Abstract
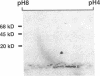
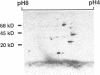
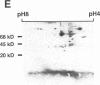
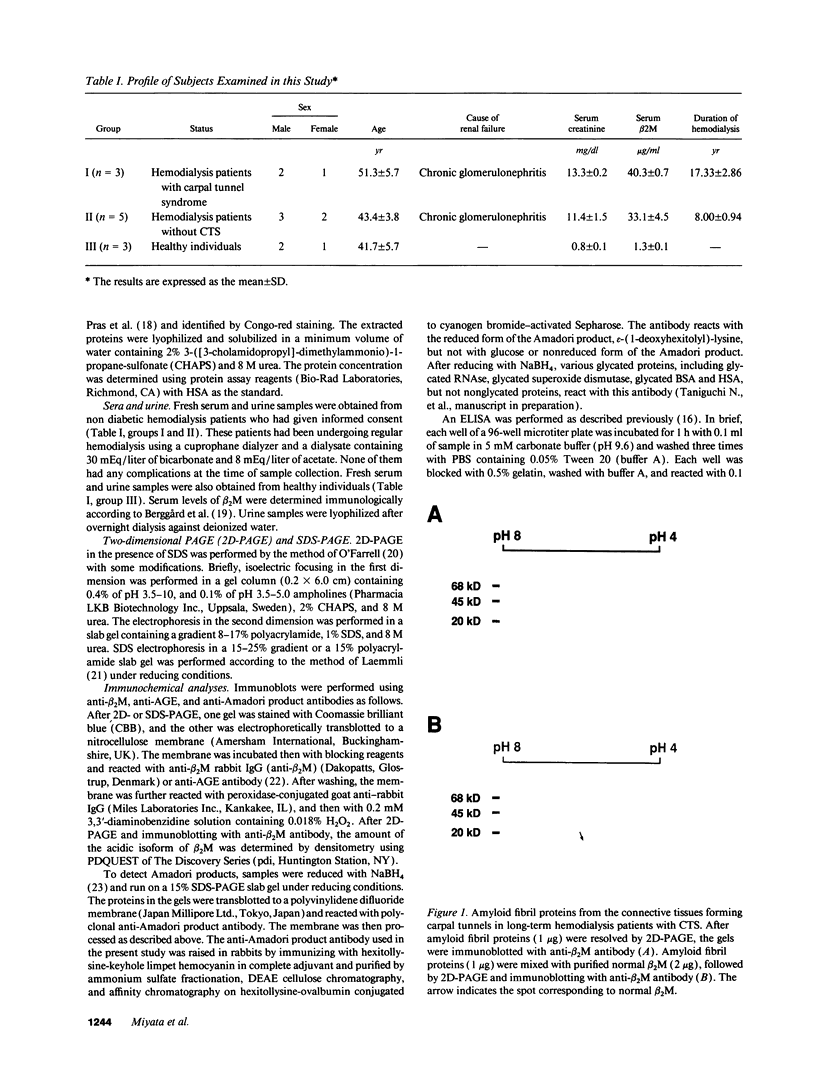
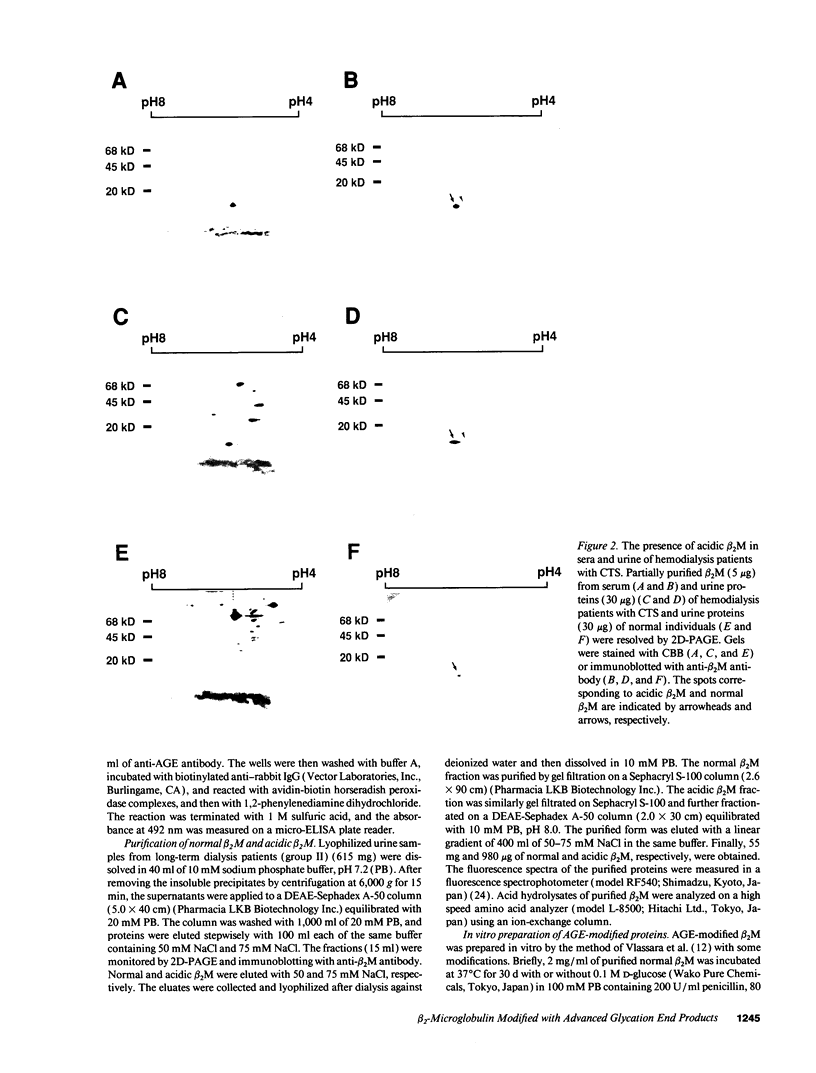
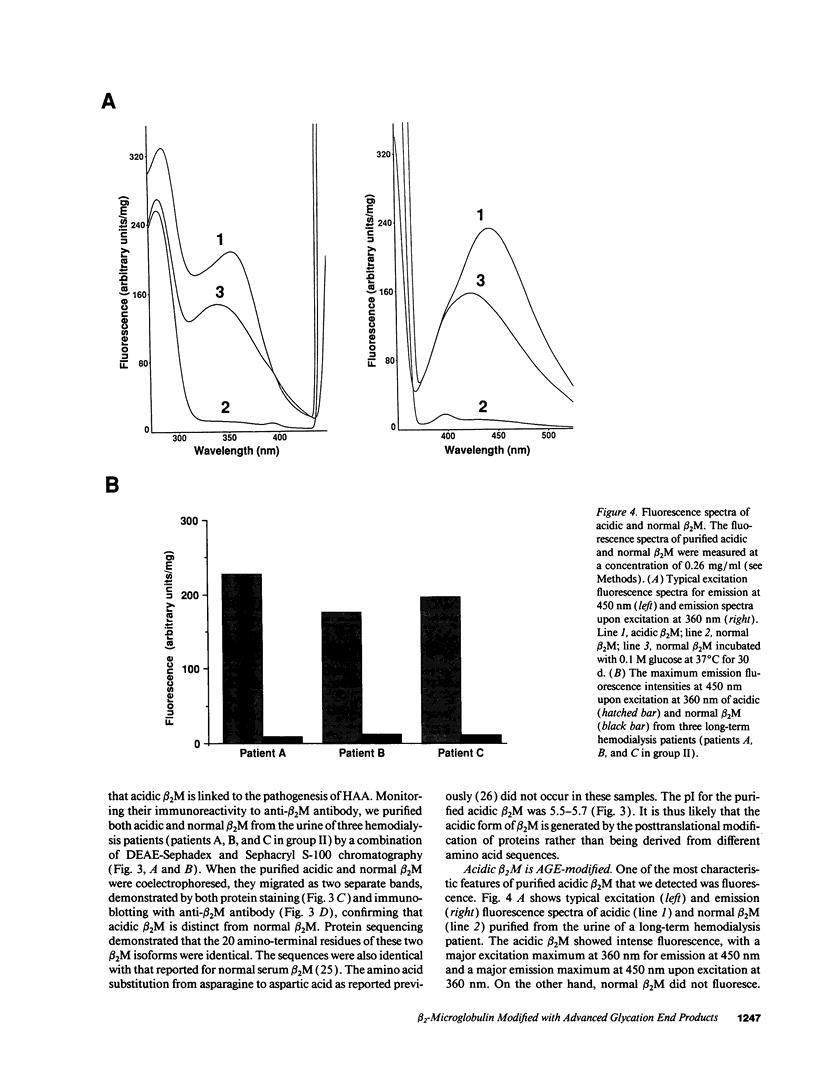
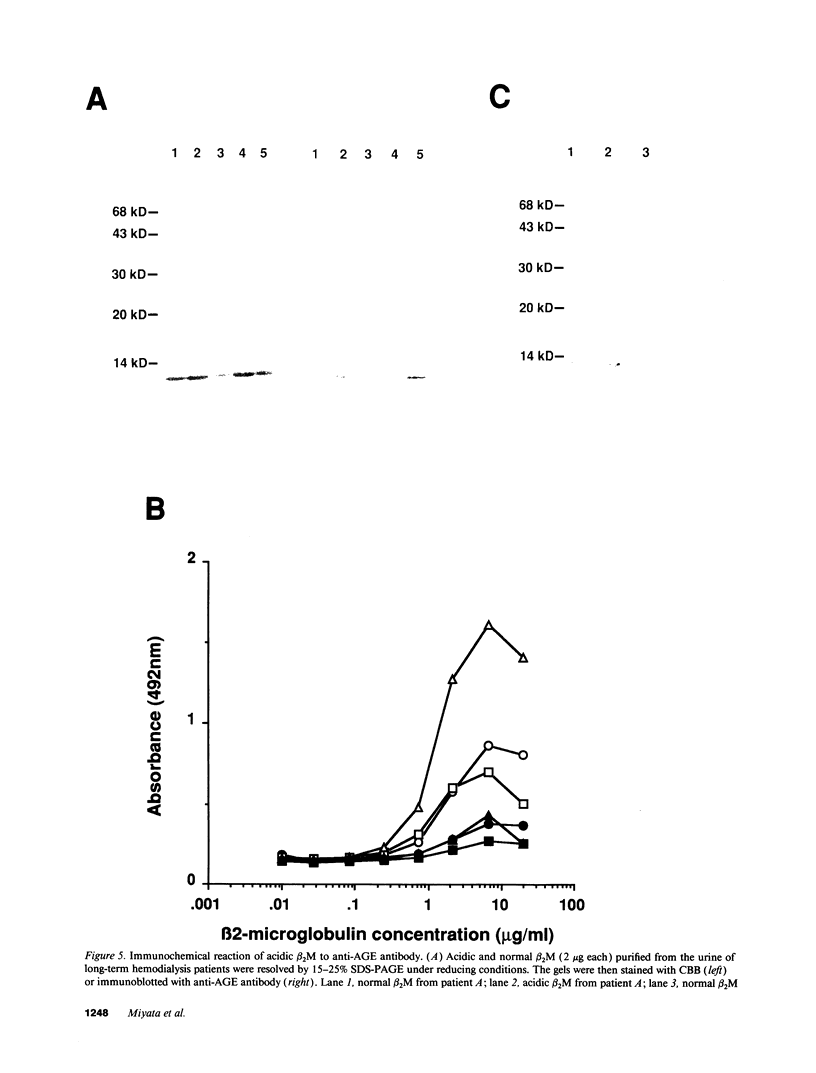
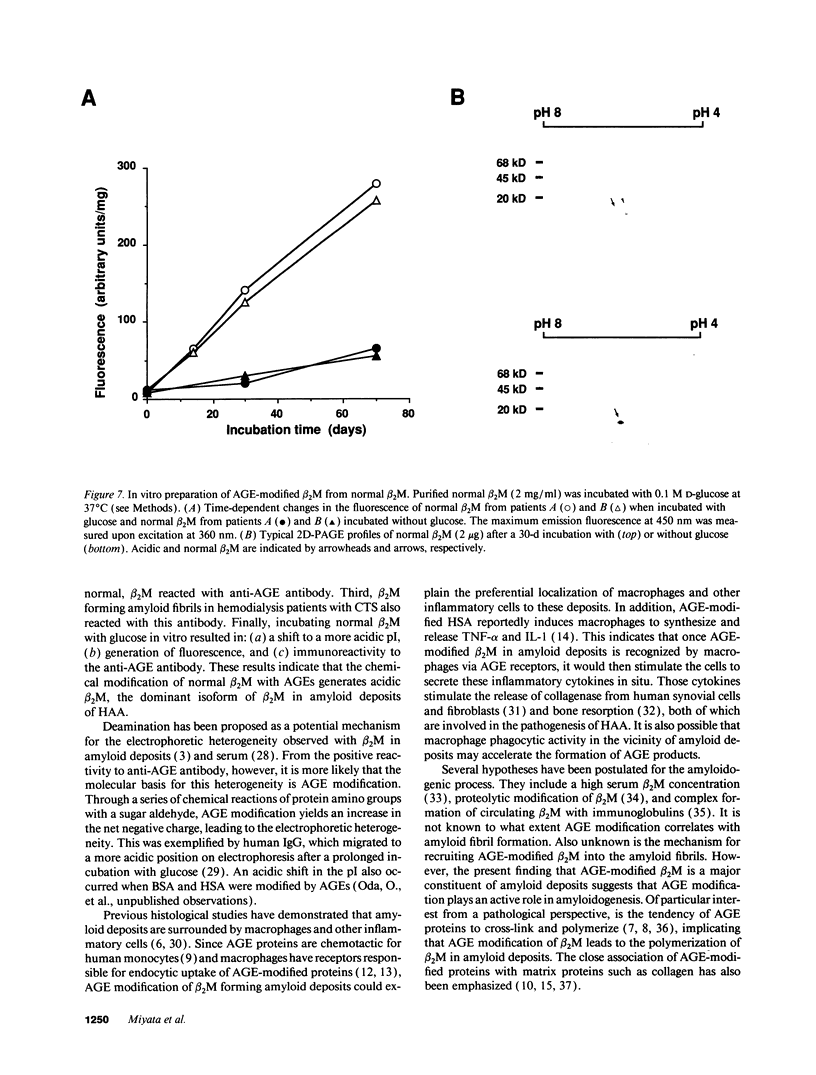
beta 2-Microglobulin (beta 2M) is a major constituent of amyloid fibrils in hemodialysis-associated amyloidosis, a complication of long-term hemodialysis patients. Amyloid fibril proteins were isolated from connective tissues forming carpal tunnels in hemodialysis patients with carpal tunnel syndrome. Two-dimensional polyacrylamide gel electrophoresis and Western blotting demonstrated that most of the beta 2M forming amyloid fibrils exhibited a more acidic pI value than normal beta 2M. This acidic beta 2M was also found in a small fraction of beta 2M in sera and urine from these patients, whereas heterogeneity was not observed in healthy individuals. We purified acidic and normal beta 2M from the urine of long-term hemodialysis patients and compared their physicochemical and immunochemical properties. Acidic beta 2M, but not normal beta 2M, was brown in color and fluoresced, both of which are characteristics of advanced glycation end products (AGEs) of the Maillard reaction. Immunochemical studies showed that acidic beta 2M reacted with anti-AGE antibody and also with an antibody against an Amadori product, an early product of the Maillard reaction, but normal beta 2M did not react with either antibody. Incubating normal beta 2M with glucose in vitro resulted in a shift to a more acidic pI, generation of fluorescence, and immunoreactivity to the anti-AGE antibody. The beta 2M forming amyloid fibrils also reacted with anti-AGE antibody. These data provided evidence that AGE-modified beta 2M is a dominant constituent of the amyloid deposits in hemodialysis-associated amyloidosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki N., Ueno N., Chakrabarti B., Morino Y., Horiuchi S. Immunochemical evidence for the presence of advanced glycation end products in human lens proteins and its positive correlation with aging. J Biol Chem. 1992 May 25;267(15):10211–10214. [PubMed] [Google Scholar]
- Berggård I., Bearn A. G. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem. 1968 Aug 10;243(15):4095–4103. [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Connors L. H., Shirahama T., Skinner M., Fenves A., Cohen A. S. In vitro formation of amyloid fibrils from intact beta 2-microglobulin. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1063–1068. doi: 10.1016/0006-291x(85)90198-6. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depierreux M., Goldman M., Fayt I., Richard C., Quintin J., Dhaene M., Van Herweghem J. L. Osteoarticular amyloidosis associated with haemodialysis: an immunoultrastructural study. J Clin Pathol. 1988 Feb;41(2):158–162. doi: 10.1136/jcp.41.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drüeke T. B. Beta-2-microglobulin amyloidosis and renal bone disease. Miner Electrolyte Metab. 1991;17(4):261–272. [PubMed] [Google Scholar]
- Falus A., Rasmussen J. M., Svehag S. E. Partial characterization of circulating macromolecular beta 2-microglobulin complexes by density gradient ultracentrifugation and page-blotting. Immunol Lett. 1983;7(2):61–64. doi: 10.1016/0165-2478(83)90034-2. [DOI] [PubMed] [Google Scholar]
- Gejyo F., Odani S., Yamada T., Honma N., Saito H., Suzuki Y., Nakagawa Y., Kobayashi H., Maruyama Y., Hirasawa Y. Beta 2-microglobulin: a new form of amyloid protein associated with chronic hemodialysis. Kidney Int. 1986 Sep;30(3):385–390. doi: 10.1038/ki.1986.196. [DOI] [PubMed] [Google Scholar]
- Gejyo F., Yamada T., Odani S., Nakagawa Y., Arakawa M., Kunitomo T., Kataoka H., Suzuki M., Hirasawa Y., Shirahama T. A new form of amyloid protein associated with chronic hemodialysis was identified as beta 2-microglobulin. Biochem Biophys Res Commun. 1985 Jun 28;129(3):701–706. doi: 10.1016/0006-291x(85)91948-5. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Munoz P. C., Casey T. T., DiRaimondo C. R., Stone W. J., Prelli F. C., Rodrigues M. M., Poulik M. D., Frangione B. Polymerization of intact beta 2-microglobulin in tissue causes amyloidosis in patients on chronic hemodialysis. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7908–7912. doi: 10.1073/pnas.83.20.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güssow D., Rein R., Ginjaar I., Hochstenbach F., Seemann G., Kottman A., Ploegh H. L. The human beta 2-microglobulin gene. Primary structure and definition of the transcriptional unit. J Immunol. 1987 Nov 1;139(9):3132–3138. [PubMed] [Google Scholar]
- Horiuchi S., Araki N., Morino Y. Immunochemical approach to characterize advanced glycation end products of the Maillard reaction. Evidence for the presence of a common structure. J Biol Chem. 1991 Apr 25;266(12):7329–7332. [PubMed] [Google Scholar]
- Kirstein M., Brett J., Radoff S., Ogawa S., Stern D., Vlassara H. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9010–9014. doi: 10.1073/pnas.87.22.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linke R. P., Hampl H., Lobeck H., Ritz E., Bommer J., Waldherr R., Eulitz M. Lysine-specific cleavage of beta 2-microglobulin in amyloid deposits associated with hemodialysis. Kidney Int. 1989 Oct;36(4):675–681. doi: 10.1038/ki.1989.245. [DOI] [PubMed] [Google Scholar]
- Makita Z., Radoff S., Rayfield E. J., Yang Z., Skolnik E., Delaney V., Friedman E. A., Cerami A., Vlassara H. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991 Sep 19;325(12):836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- Makita Z., Vlassara H., Rayfield E., Cartwright K., Friedman E., Rodby R., Cerami A., Bucala R. Hemoglobin-AGE: a circulating marker of advanced glycosylation. Science. 1992 Oct 23;258(5082):651–653. doi: 10.1126/science.1411574. [DOI] [PubMed] [Google Scholar]
- Miyata S., Monnier V. Immunohistochemical detection of advanced glycosylation end products in diabetic tissues using monoclonal antibody to pyrraline. J Clin Invest. 1992 Apr;89(4):1102–1112. doi: 10.1172/JCI115690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981 Jan 30;211(4481):491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Monnier V. M. Toward a Maillard reaction theory of aging. Prog Clin Biol Res. 1989;304:1–22. [PubMed] [Google Scholar]
- Njoroge F. G., Sayre L. M., Monnier V. M. Detection of D-glucose-derived pyrrole compounds during Maillard reaction under physiological conditions. Carbohydr Res. 1987 Sep 15;167:211–220. doi: 10.1016/0008-6215(87)80280-x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Odani H., Oyama R., Titani K., Ogawa H., Saito A. Purification and complete amino acid sequence of novel beta 2-microglobulin. Biochem Biophys Res Commun. 1990 May 16;168(3):1223–1229. doi: 10.1016/0006-291x(90)91159-p. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Saito A., Oda O., Nakajima M., Chung T. G. Detection of novel beta 2-microglobulin in the serum of hemodialysis patients and its amyloidogenic predisposition. Clin Nephrol. 1988 Sep;30(3):158–163. [PubMed] [Google Scholar]
- Pongor S., Ulrich P. C., Bencsath F. A., Cerami A. Aging of proteins: isolation and identification of a fluorescent chromophore from the reaction of polypeptides with glucose. Proc Natl Acad Sci U S A. 1984 May;81(9):2684–2688. doi: 10.1073/pnas.81.9.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. End-stage renal disease and diabetes catalyze the formation of a pentose-derived crosslink from aging human collagen. J Clin Invest. 1990 Feb;85(2):380–384. doi: 10.1172/JCI114449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989 Dec 25;264(36):21597–21602. [PubMed] [Google Scholar]
- Shirahama T., Skinner M., Cohen A. S., Gejyo F., Arakawa M., Suzuki M., Hirasawa Y. Histochemical and immunohistochemical characterization of amyloid associated with chronic hemodialysis as beta 2-microglobulin. Lab Invest. 1985 Dec;53(6):705–709. [PubMed] [Google Scholar]
- Skolnik E. Y., Yang Z., Makita Z., Radoff S., Kirstein M., Vlassara H. Human and rat mesangial cell receptors for glucose-modified proteins: potential role in kidney tissue remodelling and diabetic nephropathy. J Exp Med. 1991 Oct 1;174(4):931–939. doi: 10.1084/jem.174.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theaker J. M., Raine A. E., Rainey A. J., Heryet A., Clark A., Oliver D. O. Systemic amyloidosis of beta 2-microglobulin type: a complication of long-term haemodialysis. J Clin Pathol. 1987 Oct;40(10):1247–1251. doi: 10.1136/jcp.40.10.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. High-affinity-receptor-mediated uptake and degradation of glucose-modified proteins: a potential mechanism for the removal of senescent macromolecules. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5588–5592. doi: 10.1073/pnas.82.17.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Manogue K. R., Dinarello C. A., Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988 Jun 10;240(4858):1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Moldawer L., Chan B. Macrophage/monocyte receptor for nonenzymatically glycosylated protein is upregulated by cachectin/tumor necrosis factor. J Clin Invest. 1989 Dec;84(6):1813–1820. doi: 10.1172/JCI114366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Makita Z., Horii Y., Brunelle S., Cerami A., Sehajpal P., Suthanthiran M., Vlassara H. Two novel rat liver membrane proteins that bind advanced glycosylation endproducts: relationship to macrophage receptor for glucose-modified proteins. J Exp Med. 1991 Sep 1;174(3):515–524. doi: 10.1084/jem.174.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]