):149–50. [PubMed] [Google Scholar]
- 23.Geronimus AT, Bound J, Waidmann TA, Colen CG, Steffick D. Inequality in life expectancy, functional status, and active life expectancy across selected black and white populations in the United States. Demography. 2001;38:227–51. doi: 10.1353/dem.2001.0015. [DOI] [PubMed] [Google Scholar]
- 24.Geronimus AT, Bound J, Waidmann TA, Hillemeier MM, Burns PB. Excess mortality among blacks and whites in the United States. N Engl J Med. 1996;335:1552–8. doi: 10.1056/NEJM199611213352102. [DOI] [PubMed] [Google Scholar]
- 25.Rich-Edwards JW, Buka SL, Brennan RT, Earls F. Diverging associations of maternal age with low birthweight for black and white mothers. Int J Epidemiol. 2003;32:83–90. doi: 10.1093/ije/dyg008. [DOI] [PubMed] [Google Scholar]
- 26.Rauh VA, Andrews HF, Garfinkel RS. The contribution of maternal age to racial disparities in birthweight: a multilevel perspective. Am J Public Health. 2001;91:1815–24. doi: 10.2105/ajph.91.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGlade MS, Saha S, Dalhstrom ME. The Latina paradox: an opportunity for restructuring prenatal care delivery. Am J Public Health. 2004;94:2062–5. doi: 10.2105/ajph.94.12.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hack M, Klein NK, Taylor HG. Long-term developmental outcomes of low birth weight infants. Future Child. 1995;5:176–96. [PubMed] [Google Scholar]
- 29.Osmond C, Barker DJ, Winter PD, Fall CH, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ. 1993;307:1519–24. doi: 10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker DJ, Martyn CN, Osmond C, Hales CN, Fall CH. Growth in utero and serum cholesterol concentrations in adult life. BMJ. 1993;307:1524–7. doi: 10.1136/bmj.307.6918.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, et al. Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol. 2004;190:1091–7. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]