Abstract

A divergent fluorous mixture synthesis (FMS) of asymmetric fluorinated dendrimers has been developed. Four generations of fluorinated dendrimers with the same fluorinated moiety were prepared with high efficiency, yield and purity. Comparison of the physicochemical properties of these dendrimers provided valuable information for their application and future optimization. This strategy has not only provided a practical method for the synthesis and purification of dendrimers, but also established the possibility of utilizing the same fluorinated moiety for FMS.
Introduction
In recent years, fluorinated dendrimers1 have attracted considerable attention in the fields of catalysis,2 materials3 and biology.4 Introduction of fluorocarbons and manipulation of the fluorine content in dendrimers have a great influence on the shape and stability of fluorinated dendrimeric assemblies.5 Fluorinated dendrimers often exhibit unique physicochemical and biological properties as compared to their non-fluorinated counterparts.1-5 For example, Percec and coworkers replaced certain hydrogen atoms with fluorine atoms at the tail end of a dendron and found an unexpected change in its structural motif and self-assembling behavior.1b,5d However, most, if not all, reported fluorinated dendrimers are water insoluble, an issue that severely limits their bioavailability. Further, efficient synthesis of multi-generational fluorinated dendrimers remains a challenge.2-5 In this work, we enhance water solubility by synthesizing hydrophilic asymmetric dendrimers and improve synthesis efficiency by synthesizing several generations of fluorinated dendrimers in one pot using the so-called fluorous mixture synthesis (FMS) method developed for small molecules.6
Our interest in fluorinated dendrimers is to employ them as delivery vehicles for 19F magnetic resonance image (MRI) guided drug therapy. To this end, we have recently developed a dendritic 19F imaging tracer, 19FIT (Figure 1, G2).7 19FIT is water soluble and, in animal studies, was rapidly excreted intact. Structurally, 19FIT is an amphiphilic dendrimer, belonging to the family of asymmetric or Janus dendrimers.8 In 19FIT, the two dissimilar dendrons are connected by an amide bond, which is metabolically more stable but synthetically less facile than the disulfide bond used previously for connecting dissimilar dendrons.8 One way to modulate the physicochemical properties of 19FIT is to change the number of branches in its hydrophilic dendron, essentially forming dendrimers of different generations.9 Thus, a method for the simultaneous synthesis of multiple generations of fluorinated dendrimers is of great value. Herein, we report a FMS strategy for the synthesis of four generations of fluorinated dendrimers in one pot.
FIGURE 1. Structures and fluorine contents of four fluorinated dendrimers.

Results and Discussion
Conventional FMS is based on conjugating fluorocarbon tags (linear perfluorocarbons of different chain lengths) to non-fluorinated substrates (structural isomers or stereoisomers) for the convenience of purification.6 The basis for separation is that molecules tagged with different fluorocarbons have different fluorine content (F%) and therefore different retention times in fluorous chromatography. However, for dendrimers, sufficient differences in fluorine content and fluorous chromatography retention time can be produced even with the same fluorocarbon moiety. This approach, i.e., using the same fluorocarbon moiety for the separation of multi-generational dendrimers, is the strategy we employed in this work (Scheme 1). An FMS of four generations of dendrimers (G0 – G3, Figure 1) has been developed to illustrate this strategy. The difference in the fluorine content between successive generations is over 10%, which would be sufficient for separating the dendrimer mixture with fluorous HPLC.
SCHEME 1. Stratety for the synthesis of fluorinated dendrimers.i.

i 2, 4, 6 and 8 were synthesized individually using fluorous mono-component synthesis (along the Y-axis). 2, 4, 6 and 8 were then mixed and underwent fluorous multi-component synthesis (along the X-axis) to give G0, G1, G2 and G3 as a mixture, which was then separated using preprative HPLC. Each pair of starting material and product is connected by a solid or dashed arrow in mon- and multi-component synthesis, repsectively.
With these ideas in mind, the intermediates for dendrimers G0-G3 were synthesized sequentially: 1 → 2 → 3 → 4 → 5 → 6 → 7→ 8 (Scheme 2). The treatment of alcohol 17, 10 with potassium hydride and tert-butyl bromoacetate afforded ester 2 which then underwent a deprotection-coupling cycle (b-c) three times to give the octaester 8 with a 74% yield over six steps. During the synthesis, intermediates 2, 4, and 6 were split into two portions; one for the following step and the other for FMS. All the intermediates (2-8) were conveniently purified by either fluorous liquid- or solid-phase extractions.
SCHEME 2. Fluorous synthesis of intermediates 2, 4, 6, 8.j h.

j Reagents and Conditions: (a) KH, BrCH2CO2tBu, THF, rt.; (b) TFA, anisole, CH2Cl2, rt.; (c) DIC, HOBt, HN(CH2CO2tBu)2, DMF/THF (1/1), rt. h Synthesis of M0, M1, M2 and M3 (in italic) is illustrated in SCHEME 3. While, the chemical structures of M0, M1, M2 and M3 are indicated here.
Once all the intermediates were prepared, we commenced FMS of four generations of dendrimers (Scheme 3). The four intermediates 2 (452 mg, 0.5 mmol), 4 (430 mg, 0.4 mmol), 6 (425 mg, 0.3 mmol), 8 (421 mg, 0.2 mmol) were mixed and the tert-butyl group in the resulting mixture were removed with trifluoroacetic acid to yield a mixture of four acids, 3 + 5 + 7 + 9, which was then coupled with 1-phenyl-2,5,8,11-tetraoxa-tridecan-13-amine7, 10 to yield a mixture of four benzyl ethers, M0 + M1 + M2 + M3. After removal of the benzyl groups through Pd-catalyzed hydrogenolysis, a mixture of four fluorinated dendrimers, G0 + G1 + G2 + G3, was obtained. Each of the three steps in Scheme 3 was conducted twice to facilitate complete conversion of the starting materials. After each reaction, excess reagents were removed by solid-phase extraction on fluorous silica gel with the H2O-MeOH-THF eluent system to yield the resulting mixture. Since the synthesis started with the fluorinated core and ended with the surface groups, the result is a divergent FMS of dendrimers.
SCHEME 3. FMS of fluorinated dendrimers G0 to G3.
To finish the FMS, the dendrimer mixture, G0 + G1 + G2 + G3, was demixed/purified by HPLC to obtain individual dendrimers. Column selection is crucial for demixing. Unlike conventional FMS where separation is driven solely by the fluorine content, two additional factors, polarity and molecular size, may also influence the separation of G0-G3. This prompted us to explore separating the mixture by HPLC using three types of columns: FluroFlash®, normal-phase (NP) amide-80 and reversed-phase (RP) C18 columns. The analytical chromatograms showed that all three columns can separate the dendrimer mixture (Figure 2), yet, there were some differences in the separation profiles. FluoroFlash® and NP-amide-80 columns resulted in the opposite elution order of G0-G3, but all peaks were sharp. In contrast, the RP-C18 column resulted in the same elution order as FluoroFlash®, but the peaks were broad. The fluorinated dendrimer mixture was then separated on a preparative FluoroFlash® column to give individual fluorinated dendrimers. Surprisingly, the overall yield was good for all four dendrimers: G0 (445 mg, 0.435 mmol, 87% yield), G1 (462 mg, 0.352 mmol, 88%), G2 (460 mg, 0.243 mmol, 81%), G3 (434 mg, 0.142 mmol, 71%).
FIGURE 2. HPLC separation of fluorinated dendrimer mixture (G0 + G2 + G3 + G4).11.
(Signals were vertically displaced for display. Columns: FluoroFlash® (top); NP-amide-80 (middle); RP-C18 (bottom))
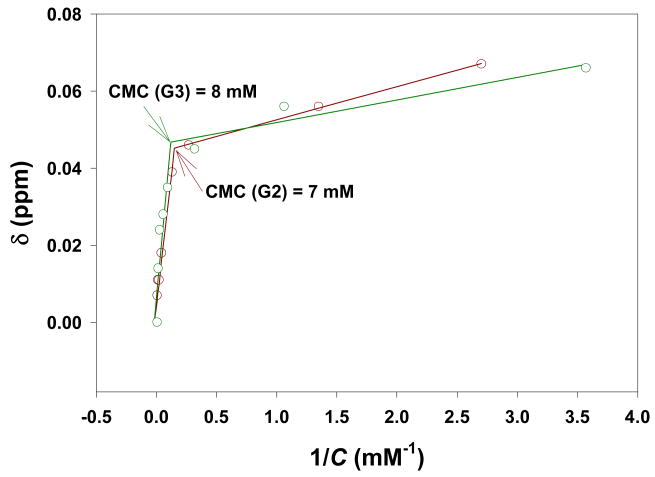
Each dendrimer emitted a sharp singlet 19F NMR signal with a peak width of less than 0.03 ppm (see Supporting Information). 19F NMR relaxation measurements indicated that both the longitudinal relaxation time T1 and the transverse relaxation time T2 decreased from G0 to G3 (Figure 3). A similar decrease of 19F T1 value with generational number has been previously reported for another class of fluorinated dendrimers.9b Such genrational dependency of 19F relaxation times might be a reflection of the intrinsic nano-periodic property pattern of dendrimers.12 Reduced T1 can increase 19F signal intensity in MRI by allowing collecting more signal transients without prolonging data acquisition time. G0 and G1 have very limited water solubility, while G2 and G3 have good water solubility (a 150 mM aqueous solution of G2 has been used in animal studies7). Using 19F NMR spectroscopy,13 the critical micelle concentrations of G2 and G3 were determined to be 7 mM and 8 mM, respectively (Figure 4). An ideal 19F imaging agent is a compound with a singlet 19F signal from multiple fluorine atoms, short T1, and good water solubility. Based on the data in hand, G2 and G3 have the potential as imaging agents for 19F MRI.
FIGURE 3. 19F T1 (filled circles) and T2 (hollow circles) of G0, G1, G2 and G3 (left to right) (19F NMR, 376 MHz, 25°C, 25 mM in CD3OD).
FIGURE 4. 19F NMR (376 MHz) determination of the critical micelle concentration (CMC) for G2 (dark red) and G3 (dark green) in water.
Conclusion
In summary, a fluorous mixture synthesis (FMS) strategy for the rapid preparation of multigenerational fluorinated dendrimers has been developed, which significantly simplifies dendrimer synthesis and purification. If necessary, this method could be adapted for the synthesis of non-fluorinated dendrimers by adopting a removable fluorocarbon moiety.
Experimental Section
tert-Butyl ester 2 (step a)
A suspension of potassium hydride (30%, 3.2 g, 24.0 mmol) was added slowly to a stirring solution of alcohol 1 (15.8 g, 20.0 mmol) in tetrahydrofuran (200 mL) at 0 °C. After 10 min, tert-butyl bromoacetate (5.9 mL, 7.8 g, 40.0 mmol) was added to the suspension in one portion at rt and the resulting mixture was stirred at rt overnight. After quenching the reaction with water (20 mL), the mixture was transferred into a separatory funnel and the lower phase was collected as clear oil. Removal of low boiling point impurities from the oil under vacuum gave the tert-butyl ester 2 as clear oil (14.1 g, 78% yield). 1H NMR (400 MHz, CDCl3) δ 4.14 (s, 6H), 3.91 (s, 2H), 3.57 (s, 2H), 1.46 (s, 9H).
General Procedure for the transformation of tert-butyl ester into acid. Preparation of acid 3 (step b)
To a stirred solution of tert-butyl ester 2 (13.6 g, 15.0 mmol) and anisole (3.0 mL) in dichloromethane (100 mL) at rt was added trifluoroacetic acid (30 mL) and the resulting solution was stirred at rt for 1 h. After evaporation to dryness under vacuum, the residue was dissolved in methanol/toluene (50 mL/30 mL) and evaporated to dryness under vacuum to give the pure acid 3 as reddish oil (12.6 g, 99% yield) which was used in the next step without further purification. 1H NMR (400 MHz, Acetone-d6) δ 4.29 (s, 6H), 4.14 (s, 2H), 3.73 (s, 2H).
General Procedure for coupling acid and amine to yield amide (step c). Preparation of amide 4
N, N-diisopropylcarbodiimide (6.6 mL, 5.4 g, 42.5 mmol) was added to a stirring solution of N-hydroxybenzotriazole (5.7 g, 42.5 mmol) and acid 3 (12.0 g, 14.2 mmol) in dry N, N-dimethylformamide (200 mL) at rt. After stirring for 15 min, di-tert-butyl iminodiacetate (10.4 g, 42.5 mmol) was added and the resulting mixture was stirred at rt for 6 h. Water (20 mL) was added to the reaction mixture and the resulting mixture was purified by solid-phase extraction on FlouroFlash® silica gel to give the amide 4 as clear oil (14.1 g, 92% yield) which was used in the next step without further purification. 1H NMR (400 MHz, CDCl3) δ 4.12 (s, 6H), 4.10 (s, 2H), 4.02 (s, 2H), 3.92 (s, 2H), 3.59 (s, 2H), 1.44 (s, 9H), 1.42 (s, 9H); 19F NMR (376 MHz, CDCl3) δ -73.29 (s).
Diacid 5
This compound was prepared by employing step b with a 99% yield. 1H NMR (400 MHz, CD3OD) δ 4.22 (s, 8H), 4.16 (s, 2H), 4.14 (s, 2H), 3.60 (s, 2H).
Tetra-tert-butyl ester 6
This compound was prepared by employing step c with a 93% yield. 1H NMR (400 MHz, CDCl3) δ 3.80-4.13 (m, 20H), 3.47 (s, 2H), 1.28-1.31 (m, 36H).
Tetraacid 7
This compound was prepared by employing step b with a 99% yield. 1H NMR (400 MHz, CD3OD) δ 4.15 (s, 2H), 4.05-4.09 (m, 10H), 3.90-4.01 (m, 6H), 3.53-3.59 (m, 2H), 3.43 (s, 2H).
Octa-tert-butyl ester 8
This compound was prepared by employing step c with an 89% yield. 1H NMR (400 MHz, CD3OD) δ 4.40 (s, 2H), 4.34 (s, 2H), 4.28 (s, 4H), 4.25 (s, 8H), 4.12-4.17 (m, 12H), 4.03-4.07 (m, 8H), 3.59 (s, 2H), 1.45-1.50 (m, 72H); 19F NMR (376 MHz, CD3OD) δ -71.16 (s); 13C NMR (100.7 MHz, CD3OD) δ 172.0, 171.6, 171.2, 171.1, 171.0, 170.8, 170.6, 169.7, 169.6, 169.55, 169.43, 169.4, 121.6 (q, J = 293.3 Hz), 84.1, 84.0, 83.8, 83.7, 83.1, 83.0, 82.9, 80.3-81.5 (m), 69.5, 68.6, 68.3, 51.6, 51.5, 51.0, 50.8, 50.77, 50.1, 49.9, 49.1, 48.4, 47.2, 28.4, 28.3, 28.29; MS (MALDI-TOF) m/z 2125 ((M+Na)+); HRMS (MALDI-TOF) calcd for C79H110F27N7NaO27 2124.6916, found 2124.7023.
Procedure for the fluorous mixture synthesis of fluorinated dendrimers G0 – G3
A mixture of monoester 2 (452 mg, 0.5 mmol), diester 4 (430 mg, 0.4 mmol), tetraester 6 (425 mg, 0.3 mmol) and octaester 8 (421 mg, 0.2 mol) was dissolved in dichloromethane (24 mL). This mixture was treated with anisol (1 mL) and trifluoroacetic acid (8 mL) at rt (step b) to give a wax of acids (3, 5, 7 and 9). The above procedure was repeated to facilitate the complete conversion of the starting materials. Then the wax was put into the coupling step with 1-phenyl-2,5,8,11-tetraoxatridecan-13-amine (3.5 g, 12.3 mmol) by employing the general coupling procedure (step c) twice to give a mixture of intermediates (M0, M1, M2 and M3). The mixture of intermediates was hydrogenolysized twice with palladium on carbon (10%, 1.0 g) in methanol over 20 h to give a mixture of fluorinated dendrimers and their side products. After filtration using a pad of celite, the dendimer mixture was then purified by HPLC on preparative FluoroFlash® column (21.1 × 250 mm, flow rate 5 mL/min, gradient from 60% MeOH in water to 100% MeOH in 170 min, then 100% MeOH for 40 min, detection wavelength at 210 nm). After lyophilization, individual fluorinated dendrimers were obtained: G0 (445 mg, 0.435 mmol, 87% yield), G1 (462 mg, 0.352 mmol, 88% yield), G2 (460 mg, 0.243 mmol, 81% yield), G3 (349 mg, 0.142 mmol, 71% yield).
Fluorinated dendrimer G0
1H NMR (400 MHz, CD3OD) δ 4.03 (s, 6H), 3.89 (s, 2H), 3.56-3.64 (m, 10H), 3.53-3.55 (m, 6H), 3.44 (s, 2H); 19F NMR (376 MHz, CD3OD) δ -71.11 (s); 13C NMR (100.7 MHz, CDCl3) δ 168.4, 120.0 (q, J = 293.3 Hz), 79.4-79.9 (m), 72.4, 71.0, 70.4, 70.2, 70.1, 70.08, 66.5, 64.9, 61.5, 46.2, 38.7; MS (MALDI-TOF) m/z 1046 ((M+Na)+), 1024 ((M+H)+); HRMS (MALDI-TOF) calcd for C27H28F27NNaO9 1046.1231, found 1046.1280; T1 (19F, 376 MHz, 26 mM in CD3OD) 1.101 second, err = 0.0577; T2 (19F, 376 MHz, 25 mM in CD3OD) 0.8779 second, err = 0.0162.
Fluorinated dendrimer G1
1H NMR (400 MHz, CD3OD) δ 4.23 (s, 6H), 4.21 (s, 2H), 4.05 (s, 2H), 4.04 (s, 2H), 3.60-3.67 (m, 22H), 3.54-3.58 (m, 8H), 3.38-3.43 (m, 4H); 19F NMR (376 MHz, CD3OD) δ -71.08 (s); 13C NMR (100.7 MHz, CD3OD) δ 172.0, 171.4, 170.8, 121.6 (q, J = 292.5 Hz), 80.4-81.4 (m), 73.7, 71.6, 71.4, 71.3, 71.2, 70.4, 70.3, 69.7, 68.7, 68.1, 62.2, 52.4, 47.3, 40.6, 40.4; MS (MALDI-TOF) m/z 1336 ((M+Na)+); HRMS (MALDI-TOF) calcd for C39H50F27N3NaO15 1336.2708, found 1336.2671; T1 (19F, 376 MHz, 25 mM in CD3OD) 0.9453 second, err = 0.0460; T2 (19F, 376 MHz, 25 mM in CD3OD) 0.7168 second, err = 0.0251.
Fluorinated dendrimer G2
1H NMR (400 MHz, CD3OD) δ 4.26 (s, 2H), 4.25 (s, 8H), 4.22 (s, 2H), 4.18 (s, 2H), 4.15 (s, 2H), 4.05 (s, 2H), 4.04 (s, 2H), 3.59-3.67 (m, 42H), 3.53-3.58 (m, 16H), 3.44 (t, J = 4.2 Hz, 4H), 3.38 (t, J = 5.6 Hz, 4H); T1 (19F, 376 MHz, 25 mM in CD3OD) 0.7888 second, err = 0.0158; T2 (19F, 376 MHz, 25 mM in CD3OD) 0.6255 second, err = 0.00819.
Fluorinated dendrimer G3
1H NMR (400 MHz, CD3OD) δ 4.39 (s, 2H), 4.35 (s, 2H), 4.21-4.24 (m, 14H), 4.18 (s, 6H), 4.05-4.10 (m, 10H), 3.54-3.67 (m, 116H), 3.42-3.47 (m, 8H), 3.36-3.40 (m, 8H); 19F NMR (376 MHz, CD3OD) δ -71.03 (s); 13C NMR (100.7 MHz, CD3OD) δ 172.1, 171.7, 171.6, 171.59, 171.3, 171.26, 171.1, 171.06, 170.8, 170.7, 170.6, 170.5, 121.5 (q, J = 292.6 Hz), 80.2-81.6 (m), 73.6, 71.5, 71.3, 71.2, 71.17, 71.1, 70.35, 70.3, 70.2, 69.4, 68.6, 68.3, 62.2, 53.3, 53.2, 53.1, 53.08, 52.9, 52.7, 50.5, 50.3, 49.5, 49.3, 48.1, 47.2, 40.5, 40.47, 40.4; MS (MALDI-TOF) m/z 3077 ((M+Na)+); HRMS (MALDI-TOF) calcd for C111H182F27N15NaO51 3078.1602, found 3078.1702; T1 (19F, 376 MHz, 25 mM in CD3OD) 0.7415 second, err = 0.00713; T2 (19F, 376 MHz, 25 mM in CD3OD) 0.4999 second, err = 0.00365.
Supplementary Material
Acknowledgments
This work was supported by NIH grant EB004416 and the Maryland Nano-Biotechnology Fund to YBY. We thank Y. Feng, L. Hyland and K. Joyner for assistance with manuscript preparation.
Footnotes
Supporting Information Available: Characterization data of synthesized compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.General reviews: Caminade AM, Turrin CO, Sutra P, Majoral JP. Curr Opin Colloid Interface Sci. 2003;8:282–295.Tomalia DA. Nature Materials. 2003;2:711–712. doi: 10.1038/nmat1004.
- 2.(a) Chechik V, Crooks RM. J Am Chem Soc. 2000;122:1243–1244. [Google Scholar]; (b) Mager M, Becke S, Windisch H, Denninger U. Angew Angew Chem Int Ed. 2001;40:1898–1902. [PubMed] [Google Scholar]; (c) Yeung LK, Crooks RM. Nano Lett. 2001;1:14–17. [Google Scholar]; (d) Roestler R, Har BJN, Piers WE. Organometallics. 2002;21:4300–4302. [Google Scholar]; (e) Puniredd SR, Wai YK, Satyanarayana N, Sinha SK, Srinivasan MP. Langmuir. 2007;23:8299–8303. doi: 10.1021/la0635707. [DOI] [PubMed] [Google Scholar]
- 3.(a) Miller TM, Neenan TX, Zayas R, Bair HE. J Am Chem Soc. 1992;114:1018–1025. [Google Scholar]; (b) Cooper AI, Londono JD, Wignall G, McClain JB, Samulski ET, Lin JS, Dobrynin A, Rubinstein M, Burke ALC, Fréchet JMJ, DeSimone JM. Nature. 1997;389:368–371. [Google Scholar]; (c) Sakamoto Y, Suzuki T, Miura A, Fujikawa H, Tokito S, Taga Y. J Am Chem Soc. 2000;122:1832–1833. [Google Scholar]; (d) Luo J, Ma H, Haller M, Jen AKY, Barto RR. Chem Commun. 2002:888–889. doi: 10.1039/b200851c. [DOI] [PubMed] [Google Scholar]; (e) Pitois C, Vestberg R, Rodlert M, Malmstrom E, Hult A, Lindgren M. Opt Mater. 2003;21:499–506. [Google Scholar]; (f) Zhao Z, Li JH, Lu P, Yang Y. Org Lett. 2008;10:3041–3044. doi: 10.1021/ol801001h. [DOI] [PubMed] [Google Scholar]
- 4.(a) Zhuo RX, Du B, Lu ZR. J Controlled Release. 1999;57:249–257. doi: 10.1016/s0168-3659(98)00120-5. [DOI] [PubMed] [Google Scholar]; (b) Tripathi PK, Khopade AJ, Nagaich S, Shrivastava S, Jain S, Jain NK. Pharmazie. 2002;57:261–264. [PubMed] [Google Scholar]
- 5.(a) Johansson G, Percec V, Ungar G, Zhou JP. Macromolecules. 1996;29:646–660. [Google Scholar]; (b) Percec V, Johansson G, Ungar G, Zhou J. J Am Chem Soc. 1996;118:9855–9866. [Google Scholar]; (c) Percec V, Glodde M, Bera TK, Mlura Y, Shlyanovskaya I, Singer KD, Balagurusamy VSK, Helney PA, Schnell I, Rapp A, Spless HW, Hudson SD, Duan H. Nature. 2002;419:384–387. doi: 10.1038/nature01072. [DOI] [PubMed] [Google Scholar]; (d) Percec V, Glodde M, Johansson G, Balagurusamy VSK, Heiney PA. Angew Chem Int Ed. 2003;42:4338–4342. doi: 10.1002/anie.200351804. [DOI] [PubMed] [Google Scholar]; (e) Percec V, Glodde M, Peterca M, Rapp A, Schnell I, Spiess HW, Bera TK, Miura Y, Balagurusamy VSK, Aquad E, Heiney PA. Chem Eur J. 2006;12:6298–6314. doi: 10.1002/chem.200501195. [DOI] [PubMed] [Google Scholar]; (f) Percec V, Aqad E, Peterca M, Imam MR, Glodde M, Bera TK, Miura Y, Balagurusamy VSK, Ewbank PC, Wurthner F, Heiney PA. Chem Eur J. 2007;13:3330–3345. doi: 10.1002/chem.200600901. [DOI] [PubMed] [Google Scholar]; (g) Chvalun SN, Shcherbina MA, Yakunin AN, Blackwell J, Percec V. Polym Sci Ser A. 2007;49:158–167. [Google Scholar]
- 6.(a) Curran DP. In: Handbook of Fluorous Chemistry. Gladysz JA, Curran DP, Horváth IT, editors. Wiley-VCH; Weinheim: 2003. pp. 142–149. [Google Scholar]; (b) Curran DP. Quimica. 2008 10:10. And references therein. [Google Scholar]
- 7.Jiang ZX, Liu X, Jeong EK, Yu YB. Angew Chem Int Ed. 2009;48:4755–4758. doi: 10.1002/anie.200901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Tomalia DA, Huang B, Swanson DR, Brothers HM, II, Klimash JW. Tetrahedron. 2003;59:3799–3813. [Google Scholar]; (b) DeMattei CR, Huang B, Tomalia DA. Nano Lett. 2004;4:771–777. [Google Scholar]; (c) Saez IM, Goodby JW. Chem Eur J. 2002;9:4869–4877. doi: 10.1002/chem.200305100. [DOI] [PubMed] [Google Scholar]; (d) Percec V, Imam MR, Bera TK, Balagurusamy VSK, Peterca M, Heiney PA. Angew Chem Int Ed. 2005;44:4739–4745. doi: 10.1002/anie.200501254. [DOI] [PubMed] [Google Scholar]; (e) Rosen BM, Wilson CJ, Wilson DA, Peterca M, Imam MR, Percec V. Chem Rev. 2009;106:6275–6540. doi: 10.1021/cr900157q. [DOI] [PubMed] [Google Scholar]
- 9.(a) Lorenz K, Frey H, Stühn B, Mülhaupt R. Macromolecules. 1997;30:6860–6868. [Google Scholar]; (b) Huang Z, Sengar RS, Wiener EC. Contrast Media Mol Imaging. 2007;2:288–289. [Google Scholar]
- 10.(a) Jiang ZX, Yu YB. Tetrahedron. 2007;63:3982–3988. doi: 10.1016/j.tet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jiang ZX, Yu YB. Synthesis. 2008:215–220. doi: 10.1055/s-2007-1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FluoroFlash® column (4.6 × 150 mm, 5 μm), gradient 60% MeOH-H2O to 100% MeOH in 15 min and then maintain 100% MeOH, flow rate 1 mL/min; NP-amide-80 column (4.6 × 250 mm, 5 μm), gradient 100% CH3CN to 70% CH3CN-H2O in 30 min, flow rate 1 mL/min; RP-C18 column (4.6 × 250 mm, 5 μm), gradient 40% CH3CN-H2O to 70% CH3CN-H2O in 30 min, flow rate 1 mL/min, rt. for all runs. The sample was prepared by dissolving and mixing pure fluorinated dendrimers (5 μmol each) in methanol (5 mL).
- 12.Tomalia DA. J Nanopart Res. 2009;11:1251–1310. doi: 10.1007/s11051-009-9632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller N, Birkhahn RH. J Phys Chem. 1967;71:957. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.