Abstract
Cardiovascular disease is a major complication of diabetes mellitus, and improved strategies for prevention and treatment are needed. Endothelial dysfunction contributes to the pathogenesis and clinical expression of atherosclerosis in diabetes mellitus. This article reviews the evidence linking endothelial dysfunction to human diabetes mellitus and experimental studies that investigated the responsible mechanisms. We then discuss the implications of these studies for current management and for new approaches for the prevention and treatment of cardiovascular disease in patients with diabetes mellitus.
Keywords: Endothelium, Nitric oxide, Inflammation, Diabetes mellitus, Mitochondria, Protein kinase C
Type 2 diabetes mellitus is a growing public health problem [1] and a major cause of cardiovascular disease in the United States [2]. Type 2 diabetes is associated with systemic insulin resistance, which promotes hyperglycemia and dyslipidemia [3], and it has been proposed that these metabolic abnormalities account for increased cardiovascular risk. Endothelial dysfunction contributes to the pathogenesis and clinical expression of atherosclerosis and has been linked to Type 2 diabetes mellitus and insulin resistance in experimental and clinical studies [4]. This article will review the concept of endothelial dysfunction and the evidence linking it to human diabetes mellitus. We will then review mechanisms of endothelial dysfunction in diabetes mellitus and the implications of this work for the management of patients with diabetes mellitus.
1 What is endothelial dysfunction?
Once thought to be simply a passive lining for blood vessels, it is now recognized that the vascular endothelium is a key determinant of vascular health. Broadly speaking, the term “endothelial dysfunction” refers to an impairment of the ability of the endothelium to properly maintain vascular homeostasis [5]. Although the term is often used in reference to a loss of bioavailable nitric oxide (NO), endothelial dysfunction also reflects increased production of vasoconstrictors and disturbed regulation of inflammation, thrombosis, and cell growth in the vascular wall [5, 6]. Numerous studies have linked endothelial dysfunction and resultant atherosclerosis with insulin resistant states such as obesity and diabetes [7–10].
The endothelium plays a key role in the regulation of arterial tone and blood flow. In this regard, the endothelium orchestrates the production of vasodilator molecules such as NO, prostacyclin, and endothelium-derived hyperpolarizing factor (EDHF), and vasoconstrictors, including endothelin-1 (ET-1) and angiotensin II [5]. Stimuli for production of endothelium-dependent vasodilators include physiologically relevant factors such as acetylcholine, thrombin, serotonin, angiotensin II, and alpha adrenergic agonists. In general, these factors also promote vasoconstriction via direct effects on vascular smooth muscle. Endothelium-derived NO and other vasodilators oppose such vasoconstrictor effects, and thus act in a homeostatic fashion to maintain normal arterial patency and compliance despite local production of vasoconstrictors. When the endothelium is dysfunctional, the vasoconstrictor effects are unopposed and arterial tone is increased. In addition, pathological states are associated with increased endothelial production of endothelin-1 and other endothelium-derived vasoconstrictors that may further promote vasospasm and increase arterial stiffness.
Another key stimulus for endothelial production of NO is increased shear stress, which is the frictional force at the endothelial surface produced by flowing blood [11]. Shear stress relates directly to arterial flow and inversely to the third power of the arterial diameter, and thus for a given level of flow, a small change in diameter has a large effect on local shear stress. In healthy conduit arteries, an increase in arterial flow stimulates “flow-mediated dilation”. The resultant increase in lumen diameter acts in a homeostatic manner to limit the increase in shear stress that results from increased flow. This response can be measured in humans using non-invasive methods, including ultrasound imaging of the brachial artery diameter during reactive hyperemia [12]. Flow-mediated dilation has emerged as an important indicator of endothelial function in clinical studies of diabetes mellitus and vascular disease [5, 12].
In addition to responding to acute changes in flow by stimulating vasodilation, the endothelium also is responsible for chronic changes in arterial structure and lumen dimension that are produced by chronic changes in blood flow [13, 14]. Like flow-mediated dilation, flow-induced remodeling is stimulated by changes in shear stress. The remodeling response to flow involves a complex interplay between vasodilator factors, local inflammation, and factors that modify the intercellular matrix [13, 15–17]. Impaired remodeling has been observed in animal models of diabetes [18] and may, in part, explain why atherosclerosis tends to produce diffusely narrowed and small caliber arteries in patients with diabetes [13].
Another important function of the endothelium is the regulation of tone in smaller resistance vessels that control blood flow and maintain the balance between blood supply and tissue demand. This complex process depends in large part on the production of non-endothelium-dependent vasodilators, such as adenosine, that dilate resistance vessels and increase tissue blood flow in response to increased oxygen demand [19, 20]. However, endothelium-derived NO also contributes to ischemia-mediated vasodilation and the hyperemic response to exercise [20]. By this mechanism, endothelial dysfunction may impair the regulation of blood flow and contribute to impaired exercise capacity in certain pathological states, including heart failure and peripheral arterial disease.
The importance of inflammation for the pathogenesis of atherosclerosis is well recognized [21]. Under physiological conditions, NO prevents leukocyte adhesion and maintains the endothelium in a quiescent, anti-inflammatory state [5]. In the presence of risk factors, the endothelium can be activated to express adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), which are required for the adhesion of leukocytes to the endothelial surface [22]. The activated endothelium also expresses chemotactic factors, such as monocyte chemoattractant protein-1, and other proinflammatory cytokines, like macrophage colony-stimulating factor, and tumor necrosis factor-beta (TNF-β) [22]. Endothelial expression of these factors contributes to the development of inflammation within the arterial wall and promotes atherogenesis [23].
In addition to regulating vessel wall inflammation, the vascular endothelium produces a host of other molecules that affect blood fluidity and thrombosis [5, 24, 25]. Endothelial production of pro-thrombotic molecules such as plasminogen activator inhibitor-1 (PAI-1), thromboxane, tissue factor, and von Willibrand’s factor (vWF), is balanced by the production of antithrombotic molecules such as NO, heparans, prostacyclin, tissue plasminogen activator, and thrombomodulin. Risk factors, including diabetes mellitus, are associated with a shift in this balance toward a pro-thrombotic, anti-fibrinolytic state.
Thus, the dysfunctional endothelium contributes to the pathogenesis of atherosclerotic vascular disease by promoting inflammation, thrombosis, arterial stiffness, and impaired regulation of arterial tone and flow. Several lines of evidence support the relevance of endothelial dysfunction to human disease, and most of these studies have focused on endothelium-dependent vasodilation as a surrogate measure of endothelial health. First, endothelium-dependent dilation is impaired in patients with documented coronary artery disease [26, 27] and in patients with classical and with more recently-recognized risk factors [28, 29]. Indeed, such abnormalities are detectable very early in the course of the disease before measurable lesions or clinical symptoms [30]. Second, lifestyle changes and drugs proven to reduce cardiovascular risk have been shown to reverse endothelial dysfunction, including exercise, smoking cessation, weight loss, cholesterol-lowering drugs, and angiotensin converting enzyme inhibitors [5]. Finally, endothelial dysfunction in the coronary or peripheral circulations predicts increased risk for cardiovascular events in patients with risk factors and in patients with established atherosclerosis [5, 31–38]. Overall, it is clear that endothelial dysfunction relates to the pathogenesis of cardiovascular disease. Since diabetes mellitus is a major cardiovascular disease risk factor, it is not surprising that clinical studies have confirmed a relationship between diabetes mellitus and endothelial dysfunction.
2 Endothelial dysfunction is present in human diabetes mellitus
A large body of evidence links endothelial dysfunction to human diabetes mellitus. As has been reported for other cardiovascular risk factors, cross-sectional studies show reduced endothelium-dependent vasodilation in coronary and peripheral arteries of patients with Type 1 [39, 40] and Type 2 diabetes mellitus [41–44]. Endothelial dysfunction is also observed in conditions associated with Type 2 diabetes including obesity [29, 45, 46], sedentary lifestyle, and the metabolic syndrome [47–49]. In addition to impaired vasodilator function, diabetes is also associated with increased circulating levels of endothelium-derived adhesion molecules and plasminogen activator inhibitor-1 [50–52], reflecting a pro-inflammatory and pro-thrombotic endothelial phenotype (Table 1).
Table 1.
Manifestations of endothelial dysfunction in diabetes mellitus
| Impaired vasodilation/increased vasoconstriction—decreased NO, increased endothelin-1 |
| Increased arterial stiffness |
| Impaired arterial remodeling |
| Endothelial activation – adhesion molecule and cytokine expression |
| Increased PAI-1 and other pro-thrombotic factors |
| Increased atherogenesis |
Insulin resistance is the defining mechanism of Type 2 diabetes mellitus, and several studies have examined the relationship between insulin resistance and endothelial dysfunction. In this regard, degree of insulin sensitivity, as determined by euglycemic clamp, correlates with increases in skin blood flow induced by acetylcholine in obese women [53] and with brachial artery flow-mediated dilation in non-diabetic subjects [54, 55]. A higher insulin response during oral glucose tolerance test, is likewise associated with endothelial dysfunction in the coronary [56, 57] and forearm circulations [58, 59]. Higher plasma insulin levels correlate inversely with brachial artery flow-mediated dilation [60]. Finally, homeostasis model assessment-insulin resistance (HOMA-IR) correlates inversely with the leg blood flow response to methacholine in non-diabetic subjects with varying levels of body mass index [47] and with skin blood flow responses to acetylcholine in diabetic compared to non-diabetic subjects [61]. In Framingham Heart Study participants, brachial artery flow-mediated dilation correlated inversely with HOMA-IR, although this relation was lost after adjusting for factors associated with the metabolic syndrome [48].
Interestingly, endothelial dysfunction may precede the development of diabetes mellitus. In this regard, healthy non-diabetic subjects who have a first degree relative with Type 2 diabetes mellitus display impaired endothelium-dependent vasodilation as well as increased plasma markers of endothelial cell activation [54, 61, 62]. Blood markers of endothelial activation and systemic inflammation are also elevated in non-diabetic offspring with evidence of insulin resistance by glucose tolerance test [62]. In addition to these cross-sectional studies, prospective studies have shown that blood markers of endothelial activation predict incident Type 2 diabetes mellitus after adjusting for other risk factors, including body mass index, level of physical activity, lipids, family history of diabetes mellitus, and glucose tolerance [52, 63]. Similarly, impaired flow-mediated dilation [64] and polymorphisms of endothelial NO synthase (eNOS) are multivariable predictors of incident Type 2 diabetes mellitus [65]. The occurrence of endothelial dysfunction prior to the development of Type 2 diabetes mellitus suggests that there are common pathophysiological mechanisms and raises that possibility of a causal link between insulin resistance and endothelial dysfunction.
Additional clinical evidence for a link between endothelial dysfunction and insulin resistance is provided by intervention studies that demonstrate improved endothelial function following treatments that improve insulin sensitivity. For example, rosiglitazone [66] and troglitazone [67] improve endothelial function in forearm microvessels of patients with Type 2 diabetes mellitus. Metformin improves endothelium-dependent dilation in patients with Type 2 diabetes mellitus [68] and in patients with the metabolic syndrome [69]. Pioglitazone improves endothelial function in non-diabetic patients with hypertension or hypercholesterolemia with or without insulin resistance at baseline [70], and rosiglitazone improves flow-mediated dilation and decreases markers of inflammation in completely healthy subjects without obesity, risk factors, or family history of diabetes [71]. Less specific interventions associated with improved insulin sensitivity and or decreased risk of diabetes mellitus also improve endothelial function including weight loss, exercise, and inhibitors of the renin-angiotensin system [45, 46, 72, 73].
Overall, it is clear that human diabetes mellitus is associated with abnormalities of endothelial function in humans, which may explain, in part, increased risk for cardiovascular disease in diabetic patients. An improved understanding of the mechanisms of endothelial dysfunction in this setting could provide new approaches for patient management.
3 Mechanisms of endothelial dysfunction in diabetes mellitus and insulin resistance
3.1 Altered cell signaling in endothelial cells
One important mechanism of endothelial dysfunction in diabetes mellitus is alteration of the signaling pathways that lead to eNOS activation in the endothelium, a process that has been extensively studied. Briefly, NO production in endothelial cells depends on the enzymatic conversion of Larginine to NO and citrulline by eNOS. The enzyme is constitutively expressed in endothelial cells and is localized to caveolae, which are specialized invaginations of the plasma membrane that are rich in specific lipids and proteins, including caveolin-1 [74]. eNOS has a low level of basal activity because of its association with caveolin-1, but it can be activated within seconds following stimulation of endothelial cells with receptor-dependent agonists such as acetylcholine and serotonin that increase intracellular calcium and promote calcium/calmodulin-dependent-displacement of caveolin-1 and activation of the enzyme [75]. Activation of eNOS is further enhanced by other protein-protein interactions, including association with heat shock protein 90 [76]. Alternatively, eNOS can be activated by bradykinin, estrogen, and shear stress via activation of the phosphoinositide-3 kinase (PI3 kinase)/Akt system. Akt phosphorylates eNOS at serine 1177, which directly increases activity and also enhances the response to other activators [77–80]. Once produced, eNOS-derived NO diffuses locally in the arterial wall and activates guanylyl cyclase in vascular smooth muscle cells, platelets, and endothelial cells to induce its biological effects [81].
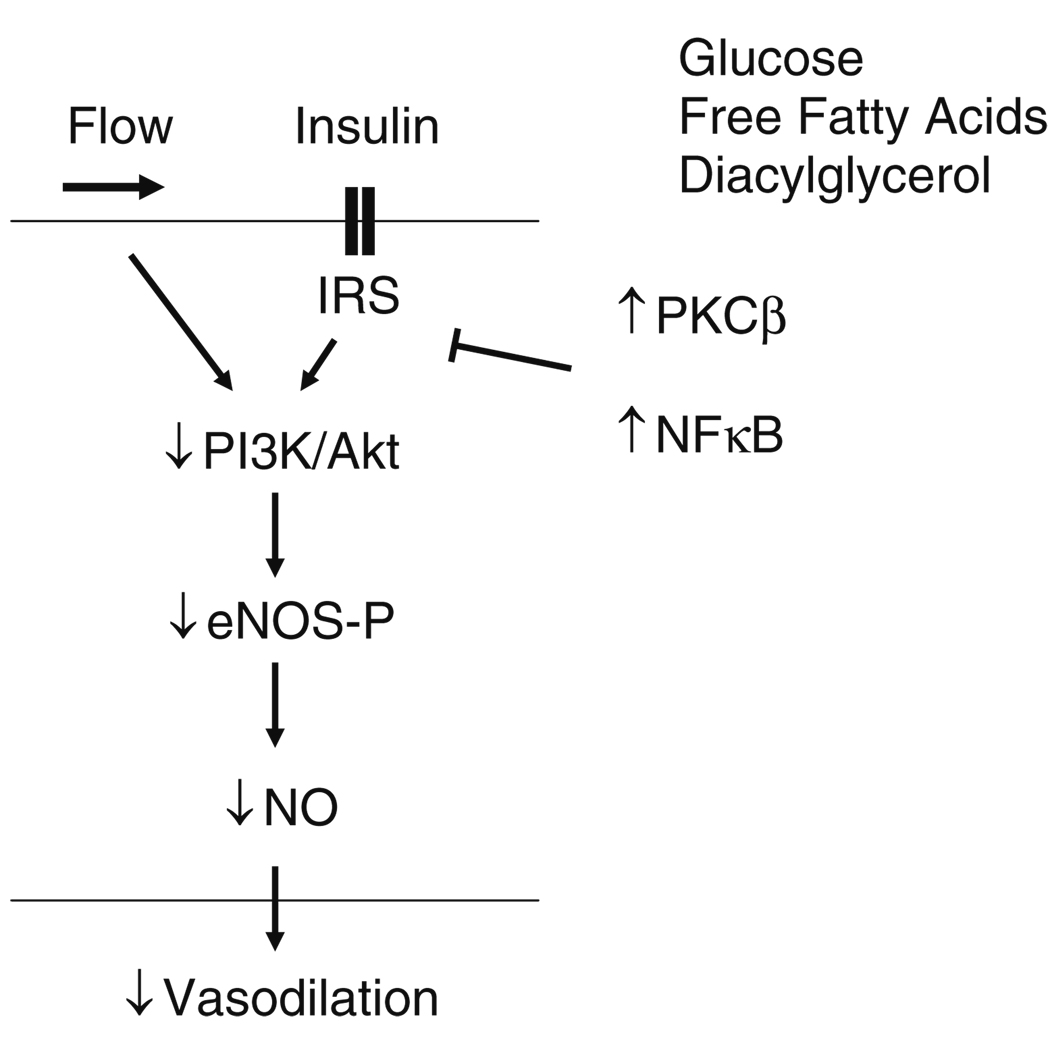
Relevant to endothelial dysfunction in diabetes, it is interesting that insulin itself is an important stimulus for eNOS activation (Fig. 1). Binding of insulin to its receptor on endothelial cells leads to phosphorylation of insulin receptor substrate-1 (IRS-1) and subsequent phosphorylation and activation of eNOS via PI3 kinase/Akt [8, 82]. Support for this mechanism is derived from studies showing that insulin-stimulated NO production is blocked by inhibitors of PI3 kinase or Akt [77, 83]. In addition, mutations in IRS-1 decrease insulin-stimulated eNOS phosphorylation and eNOS gene expression in cultured endothelial cells [84]. Furthermore, mice with endothelial specific knockout of the insulin receptor display decreased eNOS expression and impaired endothelial vasodilator function [85–87]. Animal models of insulin resistance, including the obese Zucker rat display defects in the PI3 kinase/Akt system and impaired NO bioavailability [88].
Fig. 1.
Displayed is the signaling pathway associated with insulin-mediated activation of endothelial nitric oxide synthase (eNOS). Binding of insulin to the insulin receptor leads to the phosphorylation of the insulin receptor substrate (IRS) and activation of phosphoinositide-3 kinase (PI3K) and Akt. Akt, in turn, phosphorylates eNOS. In the setting of diabetes mellitus activation of PKCβ leads to the activation of NFκB, blocks insulin signaling and reduces synthesis of nitric oxide (NO)
Insulin activates other cellular signaling pathways in addition to PI3 kinase/Akt. For example, insulin activates the mitogen-activated protein kinases (MAPK) via the small GTPase Ras [89]. The Ras/MAPK insulin-signaling pathway generally leads to cellular growth and proliferation. In endothelial cells, activation of this pathway has been linked to the expression of endothelin-1, which is a potent vasoconstrictor and mitogen, and to the expression of pro-inflammatory adhesion molecules such as ICAM-1 [90]. In the setting of diabetes and insulin resistance, insulin-mediated activation of eNOS via PI3 kinase/Akt is inhibited, while the adverse effects of insulin remain unopposed, which may promote vascular disease [90, 91]. Flow and other physiological stimuli activate eNOS via PI3 kinase/Akt, and thus, an impairment of this signaling mechanism in diabetes may have broad implications for vascular dysfunction. Furthermore, study of flow-mediated dilation may have particular relevance in studies of the mechanisms of vascular disease in human diabetes mellitus.
Human studies support the relevance of these mechanisms. In healthy humans, insulin infusion stimulates vasodilation and increases blood flow in peripheral tissues, and this effect is blunted in patients with diabetes mellitus and insulin resistance [92, 93]. In insulin clamp experiments, the time course and magnitude of insulin-mediated vasodilation appear to be closely linked to the rate of glucose uptake in the limb [94–96]. These findings suggest that the actions of insulin in tissues and the vasculature are tightly coupled and that an impaired ability to dilate in response to insulin may contribute to impaired glucose metabolism [96].
Additional human studies further support the relevance of altered insulin signaling to endothelial dysfunction. Akt phosphorylation and eNOS expression are impaired in arterial tissue collected from diabetic patients at the time of coronary bypass surgery [97]. As observed in experimental systems, insulin infusion increases circulating levels of endothelin-1 in humans, a response that may be increased in the setting of insulin resistance [98]. Thus, selective impairment of PI3 kinase/Akt signaling characterizes human insulin resistance and may contribute to endothelial dysfunction and vascular disease in Type 2 diabetes mellitus [90].
Diabetes mellitus alters a number of other mechanisms related to eNOS activation. For example, diabetes and obesity are associated with increased expression of caveolin-1 in the endothelium in experimental animals [99, 100] and in adipose tissue of diabetic patients [101]. This effect is associated with impaired activation of eNOS and impaired vasodilator function. Diabetes also impairs the interaction between eNOS and heat shock protein 90, which also impairs NO production [102]. Finally, diabetes mellitus is associated with increased levels of endogenous inhibitors of eNOS, such as asymmetric dimethyl arginine (ADMA) [103]. Thus, diabetes has multiple effects on the cell signaling and enzyme activity that result in an impaired ability to produce NO in response to physiological stimuli.
3.2 Increased oxidative stress
Increased oxidative stress in the vasculature is another important mechanism of endothelial dysfunction in diabetes mellitus and associated conditions. Exposure of arterial tissue to increased glucose or free fatty acid concentrations induces superoxide production and impairs NO bioavailability in the vascular wall, while antioxidant treatment acts to restore endothelial function under these conditions[104, 105]. Increased oxidative stress has the potential to impair NO bioavailability in several ways [106, 107]. First, superoxide anion may react with NO to form peroxynitrite and eliminate the biological activity of NO. Peroxynitrite is a highly reactive oxidant that may alter the function of a variety of cellular enzymes [108]. In particular, peroxynitrite can alter the catalytic activity of eNOS in endothelial cells and guanylyl cyclase in vascular smooth muscle cells. As a result, peroxynitrite reduces both the production of NO and the responsiveness of target tissues to NO [109, 110]. Increased production of reactive oxygen species may also influence the redox status of critical eNOS co-factors, including tetrahydrobiopterin. Loss of tetrahydrobiopterin uncouples eNOS and promotes production of superoxide, rather than NO [111]. Reactive oxygen species may increase lipid peroxidation products that interfere with receptor dependent activation of eNOS, inactivate NO, and decrease the responsiveness of target tissues [112, 113].
A number of enzymatic sources of superoxide in the diabetic vasculature have been identified. Nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) is a membrane-associated multi-subunit complex that generates superoxide anion and is involved in the oxidative burst in inflammatory cells and normal cell signaling in endothelial cells [106]. Under pathological conditions, including diabetes mellitus, NADPH oxidase activity and superoxide production is increased [114, 115]. Increased free fatty acid concentration activates NADPH oxidase and promotes activation of the pro-inflammatory transcription factor NFκB [116]. NADPH oxidase expression is also upregulated by angiotensin II [117], and it is interesting that angiotensin converting enzymes inhibitors have favorable vascular effects in diabetes [118, 119].
Uncoupled eNOS is another important source of oxidative stress in the diabetic vasculature. Under physiological conditions, eNOS exists as a dimer and produces NO, however, the enzyme reduces oxygen to superoxide anion when there is decreased availability of the cofactor tetrahydrobiopterin [120]. Diabetes is associated with eNOS uncoupling and decreased tetrahydrobiopterin levels. Since tetrahydrobiopterin is readily oxidized, a vicious cycle is established whereby oxidative stress leads to eNOS uncoupling and increased production of superoxide anion, which in turn may reduce the availability of tetrahydrobiopterin and promote further oxidative stress. Consistent with this mechanism, tetrahydrobiopterin supplementation improves NO production and endothelial function in experimental models [121, 122] and in human subjects with Type 2 diabetes mellitus [123]. A number of other sources of reactive oxygen species have been identified. For example, hyperglycemia and diabetes are associated with increased production of superoxide anion by the aldose reductase system and, as discussed below, components of the mitochondrial electron transport chain [124].
Studies in humans support the importance of increased oxidative stress as a mechanism of endothelial dysfunction in diabetes mellitus and insulin resistance. For example, circulating markers of oxidative stress, including F2 isoprostanes and antibodies against oxidized low density lipoprotein, are increased in humans with diabetes, obesity, and insulin resistance [125, 126]. Clinical studies have shown improved endothelial function with antioxidant treatment. In this regard, infusion of ascorbic acid in concentrations sufficiently high to scavenge superoxide anion, improves endothelial function in the forearm of patients with diabetes mellitus [42]. Alpha tocopherol treatment improved endothelial function in the coronary arteries of patients with Type 1 diabetes mellitus, although there was no benefit in Type 2 diabetes [127].
Although oxidative stress is clearly a contributing mechanism and antioxidants may improve endothelial function in certain situations, clinical trials have failed to show a benefit of alpha tocopherol therapy on outcomes in large-scale clinical trials [128, 129]. These findings may have important implications for prevention and management of vascular disease in diabetes mellitus. A strategy designed to inhibit enzymatic sources of reactive oxygen species is likely to provide greater benefit than treatment with scavengers of reactive oxygen species such as ascorbic acid, alpha tocopherol, and other antioxidant compounds. In support of this concept, angiotensin converting enzyme inhibitors and angiotensin receptor blockers limit angiotensin II-induced expression of NADPH oxidase and decrease superoxide anion production in the endothelium [130]. These drugs have been shown to improve endothelial function and reduce cardiovascular risk in patients with diabetes mellitus [131].
3.3 Pro-inflammatory activation of the endothelium
Atherosclerosis is recognized to be an inflammatory disease [21, 22] and the vascular endothelium is both affected by and contributes to the inflammatory process [132]. Endothelial cells can be “activated” by pro-inflammatory factors, including tumor necrosis factor-alpha (TNF-α) and C-reactive protein to promote an atherogenic phenotype [132, 133]. The activated endothelium expresses adhesion molecules and other factors that accelerate the inflammatory process. An important consequence of endothelial activation is decreased expression of eNOS and loss of NO bioactivity. In this regard, inflammatory mediators including TNF-α decrease expression of eNOS in cultured endothelial cells [134]. In humans, acute inflammatory states, such as infusion of low-dose lipo-polysaccharide or vaccination have been shown to impair endothelium-dependent vasodilation [135, 136]. On the basis of such studies, investigators argue that a broad alteration of endothelial function, including loss of NO under pro-inflammatory conditions, might be a critical mechanism that links systemic inflammation to atherosclerosis [137].
Diabetes is associated with a systemic inflammatory state that may impair endothelial function and contribute to atherosclerosis [138]. In experimental systems, increased concentrations of glucose or free fatty acids activate the endothelium [116, 139, 140]. Patients with diabetes mellitus or obesity have increased circulating levels of inflammatory markers, including C-reactive protein, TNF-α, interleukin-6, and intercellular adhesion molecule-1 [50, 141–143]. Furthermore, increased levels of inflammatory markers predict cardiovascular risk in diabetic patients [144]. Interestingly, increased levels of circulating inflammatory markers also relate to the incidence of new diabetes [52, 145–147].
The transcription factor NFκB is a key regulator of endothelial activation and, interestingly, has also been linked to the pathogenesis of insulin resistance [148, 149]. NFκB is activated by free fatty acids, inflammatory cytokines, and the receptor for advanced glycation end products (RAGE) [8, 150–152]. NFκB activation involves phosphorylation and subsequent degradation of the inhibitory subunit IκB by IκB kinase (IKK-β), which allows translocation of the regulatory subunits p50 and p65 to the nucleus, where they promote expression of inflammatory genes. In skeletal muscle, TNF-α or over expression of IKK-β produces insulin resistance [153]. Conversely, genetic suppression or pharmacological inhibition of IKK-β with salicylates prevents insulin resistance [148, 154]. Studies in cultured endothelial cells and experimental animals support links between activation of NFκB, development of an inflammatory phenotype, insulin resistance, and impaired bioactivity of NO [155, 156].
Several recent human studies support the clinical relevance of these mechanisms. Treatment of obese human subjects with salsalate improved insulin sensitivity and reduced circulating markers of inflammation [157]. Increased expression of p65 and decreased abundance of IκB, reflecting activation of NFκB has been observed in endothelial cells isolated from older and obese individuals, and these findings relate to impaired endothelium-dependent vasodilation [158, 159]. Salsalate treatment of obese human subjects reduced NFκB activation in freshly isolated endothelial cells [160]. This effect was associated with improved insulin sensitivity and endothelium-dependent vasodilation [160]. Such studies raise the interesting possibility that treatment of diabetics with salsalate or other anti-inflammatory drugs might improve glucose control and reduce the risk for vascular disease. Clinical trials are currently underway to address these possibilities (TINSAL-CVD and TINSAL-T2D).
3.4 Activation of protein kinase C
Activation of protein kinase C beta (PKCβ) may explain the links between inflammation, endothelial dysfunction, and insulin resistance in diabetes mellitus [161, 162]. The PKC’s are a family of serine/threonine kinases that act at the plasma membrane in the regulation of signal transduction in a wide variety of cell types. PKCβ is an important isoform in endothelial cells and is activated by diacylglycerol under conditions of increased glucose and fatty acid concentrations [163, 164]. Interestingly, accumulation of diacylglycerol in this setting has been attributed to impaired mitochondrial substrate utilization [165]. PKCβ inhibits PI3 kinase and Akt, thereby reducing eNOS phosphorylation [166, 167]. PKCβ also activates NFκB [168, 169]. Inhibiting PKCβ improves NO bioavailability and reduces inflammatory activation of the endothelium in experimental models [170–174]. In humans, treatment with a PKCβ inhibitor prevented the development of endothelial dysfunction following glucose infusion in the forearm of healthy volunteers [175] and improved brachial artery flow-mediated dilation in patients with diabetes mellitus [176].
3.5 Mitochondrial dysfunction
Recent studies have shed light on abnormalities of mitochondrial function as a proximate mechanism of increased oxidative stress and PKC activation in the diabetic vasculature. Although well-recognized for their role in the production of adenosine-triphosphate (ATP), mitochondria have many other cellular functions including a role in cell signaling via production of reactive oxygen species [177–179]. While most of the oxygen consumed by mitochondria relates to ATP generation, 1–2% is converted to superoxide anion under physiological conditions [180]. Mitochondrial-derived reactive oxygen species affect cell growth, differentiation, and programmed cell death [177, 179, 181–185]. Relevant to diabetes and insulin resistance, mitochondrial-derived reactive oxygen species play a role in the activation of AMP kinase a central regular of cellular energy status[185, 186]. Interestingly, mitochondrial-derived hydrogen peroxide contributes to endothelium-dependent dilation in response to shear stress in specific vascular beds [187]. While physiological levels of mitochondria-derived reactive oxygen species function in normal signaling, increased levels have pathological effects in diabetes [124]. In support of this concept, mitochondria-directed antioxidants, including lipoic acid, reduce radical production, improve insulin sensitivity, increase Akt activation, and improve NO-mediated vasodilation [188–191].
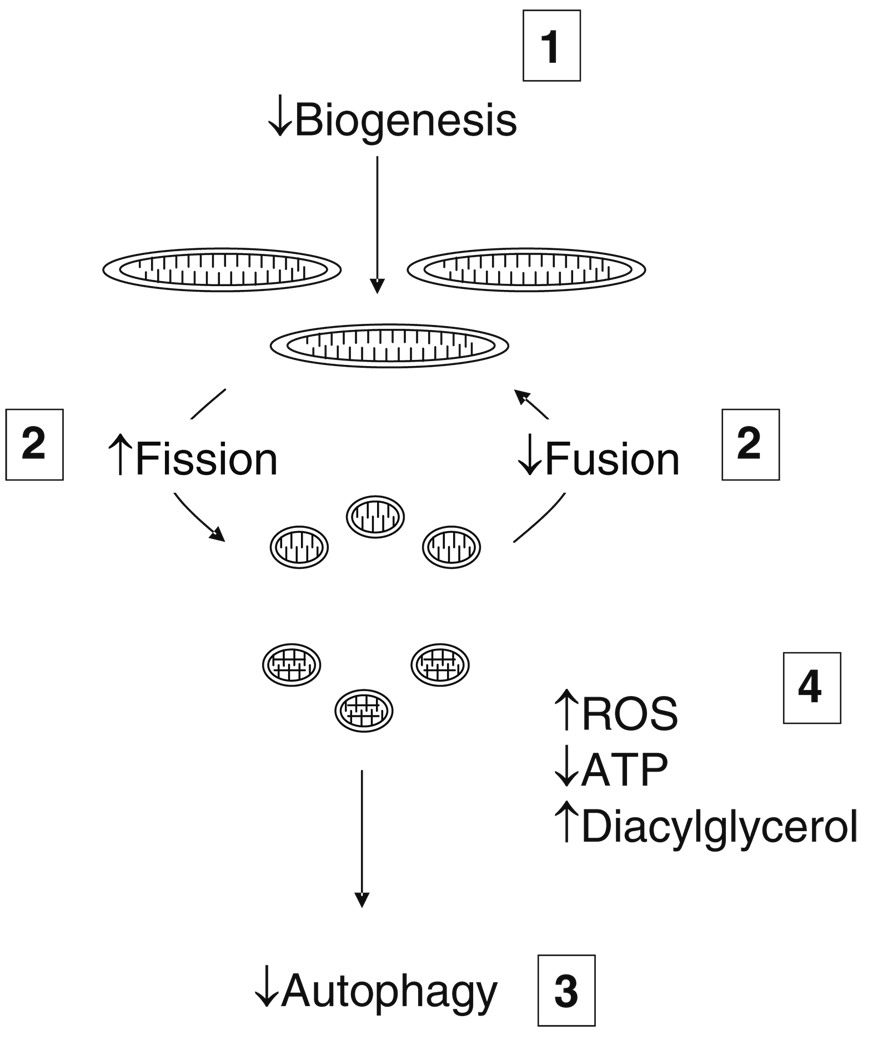
There is growing recognition of the importance of mitochondrial biogenesis and dynamics for energetics and reactive oxygen species production in diabetes (Fig. 2) [192–194]. Formation of new mitochondria (biogenesis) is regulated by peroxisomal proliferator activator receptor gamma coactivator-1alpha (PGC-1α) and nuclear respiratory factor-1 (NRF-1). Interestingly, this process depends on eNOS and bioavailable NO [195–198]. Mitochondria have a life span lasting hours to days, and as part of their life cycle, undergo cycles of fusion to form complex networks and fission to form smaller individual mitochondria. The balance between these processes is referred to as mitochondrial dynamics [192, 199]. Fusion may be beneficial and allow for distribution of metabolites and DNA throughout the network. Toward the end of the life cycle, dysfunctional daughter mitochondrial produced by fission undergo autophagy eliminating them from the cell. For this reason, fission is an adaptive process that sequesters and targets damaged mitochondrial components for elimination [200]. Under pathological conditions, however, there is a shift toward fission and inhibition of autophagy leading to the loss of mitochondrial networks and accumulation of dysfunctional mitochondria in the cell. These dysfunctional mitochondria have increased production of reactive oxygen species and impaired production of ATP [192, 200–202].
Fig. 2.
The mitochondrial life cycle and dynamics in diabetes mellitus: 1. Mitochondrial biogenesis is reduced; 2. the balance between fission and fusion is disturbed leading to increased fission, decreased fusion, and loss of normal mitochondrial networks; 3. autophagy normally removes damaged and senescent mitochondria, but diabetes impairs autophagy leading to the accumulation of dysfunctional mitochondria. 4. The net effect is a predominance of fragmented, dysfunctional mitochondria that produce increased amounts of reactive oxygen species (ROS) and decreased amounts of adenosine triphosphate (ATP). Impaired mitochondrial energetics also leads to increased levels of diacylglycerol that may activate PKCβ and impair nitric oxide production as shown in Fig. 1
Diabetes and insulin resistance are strongly linked to abnormalities of mitochondrial function [203–205]. Decreased fatty acid oxidation by mitochondria and/or decreased mitochondrial mass may lead to increased diacylglycerol concentrations and activation of PKC, which, as described above, blocks insulin signaling and activates NFκB [148, 203]. Increased glucose concentrations increase mitochondrial membrane potential and radical production [124, 206]. Mitochondrial-derived reactive oxygen species may damage mitochondrial DNA (mtDNA), which lacks protective histones, leading to impaired expression of oxidative phosphorylation enzymes and decreased substrate utilization [204]. Mitochondrial uncoupling proteins (UCP’s) act to prevent membrane hyperpolarization and limit superoxide production [182], and over-expression of uncoupling proteins in skeletal muscle prevents the development of diet-induced obesity and diabetes [207]. Diabetic conditions impair mitochondrial biogenesis, mitochondrial fusion, and autophagy, leading to cells with decreased mitochondrial mass and predominance of fragmented and dysfunctional mitochondria [205, 208]. Finally, interventions that promote mitochondrial biogenesis, such as resveratrol and other activators of the histone deacetylase SIRT1, have been shown to improve insulin sensitivity [209].
Clinical studies have demonstrated impaired mitochondrial function in patients with diabetes. For example, diabetics [210] and offspring of diabetic patients have decreased oxidative phosphorylation [211] and decreased expression of oxidative phosphorylation genes in skeletal muscle [212, 213]. Skeletal muscle from patients with diabetes is characterized by smaller mitochondria, decreased mitochondrial mass, and decreased expression of genes related to mitochondrial biogenesis [204, 212, 214–216]. Anti-diabetic changes in lifestyle, including exercise and calorie restriction lead to an increase in mitochondrial biogenesis [204, 217]. Recent preliminary work demonstrated links between endothelial dysfunction and impaired mitochondrial biogenesis and increased mitochondrial superoxide production in arterioles isolated from patients with diabetes mellitus versus healthy controls [218]. Thus, experimental studies have firmly established connections between mitochondrial dysfunction and diabetes, and these mechanisms appear to contribute to endothelial dysfunction in human diabetes mellitus.
4 Clinical implications
Type 2 diabetes mellitus is a major risk factor for cardiovascular disease. This condition is associated with insulin resistance and related metabolic abnormalities, including hyperglycemia, hypertension, visceral adiposity, and dyslipidemia with low HDL and elevated triglycerides and free fatty acids [3, 219, 220]. Current efforts to reduce cardiovascular disease focus on risk factor reduction, but diabetics continue to have increased risk despite aggressive interventions [220–222]. Along these lines, intensive glucose control has disappointing effects on the incidence of cardiovascular events [223–226]. In addition, promising therapies to improve insulin sensitivity and glucose control have unexpectedly been associated with increased cardiovascular risk [227–229]. Thus, there is a need for new approaches to the prevention and management of cardiovascular risk in diabetes.
As we have reviewed, a key mechanism of diabetic vascular disease is the development of endothelial dysfunction, which is characterized by a loss of NO and development of a pro-inflammatory vascular phenotype that promotes atherosclerosis and cardiovascular events. Recent experimental work has shed light on the mechanisms of endothelial dysfunction, which include impaired cell signaling required for activation of eNOS, increased oxidative stress, activation of pro-inflammatory signaling mechanisms, and activation of PKCβ. A proximate mechanism that may unify many aspects of endothelial dysfunction is mitochondrial dysfunction.
To date, these insights have only partially been translated into useful management strategies. Studies of endothelial function in diabetes support several important recommendations about patient management (Table 2). In spite of the prominent role played by oxidative stress in diabetes and atherosclerosis, antioxidant therapy had no benefit in large randomized trials [128, 129, 230]. Therapy directed against enzymatic sources of superoxide anion appears to have greater utility for the prevention of vascular disease. In addition to favorable effects on the renal vasculature, decreased superoxide production by NADPH oxidase may explain the observed benefits of angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB’s) against cardiovascular disease. This mechanism may provide further rationale for the preferential use of these drugs rather than other hypertensive agents in the management of hypertension in diabetic patients. Improved endothelial function, reduced radical production, and inhibition of pro-inflammatory mechanisms may also explain the well-established benefits of statin therapy in diabetes.
Table 2.
Implications for management from studies of endothelial function in diabetes
| No benefit from traditional antioxidants (vitamin E and vitamin C) |
| ACE inhibitors and ARB’s preferred |
| Statin therapy beneficial |
| Intensive glucose control/Insulin sensitizers should be beneficial |
| Merit further clinical study: |
| PKCβ inhibitors |
| NFκB inhibitors |
| Mitochondrial directed therapy—inhibit fission, augment fusion and autophagy |
| Practical methods to monitor endothelial function |
The reviewed studies of the mechanisms of endothelial dysfunction in diabetes may point to new management strategies for the prevention of cardiovascular disease in diabetes. Anti-inflammatory drugs and PKCβ inhibitors appear to hold great promise and clinical studies are in progress, but these strategies have not yet been established as safe and effective. Recent work on mitochondrial dysfunction suggests possible new directions for therapy. Drugs that have favorable effects on excess mitochondrial superoxide production, biogenesis, dynamics, and/or autophagy might prove effective given the importance of these mechanisms for diabetes-associated inflammation, endothelial dysfunction, and insulin resistance. Resveratrol or more potent activators of SIRT1 have potential in this regard.
Finally, the reviewed work supports the concept that monitoring endothelial function might prove useful for management decisions in the care of patients with diabetes. Testing endothelial function might help with selecting types and intensity of therapy. Similarly, serial examination of endothelial function might be useful for monitoring the effectiveness of risk reduction interventions. Well-established invasive and non-invasive methods for measuring endothelial function have proven useful in the research setting. However, translation of this work to the clinic will require the development of standardized methods for measuring endothelial function in individual patients that can be applied to the day-to-day care of patients with diabetes mellitus or other risk factors for cardiovascular disease.
Acknowledgments
Drs. Tabit, Hamburg, and Vita received support from the NIH-sponsored Boston University Medical Center Leadership Program in Vascular Medicine (K12 HL083781). Dr. Vita’s work is supported by grants from the NIH (HL083801, HL081587, HL083269, and HL75795).
Contributor Information
Corey E. Tabit, Evans Department of Medicine and Whitaker Cardiovascular Institute, Boston University School of Medicine, Boston, MA, USA
William B. Chung, Evans Department of Medicine and Whitaker Cardiovascular Institute, Boston University School of Medicine, Boston, MA, USA
Naomi M. Hamburg, Evans Department of Medicine and Whitaker Cardiovascular Institute, Boston University School of Medicine, Boston, MA, USA
Joseph A. Vita, Evans Department of Medicine and Whitaker Cardiovascular Institute, Boston University School of Medicine, Boston, MA, USA Section of Cardiology, Boston Medical Center, 88 East Newton Street, Boston, MA 02118, USA, jvita@bu.edu.
References
- 1.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care. 2006;29:2114–2116. doi: 10.2337/dc06-1136. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Wassef M, Chait A, et al. Prevention conference VI: Diabetes and cardiovascular disease: writing Group II: pathogenesis of atherosclerosis in diabetes. Circulation. 2002;105:e138–e143. doi: 10.1161/01.cir.0000013954.65303.c5. [DOI] [PubMed] [Google Scholar]
- 4.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 5.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 6.Vita JA, Keaney JF., Jr Endothelial function: A barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 7.Beckman JA, Libby P, Creager MA. Diabetes mellitus, the metabolic syndrome, and atherosclerotic vascular disease. In: Zipes DP, Libby P, Bonow RO, Braunwald E, editors. Braun-wald’s heart disease: A textbook of cardiovascular medicine. Philadelphia: Elsevier Saunders; 2005. pp. 1035–1046. [Google Scholar]
- 8.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 9.Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2009;335:165–189. doi: 10.1007/s00441-008-0685-6. [DOI] [PubMed] [Google Scholar]
- 10.Calles-Escandon J, Cipolla M. Diabetes and endothelial dysfunction: a clinical perspective. Endocr Rev. 2001;22:36–52. doi: 10.1210/edrv.22.1.0417. [DOI] [PubMed] [Google Scholar]
- 11.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 12.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 13.Silver AE, Vita JA. Shear-stress-mediated arterial remodeling in atherosclerosis: too much of a good thing? Circulation. 2006;113:2787–2789. doi: 10.1161/CIRCULATIONAHA.106.634378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov’s phenomenon. Arterioscler Thromb Vasc Biol. 2007;27:1722–1728. doi: 10.1161/ATVBAHA.106.129254. [DOI] [PubMed] [Google Scholar]
- 15.Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis) Circ Res. 2004;95:449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 16.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003;23:1143–1151. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 17.Pasterkamp G, Galis ZS, de Kleijn DP. Expansive arterial remodeling: location, location, location. Arterioscler Thromb Vasc Biol. 2004;24:650–657. doi: 10.1161/01.ATV.0000120376.09047.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Chantemele EJ Belin, Vessieres E, Guihot AL, et al. Type 2 diabetes severely impairs structural and functional adaptation of rat resistance arteries to chronic changes in blood flow. Cardiovasc Res. 2009;81:788–796. doi: 10.1093/cvr/cvn334. [DOI] [PubMed] [Google Scholar]
- 19.Rubio R, Berne RM. Release of adenosine by the normal myocardium in dogs and its relationship to the regulation of coronary resistance. Circ Res. 1969;25:407–415. doi: 10.1161/01.res.25.4.407. [DOI] [PubMed] [Google Scholar]
- 20.Loscalzo J, Vita JA. Ischemia, hyperemia, exercise, and nitric oxide: complex physiology and complex molecular adaptations. Circulation. 1994;90:2556–2559. doi: 10.1161/01.cir.90.5.2556. [DOI] [PubMed] [Google Scholar]
- 21.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 22.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Cybulsky MI, Gimbrone MA, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993;13(2):197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- 24.Ruberg FL, Leopold JA, Loscalzo J. Atherothrombosis: plaque instability and thrombogenesis. Prog Cardiovasc Dis. 2002;44:381–394. doi: 10.1053/pcad.2002.123469. [DOI] [PubMed] [Google Scholar]
- 25.Libby P. Atherosclerosis: the new view. Sci Am. 2002;286:46–55. doi: 10.1038/scientificamerican0502-46. [DOI] [PubMed] [Google Scholar]
- 26.Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasocon-striction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 27.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 28.Vita JA, Treasure CB, Nabel EG, et al. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- 29.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of endothelial function in the community: the Framingham heart study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 30.Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein (a) level. J Clin Invest. 1994;93:50–55. doi: 10.1172/JCI116983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long- term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 32.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 33.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 34.Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 35.Gokce N, Keaney JF, Jr, Menzoian JO, et al. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 36.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 37.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 38.Huang AL, Silver AE, Shvenke E, et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–2119. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 40.Nicolls MR, Haskins K, Flores SC. Oxidant stress, immune dysregulation, and vascular function in type I diabetes. Antioxid Redox Signal. 2007;9:879–889. doi: 10.1089/ars.2007.1631. [DOI] [PubMed] [Google Scholar]
- 41.McVeigh GE, Brennan GM, Johnston GD, et al. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 42.Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1996;97:22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 44.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamdy O, Ledbury S, Mullooly C, et al. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care. 2003;26:2119–2125. doi: 10.2337/diacare.26.7.2119. [DOI] [PubMed] [Google Scholar]
- 46.Gokce N, Vita JA, Donnell M, et al. Effect of medical and surgical weight loss on endothelial vasomotor function in obese patients. Am J Cardiol. 2005;95:266–268. doi: 10.1016/j.amjcard.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome: determinants of endothelial dysfunction in whites and blacks. Circulation. 2005;112:32–38. doi: 10.1161/CIRCULATIONAHA.104.520130. [DOI] [PubMed] [Google Scholar]
- 48.Hamburg NM, Larson MG, Vita JA, et al. Metabolic syndrome, insulin resistance, and brachial artery vasodilator function in framingham offspring participants without clinical evidence of cardiovascular disease. Am J Cardiol. 2008;101:82–88. doi: 10.1016/j.amjcard.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 50.Keaney JF, Jr, Massaro JM, Larson MG, et al. Heritability and correlates of intercellular adhesion molecule-1 in the Framingham Offspring Study. J Am Coll Cardiol. 2004;44:168–173. doi: 10.1016/j.jacc.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 51.Meigs JB, Mittleman MA, Nathan DM, et al. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. JAMA. 2000;283:221–228. doi: 10.1001/jama.283.2.221. [DOI] [PubMed] [Google Scholar]
- 52.Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291:1978–1986. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
- 53.de Jongh RT, Serne EH, IJzerman RG, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–2535. doi: 10.1161/01.CIR.0000129772.26647.6F. [DOI] [PubMed] [Google Scholar]
- 54.Balletshofer BM, Rittig K, Enderle MD, et al. Endothelial dysfunction is detectable in young normotensive first-degree relatives of subjects with type 2 diabetes in association with insulin resistance. Circulation. 2000;101:1780–1784. doi: 10.1161/01.cir.101.15.1780. [DOI] [PubMed] [Google Scholar]
- 55.Campia U, Sullivan G, Bryant MB, Waclawiw MA, Quon MJ, Panza JA. Insulin impairs endothelium-dependent vasodilation independent of insulin sensitivity or lipid profile. Am J Physiol Heart Circ Physiol. 2004;286:H76–H82. doi: 10.1152/ajpheart.00539.2003. [DOI] [PubMed] [Google Scholar]
- 56.Shimabukuro M, Shinzato T, Higa S, et al. Enhanced insulin response relates to acetylcholine-induced vasoconstriction in vasospastic angina. J Am Coll Cardiol. 1995;25:356–361. doi: 10.1016/0735-1097(94)00381-y. [DOI] [PubMed] [Google Scholar]
- 57.Shinozaki K, Hirayama A, Nishio Y, et al. Coronary endothelial dysfunction in the insulin-resistant state is linked to abnormal pteridine metabolism and vascular oxidative stress. J Am Coll Cardiol. 2001;38:1821–1828. doi: 10.1016/s0735-1097(01)01659-x. [DOI] [PubMed] [Google Scholar]
- 58.Pasimeni G, Ribaudo MC, Capoccia D, et al. Non-invasive evaluation of endothelial dysfunction in uncomplicated obesity: relationship with insulin resistance. Microvasc Res. 2006;71:115–120. doi: 10.1016/j.mvr.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Lampinen KH, Ronnback M, Groop PH, Kaaja RJ. A relationship between insulin sensitivity and vasodilation in women with a history of preeclamptic pregnancy. Hypertension. 2008;52:394–401. doi: 10.1161/HYPERTENSIONAHA.108.113423. [DOI] [PubMed] [Google Scholar]
- 60.Ardigo D, Franzini L, Valtuena S, Monti LD, Reaven GM, Zavaroni I. Relation of plasma insulin levels to forearm flow-mediated dilatation in healthy volunteers. Am J Cardiol. 2006;97:1250–1254. doi: 10.1016/j.amjcard.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 61.Caballero AE, Arora S, Saouaf R, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48:1856–1862. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- 62.Tesauro M, Rizza S, Iantorno M, et al. Vascular, metabolic, and inflammatory abnormalities in normoglycemic offspring of patients with type 2 diabetes mellitus. Metabolism. 2007;56:413–419. doi: 10.1016/j.metabol.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 63.Meigs JB, O’Donnell CJ, Tofler GH, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55:530–537. doi: 10.2337/diabetes.55.02.06.db05-1041. [DOI] [PubMed] [Google Scholar]
- 64.Rossi R, Cioni E, Nuzzo A, Origliani G, Modena MG. Endothelial-dependent vasodilation and incidence of type 2 diabetes in a population of healthy postmenopausal women. Diabetes Care. 2005;28:702–707. doi: 10.2337/diacare.28.3.702. [DOI] [PubMed] [Google Scholar]
- 65.Monti LD, Barlassina C, Citterio L, et al. Endothelial nitric oxide synthase polymorphisms are associated with type 2 diabetes and the insulin resistance syndrome. Diabetes. 2003;52:1270–1275. doi: 10.2337/diabetes.52.5.1270. [DOI] [PubMed] [Google Scholar]
- 66.Pistrosch F, Passauer J, Fischer S, Fuecker K, Hanefeld M, Gross P. In type 2 diabetes, rosiglitazone therapy for insulin resistance ameliorates endothelial dysfunction independent of glucose control. Diabetes Care. 2004;27:484–490. doi: 10.2337/diacare.27.2.484. [DOI] [PubMed] [Google Scholar]
- 67.Caballero AE, Saouaf R, Lim SC, et al. The effects of troglitazone, an insulin-sensitizing agent, on the endothelial function in early and late type 2 diabetes: a placebo-controlled randomized clinical trial. Metabolism. 2003;52:173–180. doi: 10.1053/meta.2003.50023. [DOI] [PubMed] [Google Scholar]
- 68.Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;37:1344–1350. doi: 10.1016/s0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- 69.de Aguiar LG, Bahia LR, Villela N, et al. Metformin improves endothelial vascular reactivity in first-degree relatives of type 2 diabetic patients with metabolic syndrome and normal glucose tolerance. Diabetes Care. 2006;29:1083–1089. doi: 10.2337/diacare.2951083. [DOI] [PubMed] [Google Scholar]
- 70.Campia U, Matuskey LA, Panza JA. Peroxisome proliferator-activated receptor-gamma activation with pioglitazone improves endothelium-dependent dilation in nondiabetic patients with major cardiovascular risk factors. Circulation. 2006;113:867–875. doi: 10.1161/CIRCULATIONAHA.105.549618. [DOI] [PubMed] [Google Scholar]
- 71.Hetzel J, Balletshofer B, Rittig K, et al. Rapid effects of rosiglitazone treatment on endothelial function and inflammatory biomarkers. Arterioscler Thromb Vasc Biol. 2005;25:1804–1809. doi: 10.1161/01.ATV.0000176192.16951.9a. [DOI] [PubMed] [Google Scholar]
- 72.Cheetham C, Collis J, O’Driscoll G, Stanton K, Taylor R, Green D. Losartan, an angiotensin type 1 receptor antagonist, improves endothelial function in non-insulin-dependent diabetes [In Process Citation] J Am Coll Cardiol. 2000;36:1461–1466. doi: 10.1016/s0735-1097(00)00933-5. [DOI] [PubMed] [Google Scholar]
- 73.O’Driscoll G, Green D, Maiorana A, Stanton K, Colreavy F, Taylor R. Improvement in endothelial function by angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1999;33:1506–1511. doi: 10.1016/s0735-1097(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 74.Shaul PW, Smart EJ, Robinson LJ, et al. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 75.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Cardena G, Fan R, Shah V, et al. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 77.Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179) J Biol Chem. 2001;276:30392–30398. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- 78.Zeng G, Nystrom FH, Ravichandran LV, et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101:1539–1545. doi: 10.1161/01.cir.101.13.1539. [DOI] [PubMed] [Google Scholar]
- 79.Kuboki K, Jiang ZY, Takahara N, et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation. 2000;101:676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 80.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 81.Krumenacker JS, Hanafy KA, Murad F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res Bull. 2004;62:505–515. doi: 10.1016/S0361-9230(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 82.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 83.Hartell NA, Archer HE, Bailey CJ. Insulin-stimulated endothelial nitric oxide release is calcium independent and mediated via protein kinase B. Biochem Pharmacol. 2005;69:781–790. doi: 10.1016/j.bcp.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 84.Federici M, Pandolfi A, De Filippis EA, et al. G972R IRS-1 variant impairs insulin regulation of endothelial nitric oxide synthase in cultured human endothelial cells. Circulation. 2004;109:399–405. doi: 10.1161/01.CIR.0000109498.77895.6F. [DOI] [PubMed] [Google Scholar]
- 85.Wheatcroft SB, Shah AM, Li JM, et al. Preserved glucoregulation but attenuation of the vascular actions of insulin in mice heterozygous for knockout of the insulin receptor. Diabetes. 2004;53:2645–2652. doi: 10.2337/diabetes.53.10.2645. [DOI] [PubMed] [Google Scholar]
- 86.Vicent D, Ilany J, Kondo T, et al. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest. 2003;111:1373–1380. doi: 10.1172/JCI15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duncan ER, Crossey PA, Walker S, et al. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes. 2008;57:3307–3314. doi: 10.2337/db07-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang ZY, Lin YW, Clemont A, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nacci C, Tarquinio M, Montagnani M. Molecular and clinical aspects of endothelial dysfunction in diabetes. Intern Emerg Med. 2009;4:107–116. doi: 10.1007/s11739-009-0234-7. [DOI] [PubMed] [Google Scholar]
- 90.Wheatcroft SB, Williams IL, Shah AM, Kearney MT. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med. 2003;20:255–268. doi: 10.1046/j.1464-5491.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 91.Montagnani M, Golovchenko I, Kim I, et al. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem. 2002;277:1794–1799. doi: 10.1074/jbc.M103728200. [DOI] [PubMed] [Google Scholar]
- 92.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction: implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baron AD, Brechtel-Hook G, Johnson A, Cronin J, Leaming R, Steinberg HO. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am J Physiol. 1996;271:E1067–E1072. doi: 10.1152/ajpendo.1996.271.6.E1067. [DOI] [PubMed] [Google Scholar]
- 95.Mather K, Laakso M, Edelman S, Hook G, Baron A. Evidence for physiological coupling of insulin-mediated glucose metabolism and limb blood flow. Am J Physiol Endocrinol Metab. 2000;279:E1264–E1270. doi: 10.1152/ajpendo.2000.279.6.E1264. [DOI] [PubMed] [Google Scholar]
- 96.Baron AD. Insulin resistance and vascular function. J Diabetes Complications. 2002;16:92–102. doi: 10.1016/s1056-8727(01)00209-4. [DOI] [PubMed] [Google Scholar]
- 97.Okon EB, Chung AW, Rauniyar P, et al. Compromised arterial function in human type 2 diabetic patients. Diabetes. 2005;54:2415–2423. doi: 10.2337/diabetes.54.8.2415. [DOI] [PubMed] [Google Scholar]
- 98.Piatti PM, Monti LD, Conti M, et al. Hypertriglyceridemia and hyperinsulinemia are potent inducers of endothelin-1 release in humans. Diabetes. 1996;45:316–321. doi: 10.2337/diab.45.3.316. [DOI] [PubMed] [Google Scholar]
- 99.Lam TY, Seto SW, Lau YM, et al. Impairment of the vascular relaxation and differential expression of caveolin-1 of the aorta of diabetic+db/+db mice. Eur J Pharmacol. 2006;546:134–141. doi: 10.1016/j.ejphar.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 100.Bucci M, Roviezzo F, Brancaleone V, et al. Diabetic mouse angiopathy is linked to progressive sympathetic receptor deletion coupled to an enhanced caveolin-1 expression. Arterioscler Thromb Vasc Biol. 2004;24(24):721–726. doi: 10.1161/01.ATV.0000122362.44628.09. [DOI] [PubMed] [Google Scholar]
- 101.Catalan V, Gomez-Ambrosi J, Rodriguez A, et al. Expression of caveolin-1 in human adipose tissue is upregulated in obesity and obesity-associated type 2 diabetes mellitus and related to inflammation. Clin Endocrinol (Oxf) 2008;68:213–219. doi: 10.1111/j.1365-2265.2007.03021.x. [DOI] [PubMed] [Google Scholar]
- 102.Lei H, Venkatakrishnan A, Yu S, Kazlauskas A. Protein kinase A-dependent translocation of Hsp90 alpha impairs endothelial nitric-oxide synthase activity in high glucose and diabetes. J Biol Chem. 2007;282:9364–9371. doi: 10.1074/jbc.M608985200. [DOI] [PubMed] [Google Scholar]
- 103.Boger RH. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc Res. 2003;59:824–833. doi: 10.1016/s0008-6363(03)00500-5. [DOI] [PubMed] [Google Scholar]
- 104.Tesfamariam B, Cohen RA. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol. 1992;263:H321–H326. doi: 10.1152/ajpheart.1992.263.2.H321. [DOI] [PubMed] [Google Scholar]
- 105.Davda RK, Stepniakowski KT, Lu G, Ullian ME, Goodfriend TL, Egan BM. Oleic acid inhibits endothelial nitric oxide synthase by a protein kinase C-independent mechanism. Hypertension. 1995;26:764–770. doi: 10.1161/01.hyp.26.5.764. [DOI] [PubMed] [Google Scholar]
- 106.Stocker R, Keaney JF., Jr The role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 107.Tomasian D, Keaney JF, Jr, Vita JA. Antioxidants and the bioactivity of endothelium-derived nitric oxide. Cardiovasc Res. 2000;47:426–435. doi: 10.1016/s0008-6363(00)00103-6. [DOI] [PubMed] [Google Scholar]
- 108.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 109.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Munzel T, Daiber A, Ullrich V, Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:1551–1557. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 111.Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 112.Hamburg NM, Vita JA. Endothelial dysfunction in atherosclerosis: Mechanisms of impaired nitric oxide bioactivity. In: Loscalzo J, editor. Molecular mechanisms of atherosclerosis. London: Taylor & Francis; 2006. pp. 95–110. [Google Scholar]
- 113.Tong X, Evangelista A, Cohen RA. Targeting the redox regulation of SERCA in vascular physiology and disease. Curr Opin Pharmacol. 2009 doi: 10.1016/j.coph.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.San Martin A, Du P, Dikalova A, et al. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;292:H2073–H2082. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- 115.Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 116.Maloney E, Sweet IR, Hockenbery DM, et al. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol. 2009;29:1370–1375. doi: 10.1161/ATVBAHA.109.188813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rajagopalan S, Harrison DG. Reversing endothelial dysfunction with ACE inhibitors: a new trend? Circulation. 1996;94:240–243. doi: 10.1161/01.cir.94.3.240. [DOI] [PubMed] [Google Scholar]
- 118.Henriksen EJ. Improvement of insulin sensitivity by antagonism of the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;293:R974–R980. doi: 10.1152/ajpregu.00147.2007. [DOI] [PubMed] [Google Scholar]
- 119.Oak JH, Cai H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes. 2007;56:118–126. doi: 10.2337/db06-0288. [DOI] [PubMed] [Google Scholar]
- 120.Vasquez-Vivar J, Kalyanaraman B, Martasek P, et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pieper GM. Acute amelioration of diabetic endothelial dysfunction with a derivative of the nitric oxide synthase cofactor, tetrahydrobiopterin. J Cardiovasc Pharmacol. 1997;29:8–15. doi: 10.1097/00005344-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 122.Alp NJ, Mussa S, Khoo J, et al. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I over-expression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43:1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 124.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 125.Meigs JB, Larson MG, Fox CS, Keaney JF, Jr, Vasan RS, Benjamin EJ. Association of oxidative stress, insulin resistance, and diabetes risk phenotypes: the Framingham Offspring Study. Diabetes Care. 2007;30:2529–2535. doi: 10.2337/dc07-0817. [DOI] [PubMed] [Google Scholar]
- 126.Keaney JF, Jr, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 127.Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Keaney JF, Creager MA. Oral antioxidant therapy improves endothelial function in type 1 but not type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2003;285:H2392–H2398. doi: 10.1152/ajpheart.00403.2003. [DOI] [PubMed] [Google Scholar]
- 128.Lonn E, Yusuf S, Hoogwerf B, et al. Effects of vtamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE Study and MICRO-HOPE Substudy. Diabetes Care. 2002;25:1919–1927. doi: 10.2337/diacare.25.11.1919. [DOI] [PubMed] [Google Scholar]
- 129.MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 130.Münzel T, Keaney JF., Jr Are ACE-inhibitors a “magic bullet” against oxidative stress? Circulation. 2001;104:1571–1574. doi: 10.1161/hc3801.095585. [DOI] [PubMed] [Google Scholar]
- 131.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 132.Huang AL, Vita JA. Effects of systemic inflammation on endothelium-dependent vasodilation. Trends Cardiovasc Med. 2006;16:15–20. doi: 10.1016/j.tcm.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 134.Zhang J, Patel JM, Li YD, Block ER. Proinflammatory cytokines downregulate gene expression and activity of constitutive nitric oxide synthase in porcine pulmonary artery endothelial cells. Res Commun Mol Pathol Pharmacol. 1997;96:71–87. [PubMed] [Google Scholar]
- 135.Hingorani AD, Cross J, Kharbanda RK, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102:994–999. doi: 10.1161/01.cir.102.9.994. [DOI] [PubMed] [Google Scholar]
- 136.Bhagat K, Moss R, Collier J, Vallance P. Endothelial “stunning” following a brief exposure to endotoxin: a mechanism to link infection and infarction? Cardiovasc Res. 1996;32:822–829. [PubMed] [Google Scholar]
- 137.Vallance P, Collier J, Bhagat K. Infection, inflammation, and infarction: does acute endothelial dysfunction provide a link? Lancet. 1997;349:1391–1392. doi: 10.1016/S0140-6736(96)09424-X. [DOI] [PubMed] [Google Scholar]
- 138.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 139.Pieper GM, Riaz uH. Activation of nuclear factor-kappaB in cultured endothelial cells by increased glucose concentration: prevention by calphostin C. J Cardiovasc Pharmacol. 1997;30:528–532. doi: 10.1097/00005344-199710000-00019. [DOI] [PubMed] [Google Scholar]
- 140.Piga R, Naito Y, Kokura S, Handa O, Yoshikawa T. Short-term high glucose exposure induces monocyte-endothelial cells adhesion and transmigration by increasing VCAM-1 and MCP-1 expression in human aortic endothelial cells. Atherosclerosis. 2007;193:328–334. doi: 10.1016/j.atherosclerosis.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 141.Festa A, D’Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 142.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998;83:2907–2910. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 143.Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res. 2001;9:414–417. doi: 10.1038/oby.2001.54. [DOI] [PubMed] [Google Scholar]
- 144.Schulze MB, Rimm EB, Li T, Rifai N, Stampfer MJ, Hu FB. C-reactive protein and incident cardiovascular events among men with diabetes. Diabetes Care. 2004;27:889–894. doi: 10.2337/diacare.27.4.889. [DOI] [PubMed] [Google Scholar]
- 145.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 146.Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 147.Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 148.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Read MA, Whitley MZ, Williams AJ, Collins T. NF-kappa B and I kappa B alpha: an inducible regulatory system in endothelial activation. J Exp Med. 1994;179:503–512. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim F, Gallis B, Corson MA. TNF-alpha inhibits flow and insulin signaling leading to NO production in aortic endothelial cells. Am J Physiol Cell Physiol. 2001;280:C1057–C1065. doi: 10.1152/ajpcell.2001.280.5.C1057. [DOI] [PubMed] [Google Scholar]
- 151.Bierhaus A, Chevion S, Chevion M, et al. Advanced glycation end product-induced activation of NF-kappaB is suppressed by alpha-lipoic acid in cultured endothelial cells. Diabetes. 1997;46:1481–1490. doi: 10.2337/diab.46.9.1481. [DOI] [PubMed] [Google Scholar]
- 152.Bierhaus A, Schiekofer S, Schwaninger M, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 153.de Alvaro C, Teruel T, Hernandez R, Lorenzo M. Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner. J Biol Chem. 2004;279:17070–17078. doi: 10.1074/jbc.M312021200. [DOI] [PubMed] [Google Scholar]
- 154.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity-and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 155.Kim F, Tysseling KA, Rice J, et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKK {beta} Arterioscler Thromb Vasc Biol. 2005;25:989–994. doi: 10.1161/01.ATV.0000160549.60980.a8. [DOI] [PubMed] [Google Scholar]
- 156.Kim F, Pham M, Maloney E, et al. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–294. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Donato AJ, Eskurza I, Silver AE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 159.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFkappaB, reduced IkappaBalpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Das EN, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;55:498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 162.He Z, King GL. Protein kinase Cbeta isoform inhibitors: a new treatment for diabetic cardiovascular diseases. Circulation. 2004;110:7–9. doi: 10.1161/01.CIR.0000133428.02295.6C. [DOI] [PubMed] [Google Scholar]
- 163.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci U S A. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hyper-galactosemia. Diabetes. 1994;43:1122–1129. doi: 10.2337/diab.43.9.1122. [DOI] [PubMed] [Google Scholar]
- 165.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55:S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tesfamariam B, Brown ML, Cohen RA. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991;87:1643–1648. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Goel A, Zhang Y, Anderson L, Rahimian R. Gender difference in rat aorta vasodilation after acute exposure to high glucose: involvement of protein kinase C beta and superoxide but not of Rho kinase. Cardiovasc Res. 2007;76:351–360. doi: 10.1016/j.cardiores.2007.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rask-Madsen C, King GL. Proatherosclerotic mechanisms involving protein kinase C in diabetes and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:487–496. doi: 10.1161/01.ATV.0000155325.41507.e0. [DOI] [PubMed] [Google Scholar]
- 169.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 170.Naruse K, Rask-Madsen C, Takahara N, et al. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55:691–698. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- 171.Cotter MA, Jack AM, Cameron NE. Effects of the protein kinase C beta inhibitor LY333531 on neural and vascular function in rats with streptozotocin-induced diabetes. Clin Sci (Lond) 2002;103:311–321. doi: 10.1042/cs1030311. [DOI] [PubMed] [Google Scholar]
- 172.Hink U, Li H, Mollnau H, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 173.Ishii H, Jirousek MR, Koya D, et al. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 174.Kouroedov A, Eto M, Joch H, Volpe M, Luscher TF, Cosentino F. Selective inhibition of protein kinase Cbeta2 prevents acute effects of high glucose on vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 2004;110:91–96. doi: 10.1161/01.CIR.0000133384.38551.A8. [DOI] [PubMed] [Google Scholar]
- 175.Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Creager MA. Inhibition of protein kinase C beta prevents impaired endothelium- dependent vasodilation caused by hyperglycemia in humans. Circ Res. 2002;90:107–111. doi: 10.1161/hh0102.102359. [DOI] [PubMed] [Google Scholar]
- 176.Mehta NN, Sheetz M, Price K, et al. Selective PKC beta inhibition with ruboxistaurin and endothelial function in type-2 diabetes mellitus. Cardiovasc Drugs Ther. 2009;23:17–24. doi: 10.1007/s10557-008-6144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Duchen MR. Roles of mitochondria in health and disease. Diabetes. 2004;53:S96–S102. doi: 10.2337/diabetes.53.2007.s96. [DOI] [PubMed] [Google Scholar]
- 178.Rizzuto R, Pozzan T, Carafoli E. Ca2+ on the move: ways and means to translate a multifarious signal. Trends Pharmacol Sci. 2002;23:348–350. doi: 10.1016/s0165-6147(02)02063-1. [DOI] [PubMed] [Google Scholar]
- 179.Darley-Usmar V. The powerhouse takes control of the cell; the role of mitochondria in signal transduction. Free Radic Biol Med. 2004;37:753–754. doi: 10.1016/j.freeradbiomed.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 180.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 181.Ramachandran A, Levonen AL, Brookes PS, et al. Mitochondria, nitric oxide, and cardiovascular dysfunction. Free Radic Biol Med. 2002;33:1465–1474. doi: 10.1016/s0891-5849(02)01142-5. [DOI] [PubMed] [Google Scholar]
- 182.Brand MD, Affourtit C, Esteves TC, et al. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 183.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 184.Chen K, Thomas SR, Albano A, Murphy MP, Keaney JF., Jr Mitochondrial function is required for hydrogen peroxide-induced growth factor receptor transactivation and downstream signaling. J Biol Chem. 2004;279:35079–35086. doi: 10.1074/jbc.M404859200. [DOI] [PubMed] [Google Scholar]
- 185.Quintero M, Colombo SL, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci USA. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zou MH, Kirkpatrick SS, Davis BJ, et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo: Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279:43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- 187.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 188.El Midaoui A, de Champlain J. Prevention of hypertension, insulin resistance, and oxidative stress by alpha-lipoic acid. Hypertension. 2002;39:303–307. doi: 10.1161/hy0202.104345. [DOI] [PubMed] [Google Scholar]
- 189.Hagen TM, Ingersoll RT, Lykkesfeldt J, et al. (R)-alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999;13:411–418. doi: 10.1096/fasebj.13.2.411. [DOI] [PubMed] [Google Scholar]
- 190.Smith AR, Hagen TM. Vascular endothelial dysfunction in aging: loss of Akt-dependent endothelial nitric oxide synthase phosphorylation and partial restoration by (R)-alpha-lipoic acid. Biochem Soc Trans. 2003;31:1447–1449. doi: 10.1042/bst0311447. [DOI] [PubMed] [Google Scholar]
- 191.Heitzer T, Finckh B, Albers S, Krohn K, Kohlschutter A, Meinertz T. Beneficial effects of alpha-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: relation to parameters of oxidative stress. Free Radic Biol Med. 2001;31:53–61. doi: 10.1016/s0891-5849(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 192.Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nisoli E, Clementi E, Paolucci C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 196.Nisoli E, Falcone S, Tonello C, et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci USA. 2004;101:16507–16512. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nisoli E, Clementi E, Carruba MO, Moncada S. Defective mitochondrial biogenesis: a hallmark of the high cardiovascular risk in the metabolic syndrome? Circ Res. 2007;100:795–806. doi: 10.1161/01.RES.0000259591.97107.6c. [DOI] [PubMed] [Google Scholar]
- 198.Nisoli E, Tonello C, Cardile A, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 199.Menzies RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J Biol Chem. 1971;246:2425–2429. [PubMed] [Google Scholar]
- 200.Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 204.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zorzano A, Liesa M, Palacin M. Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int J Biochem Cell Biol. 2009;41:1846–1854. doi: 10.1016/j.biocel.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 206.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyper-glycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 207.Li B, Nolte LA, Ju JS, et al. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat Med. 2000;6:1115–1120. doi: 10.1038/80450. [DOI] [PubMed] [Google Scholar]
- 208.Goldman S, Zhang Y, Jin S. Autophagy and adipogenesis: implications in obesity and type II diabetes. Autophagy. 2010;6:179–181. doi: 10.4161/auto.6.1.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 214.Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 216.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 217.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Widlansky ME, Vita JA, Wang J, Kizhakekuttu TJ, Arthur EIL, Gutterman DD. Mitochondrial membrane hyperpolarization and reduced mitochondrial mass characterize the arteriolar endothelium and mononuclear cells of humans with Type 2 diabetes mellitus- in vivo and in vitro assessment of mitochondrial function [abstr] Circulation. 2009;120:S1107. [Google Scholar]
- 219.McCulloch DK. Overview of medical care in adults with diabetes mellitus. In: Holman RR, Mulder JE, editors. UpToDate Online. Boston: 2009. [Google Scholar]
- 220.Pearson TA, Blair SN, Daniels SR, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 221.Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114–126. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- 222.Preis SR, Hwang SJ, Coady S, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119:1728–1735. doi: 10.1161/CIRCULATIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 224.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 226.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–357. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 227.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 228.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 229.Goldfine AB. Assessing the cardiovascular safety of diabetes therapies. N Engl J Med. 2008;359:1092–1095. doi: 10.1056/NEJMp0805758. [DOI] [PubMed] [Google Scholar]
- 230.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]